Abstract
The goal of this study was to develop an in vivo sonothrombolysis model for stroke research. The rabbit carotid artery has average vessel diameters similar to human M1/M2 segments and allows generation of a thrombotic occlusion using various kinds of thrombus material as well as thrombus placement under visual control. It further allows real-time monitoring of flow and clot mechanics during the sonothrombolysis procedure using high-frequency diagnostic ultrasound. In the present study, the model will be introduced and first results to show feasibility using diagnostic as well as high-intensity focused ultrasound will be presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of ultrasound (US) to accelerate clot lysis (sonothrombolysis) in thrombotic or thrombo-embolic diseases is of high interest. The potential impact of sonothrombolysis in clinical areas, such as ischemic stroke, has been shown and first clinical studies are very encouraging [1–5]. Despite the excitement to use ultrasound as a potential thrombolytic amplifier in vessel-occlusive diseases, the current knowledge about basic mechanisms of ultrasound-induced thrombolysis is limited. Reversible disaggregation of fibrin fibers [6] as well as improved penetration of tissue plasminogen activator (tPA) molecules into the clot [7–9] has been described as a potential mechanism why ultrasound has a beneficial effect. In vitro, preferable ultrasound operating parameter combinations, acoustic intensity values, and insonation durations have been suggested [10–12] as well as the use of ultrasound in combination with lytic drugs or ultrasound microbubbles [13–15]. Various ultrasound devices and their potential use for sonothrombolysis have been tested, ranging from commercially available diagnostic [2, 4] to customized, purely therapeutic devices [16, 17] and focused ultrasound systems [18, 19]. The translation of this knowledge into appropriate in vivo sonothrombolysis models, however, is rather poor. For sonothrombolysis research in stroke, a small number of animal models have been established, including rats [20, 21], rabbits [22, 23], and pigs [24] as the preferred species. The majority of these models use a transcranial approach, which means insonation through the intact skull. Regarding acoustic absorption and phase aberration, the skull bone characteristics of these species are very different compared to those of human. Further, thrombus size, clot preparation, and intravascular placement methods vary widely, as shown in Table 1. Hence, it is questionable how the knowledge gained in these animal models can be translated to application in humans. Our current understanding of transcranial sound field characteristics in humans is sparse and little is known about the histological features of true intravascular thrombi causing ischemic strokes. Regarding the latter, a first, detailed histological characterization of intracranial thrombi harvested from interventional procedures was provided recently by Marder et al. [25].
Taking the above-mentioned fact into consideration, it might be reasonable to suggest certain requirements of how a preferred in vivo sonothrombolysis model, in this case for the application stroke, should look. These requirements might include (a) a vessel size comparable to a M1 or M2 segment of a human middle cerebral artery (MCA) [26, 27], (b) a thrombus size/volume comparable to what can be expected in proximal M1 or M2 occlusions, (c) a bifurcation to mimic MCA branching, a preferred location for embolic occlusions, (d) the possibility to mimic a human transcranial insonation, mainly to account for appropriate acoustic signal absorption, (e) the ability to monitor flow and clot mechanics, preferably at any given timepoint before, after, or during the procedure, and (f) perhaps to provide the possibility to introduce actual human thrombi to achieve vessel occlusion.
The aim of the present work was to develop a sonothrombolysis in vivo model which fulfills most of these requirements. To do so, a rabbit carotid artery model has been chosen and the preliminary knowledge gained using this model will be described in detail.
Materials and Methods
Animal Anesthesia, Preparation, Surgery
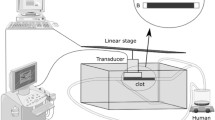
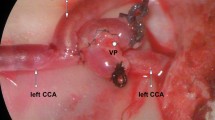
The following study protocol was approved by the University of California, San Diego Institutional Animal Care and Use Committee (protocol #S07273). For all studies, female New Zealand white rabbits with an average weight range between 1.5 and 2.5 kg were used. Animals were pre-anesthesized with ketamine (35–50 mg/kg, subcutaneous (SQ), 1x bolus) and xylazine (5.0–8.8 mg/kg, SQ, 1x bolus). Next, the neck was shaved using standard clippers (Super AGR+, „“andis Inc., Sturtevant, WI, USA). To maintain anesthesia during the procedure, propofol (3.0–10.0 mg/kg/min, IV) was administered. For the surgical procedure, the animals were placed in supine position into a customized, MRI-compatible rabbit holder and connected to a monitoring device (MouseOx™, STARR Lifesciences Corp., Oakmont, PA, USA) to control the vital signs during the procedure (Fig. 1). In the following steps, the carotid artery was separated from its most proximal accessible segment to about 0.5 cm distal to the bifurcation into the internal and external carotid artery, leading to an approximate total separation between 2.0 and 4.0 cm. Clot preparation and placement are described in the following text.
Individual experiments generally lasted 90–120 min, at which point the animals were euthanized with sodium pentobarbital (150 mg/kg, IV) without regaining consciousness.
Thrombus Preparation
Following the literature, it is apparent that there is no common agreement of how experimental thrombi used for in vivo sonothrombolysis studies should be generated and clot preparation recipes vary widely. A selected overview of current thrombus preparation techniques is given in Table 1. As part of the model development, the aim was to investigate whether different thrombus preparation techniques can be applied to the rabbit carotid artery model and to assess potential differences, for example, in fibrin fiber architecture, between different kinds of thrombi using scanning electron microscopy (SEM). The following three thrombus preparation methods were studied:
-
Approach I (rabbit, arterial)—Initially, thrombotic occlusion was accomplished as follows: a 0.5-cm segment of the common carotid artery was clamped and 2–4 IU of thrombin (King Pharmaceuticals Inc., Bristol, TN, USA) was injected into the clamped segment. After 20–45 min, the proximal clamp was released, followed by distal clamp release an additional 15 min later. Approach I was chosen because the additional use of thrombin to accelerate thrombus formation seems to be more frequently represented in the literature.
-
Approach II (rabbit, venous)—Samples of 0.5 ml venous rabbit whole blood were transferred into 1.0-ml glass syringes and incubated for 3 h in a 37°C waterbath. After incubation, the clots were transferred into a 1.0-ml plastic syringe used for insulin injections. Using a 20 G needle, the thrombotic material was injected into a 0.5-cm clamped segment of the common carotid artery. Prior to injection, the clamped vessel segment was injured using blunt forceps to induce the release of tissue factor 3. After intravascular incubation of 30–60 min, the proximal clamp was released first, followed by release of the distal clamp 15 min later. Approach II was chosen based on the consideration that additional thrombin accelerates clot formation; however, it detours the main part of the coagulation cascade, leading to artificial, fibrin-rich thrombi. Hence, an optional method using rabbit whole blood, now being generated outside the vascular system and without the addition of thrombin, was suggested.
-
Approach III (human, venous)—Venous whole blood was drawn from healthy, unmedicated donors into vacutainer citrate tubes. A UCSD-approved IRB protocol in accordance with the World Medical Association Declaration of Helsinki was in place. Each donor signed an informed consent prior to the blood draw. For 10 s, 0.5 mL of citrate blood was vortexed with 45 μL CaCl2 (210 mmol/L) and transferred into a borosilicate glass syringe. Thrombi were incubated for 3.0 h in a preheated (37°C) waterbath. After incubation, the clots were transferred into a 1.0-ml plastic insulin syringe. Using a 20 G needle, the thrombotic material was injected into a 0.5-cm clamped segment of the common carotid artery. After intravascular incubation of 30–60 min, the proximal clamp was released, followed by distal clamp release 15 min later. Approach III was chosen because of the ultimate goal to treat embolic strokes in humans. Therefore, the goal must be to study human thrombi. Since ex situ human blood clots were not available for the present study, the compromise to generate thrombi using human whole blood seemed to be reasonable.
For all three approaches, immediately after confirming total occlusion by non-contrast high frequency ultrasound, heparin was administered as a bolus of 200 units/kg in all cases to avoid re-thrombosis.
Ultrasound Monitoring
Prior to clamping of the carotid artery, an artificial stenosis of about 60 % diameter reduction was created further downstream using a surgical suture (2–0, Ethicon, Inc., Somerville, NJ, USA) and under visual control using a high-end duplex ultrasound device (iU22, Philips Healthcare, Bothell, WA, USA) and linear array transducer (X7-2). The purpose of the artificial stenosis was to avoid a potential downstream flush of the clot in case of dislocation due to the flow mechanics after clamp release. The same ultrasound equiment was used to assess vessel occlusion and to monitor potential recanalization during or after therapeutic ultrasound exposure (Fig. 2).
a Brightness mode image of the common carotid artery after a 60-% artificial stenosis (arrow) has been created under visual control and prior to thrombus placement. b A hyperechogenic thrombus in place. c Post-insonation color Doppler in combination with spectral Doppler is used to assess recanalization. d Post-recanalization wall-adherent thrombotic material can be visualized in brightness mode (arrows)
Operating Parameters—Diagnostic Ultrasound
For the sonothrombolysis studies using primary diagnostic ultrasound, a Philips iU22 was used, with its phased array transducer (S5–1). The operating parameter combination was as follows: the transmit frequency ranged from 1.6 to 2.0 MHz per ultrasound pulse (Chirp Mode), the pulse width was 5 μs with an 8-kHz pulse repetition frequency, and each insonation lasted 30 min. A distance of 5.0 cm between the transducer’s aperture and the target clot was achieved using a custom-made agar (5 %) stand-off, which had a groove at its bottom surface to surround the target vessel segment without compression. The transducer was mounted on top of the agar stand-off with the center beamline aiming at the target clot.
Operating Parameters—High-Intensity Focused Ultrasound
An ExAblate™ 4000 High-Intensity Focused Ultrasound head system (HIFU; InSightec Inc., Tirat Carmel, Israel) was used for this study. The key component of this system is a hemispheric phased array transducer with approximately 1,000 elements that can be operated individually. Differently from conventional ultrasound systems, ExAblate™ 4000 generates a sharp three-dimensional focus (4.0 mm in diameter, 6.0 mm in length) located in the center of the transducer. Due to the three-dimensionality of the HIFU beam, the target (i.e., thrombus) is insonated from all directions of the hemisphere. Using the customized rabbit holder, the animal was positioned inside the transducer in such a way that the carotid artery thrombus was exposed to the center of the focus. Whereas the transmit frequency remained constant at 220 kHz, other operating parameters such as duty cycle, pulse width, acoustic output power, or insonation duration varied, as presented in the Tables 2, 3, and 4.
Virtual Transskull Insonation
To account for acoustic signal absorption due to the skull bone, an attenuation value of 90 % was assumed. Since we did not place a piece of human skull between the transducer and the therapeutic site, we mimicked the skull attenuation by reducing the output power of the device by a factor of 10 from the FDA limit of 720 mW/cm2. That is, we measured the intensity of the acoustic field in a wet tank (AIMS, ONDA Corp., Sunnyvale, CA, USA) and adjusted the ultrasound power so as not to exceed 72 mW/cm2. These power levels were then recorded and used for subsequent sonothrombolytic studies. Although the amount of sound attenuation varies by individual, a 90 % acoustic reduction seemed reasonable based on our earlier work characterizing the acoustic field of diagnostic ultrasound devices [28].
For the HIFU sonothrombolysis studies, we mimicked the presence of the skull with a 70 % attenuation factor. This was based on prior acoustic measurements with and without a human skull specimen in the HIFU transducer. For our in vitro experiments, the acoustic power had been set to 235 W. Accordingly, to simulate the effect of the skull for the HIFU in vivo studies, the acoustic output power was reduced to 66 W. However, this reduced power setting was used in only a small subset of the total number of experiments. In the majority of studies, the acoustic output was increased to significantly higher values.
Scanning Electron Microscopy
Blood clot specimens were prepared for SEM to visualize the fibrin network structural changes induced by US. Immediately after exposure to US, for treatment groups, an insonated clot and a control clot were washed three times in 0.1 M of Dulbecco’s phosphate-buffered saline (DPBS). For primary fixation, washed clots were immersed in 2.5 % glutaraldehyde in 0.1 M of Dulbecco’s DPBS buffer with a pH of 7.4 for 60–90 min at 4°C and rinsed three times with a cold DPBS wash for 10 min for each change. Post-fixation, a secondary fix of specimens in 2 % osmium tetroxide (OsO4) in buffer solution at pH 7.4 (1 ml 2 % OsO4 + 1 ml of 0.1 M DPS) was done for 1 h. Then, clot samples were rinsed with distilled water, three times for 5 min, and dehydrated using graded serial ethanol dilutions from 30 to 100 %. Critical point drying (Autosamdri 815 A, Tousimis Research Corp., Washington, DC, USA) of the clot samples were done with 100 % ethanol. The clots were then cut in cross- or longitudinal sections, such to be mounted and coated with iridium (Ir). Sputter coating (K575X, Quorum Technologies, East Sussex, UK) was done with Ir at 85 mA sputter current with a 7-s deposition time.
Passive Cavitation Detection
We used a combination of MATLAB™ (The Mathworks, Natick, MA, USA) and AIMS to acquire signals detected by a passive cavitation detection (PCD) hydrophone. The PCD (model Y-105, Sonic Concepts, Bothell, WA, USA) had two side-facing, non-focused elements and was positioned about 1.5 cm distant to the carotid artery with the active element facing toward the thrombus. MATLAB™ was used to take remote control of AIMS, import waveform data, and perform spectral decomposition.
Results
General Impression
To date, a total of N = 43 in vivo sonothrombolysis studies using the rabbit carotid artery model have been performed. Anesthesia and monitoring, positioning of the animal using a customized rabbit holder as well as the surgical procedure itself could be performed in a timely fashion. Vessel occlusion using different thrombus preparation methods could be achieved with great confidence. In less than 10 % of all interventions, the initial thrombus dislocated further downstream after clamp release, which required a repetition of the clot formation procedure.
Diagnostic Ultrasound
Using diagnostic ultrasound, only N = 3 animals were studied until today. In all cases, vessel occlusion could be achieved. Thrombus preparation was done following the Approach II procedure as described above. The ultrasound exposure time was 30 min in each experiment and no further drugs, such as tPA or ultrasound microbubbles, were used. In all three cases, no vessel recanalization could be achieved. Per visual inspection using high-resolution duplex ultrasound, the intravascular thrombi did not change in location, echogenicity, or size. The main goal for this first study was to test the reliability and reproducibility of the surgical procedure itself and the positioning of the ultrasound transducer.
High-Intensity Focused Ultrasound
To date, we gained most knowledge about the rabbit carotid artery model and in vivo sonothrombolysis using HIFU. The primary reason to use HIFU was that we recently finished a larger series (total N = 2,000) of in vitro HIFU sonothrombolysis studies showing significant clot weight loss within seconds of ultrasound exposure [29].
Whereas animal preparation, anesthesia, surgery, and thrombus generation did not raise any concerns technically, in only N = 4 cases could vessel recanalization, full or partial, be achieved, which is 9.3 % of all HIFU in vivo studies we have performed so far (N = 40) and less than we anticipated based on our in vitro results.
In the first case of recanalization, vessel occlusion had been achieved by injection of 20 μl (2–4 IU) thrombin and incubation of 45 min (Approach I). Full recanalization was achieved after a single 30-s insonation using the following operating parameters: (a) duty cycle 5 %, (b) pulse width 100 ms, and (c) acoustic output power 100 W. This corresponded to a peak negative pressure (P neg) of 4.7 MPa and a mechanical index (MI) of 10.1.
In the second (Approach I) and third (Approach II) case, full recanalization could be achieved after subtotal occlusion was confirmed (residual, hemodynamically non-relevant flow documented by color Doppler). In these cases, insonation was performed after intravenous administration of a platelet aggregation blocker (case 2: Integrilin™, case 3: Tirofiban™). The following parameter combination was used for both studies: (a) duty cycle 5 %, (b) pulse width 0.1 ms, (c) acoustic output power 500 W, and (d) insonation duration of 30 s (P neg = 10.6 MPa, MI = 22.6). In the second case, multiple insonations (N = 4) were required to achieve recanalization, whereas a single insonation was needed for the third case.
In the fourth case (Approach III), partial recanalization could be achieved after intravenous administration of Tirofiban™ using the following parameter combination: (a) duty cycle 100 %, (b) acoustic output power 600 W, and (c) insonation duration 30 s (P neg = 11.6 MPa, MI = 24.8). A single insonation was needed to partially recanalize the vessel.
In all other studies (N = 39), no recanalization could be achieved independent from the parameter combination, single or repetitive insonations, the use or platelet blockers, or thrombus preparation method.
Comparison of Rabbit Versus Human Clots
In our efforts to understand why only a relatively small amount of vessel recanalization could be achieved, we performed selected SEM studies comparing rabbit and human thrombi before and after HIFU exposure. The comparison between human clots and rabbit clots showed that the fibrin architecture between both is quite different. Whereas in human clots the organization between fibrin fibers seemed to be more loose, thick bundles of fibrin fibers could be seen in rabbit clots. Further, it appeared that the fiber density in rabbit clots was much higher than in human clots. It is noteworthy as well that the individual fibers were much more windy, rather than straight as seen in human clots, and they seemed to be organized in a more chaotic pattern compared to the fiber organization in human clots (Fig. 3).
Scanned electron microscopic images of human (a × 10,000, c × 20,000) and rabbit (b × 10,000, d × 20,000) thrombus specimens. Whereas in human clots the organization between fibrin fibers seemed to be more loose, thick bundles of fibrin fibers can be seen in rabbit clots. Further, it appeared that the fiber density in rabbit clots was much higher than in human clots
After ultrasound exposure, the disaggregation of the fibrin fiber network could be seen with single fibers covered with cellular matrix (dust). Here again, the effect of fibrin fiber disaggregation was much more pronounced in human thrombi compared to those of rabbit specimens (Fig. 4). This disaggregation is consistent with previous studies [6].
Scanned electron microscopic images of human (a × 10,000, c × 20,000) and rabbit (b × 10,000, d × 20,000) thrombus specimens after ultrasound exposure. Disaggregation of the fibrin fiber network could be seen with single fibers covered with cellular matrix (dust). The effect of fibrin fiber disaggregation was more pronounced in human thrombi compared to rabbit specimens
Passive Cavitation Detection
We searched the literature and found that platelet aggregation can be caused by shear stress due to inertial cavitation [30]. To answer the question whether inertial cavitation might have occured during HIFU exposure, we performed PCD measurements in two animals. In both cases, inertial cavitation could be detected at acoustic power levels of 10 W and above, using the following parameter combination: duty cycle 50 %, pulse width 100 ms. Typical cavitation profiles are displayed in Fig. 5.
Spectral signals obtained by fast Fourier transform of the waveforms picked up by the PCD hydrophone. The black spectrum plot is typical of applied power being less than required to produce cavitation. The red spectrum, produced with higher applied power, indicates the presence of inertial cavitation, as seen by the overall rise in broadband signal. At 220 kHz (arrow), the transmit frequency is displayed as the highest peak
Clot Imaging Using High-Frequency Duplex Ultrasound
In all cases, high-frequency ultrasound imaging could be used to assess occlusion, recanalization, and thrombus dimensions. In case of recanalization, remaining thrombotic material adherent to the vessel wall could be visualized and the flow velocities could be assessed using spectral Doppler (Fig. 2). Compression/decompression (waterhammer effect) as well as detachment of the thrombus in dependence of the phase of the cardiac cycle could be studied in real time (Fig. 6).
Brightness mode images of thrombus in place prior to ultrasound exposure during diastole (a, b) and systole (c, d). Observation 1—the clot is attached to the vessel wall in its entire circumference. During systole (a), the clot does not detach from the vessel wall but is compressed significantly along its longitudinal axis (length, 7 mm). During diastole (c), the clot extends to its original length of 10 mm. Observation 2—c In this case, the clot detaches partially from the vessel wall during systole (arrows) and attaches back to the vessel wall during diastole (d). No change in thrombus length could be observed
Discussion
It could be demonstrated that using the rabbit carotid artery model for in vivo sonothrombolysis research is feasible. The model provides vessel dimensions (M1/M2 segments) and anatomical features (bifurcation) which are comparable to the human intracranial vasculature. Vessel occlusion can be achieved using different thrombus preparation techniques, including the placement of human clot material, and using clot dimensions that are similar to distal M1- or proximal M2-occluding thrombi in humans. High-frequency ultrasound can be used for high-resolution imaging before, after, and during the sonothrombolytic procedure. The model is compatible with different ultrasound techniques used for sonothrombolysis, including diagnostic ultrasound or high-intensity focused ultrasound.
In order to find the optimal stroke sonothrombolysis model, certain requirements should be fulfilled to mimic the human pathology of an intracranial embolic vessel occlusion. These requirements may include similar vessel sizes, comparable thrombus dimensions, and the possibility to mimic a transcranial insonation based on acoustic features (i.e., signal attenuation) as they appear inside a human skull. The preliminary results described in the present work, however, imply that the quality of an appropriate in vivo sonothrombolysis model, first and foremost, is defined by the thrombotic material itself. The results of the SEM studies show the obvious differences between human and rabbit fibrin networks and indicate why rabbit clots might be more difficult to disaggregate than human blood clots. Although no quantitative analysis was performed, the visual inspection before and after ultrasound exposure confirms the much higher fibrin fiber density in rabbit clots. The latter might lead to the conclusion that rabbit clots are inappropriate for sonothrombolysis research. A similar impression was described in a recent publication by Abramowicz et al., published in 2003 [31]. The authors compared the histology, coagulation, and lysis behavior of rabbit and human whole blood samples exposed to 1-MHz ultrasound. In this work, the fibrin architecture was not investigated specifically, but the investigators found that rabbit erythrocytes are significantly smaller and more difficult to lyse compared to human red blood cells. Cell lysis during ultrasound exposure, however, might play an important role and determines, besides fibrin disaggregation, thrombolysis efficacy. Hence, the observation by Abramowicz et al. would tend to support less lysis with rabbit blood clots. To overcome this potential bias, the intention to study human thrombi placed inside a rabbit carotid artery was put into practice and was shown to be feasible in the present work. A similar approach has been described by Alonso et al. [14]. A drawback of the approach to use human thrombi in a rabbit model is limited knowledge regarding interaction of foreign clot material with rabbit hematology.
In general, artificial thrombi are used for sonothrombolysis research, in vitro as well as in vivo. In the present study, thrombi were made out of human whole citrate blood from healthy volunteers (Approach III). Vessel-occluding thrombi in humans have quite different characteristics, for example, with regard to fibrin, cellular, or cholesterin content. A significant contribution to this field was provided by Marder et al. [25] who studied the histology of ex vivo thrombi harvested from neurointerventional procedures in acute ischemic stroke patients using the MERCI retriever device. They could show that, independent from the source of embolism, cardioembolic or atherosclerotic, most of the thromboemboli shared architectural features of random fibrin–platelet deposits with contents of nucleated cells, such as monocytes and neutrophils, as well as erythrocyte-rich regions. True red thrombi with predominant erythrocyte content were uncommon. The authors stated as well that the fibrin–platelet composition in these ex vivo clots provides a foundation for potential anticoagulant as well as antiplatelet treatment options. To our knowledge, the latter is currently not being investigated with regard to sonothrombolysis preclinically but would be of great interest based on the present observations.
Ultrasound-induced platelet aggregation has been described earlier and might be of concern with regard to sonothrombolysis. Williams et al. [32] studied the effects of therapeutic intensities of ultrasound on human platelets in whole blood by monitoring the release of the platelet-specific protein beta-thromboglobulin (beta-TG). They could show increasing beta-TG release with increasing ultrasound intensity. Kornowski et al. [33] described the effect of ultrasound-induced platelet activation which counterbalances its thrombolysis accelerating effect. The mechanism of platelet activation is based upon shear stress. The latter is induced by inertial cavitation, caused by ultrasound [30]. We detected inertial cavitation signals during HIFU exposure at acoustic output power levels of ≥10 W. Hence, we can assume that, in all HIFU studies performed, inertial cavitation occured and that this efect potentially promoted platelet activation. This could explain, besides other underlying mechanisms, why the succes rate we saw was relatively small. Unfortunately, the cavitation measurements were performed at the very end of this study series and no cavitation measurements were performed using diagnostic ultrasound for sonothrombolysis. However, regarding the latter, it is highly unlikely that the pressure levels reached during these applications could have caused inertial cavitation since output power was capped at 10 % of current FDA limits for diagnostic ultrasound.
The use of high-frequency ultrasound imaging provides the opportunity to assess vessel occlusion and recanalization using the same techniques being used for diagnostic imaging in humans. It further enables assessment of thrombus dimensions prior and wall-adherent thrombotic material post-therapeutic ultrasound exposure. Most impressive, however, is the ability to visualize the impact of fluid mechanics on the vessel-occluding thrombus. To our knowledge, the impact of flow mechanics on sonothrombolysis has not been investigated thoroughly, but it can be assumed that flow mechanics do play an important role with regard to changing clot density throughout the cardiac cycle as well as transport mechanisms towards the vessel-occluding thrombus.
The main limitations of the model are the lack of vessel wall histology to assess whether endothelial damage might be caused by ultrasound exposure. Future studies will focus on this aspect. Since the animals are euthanized immediately after the experimental procedure, there is currently no opportunity to study potential long-term effects of ultrasound exposure, such as inflammatory reactions or smooth muscle cell proliferation. The model provides the possibility to test for clot fragmentation and subsequent distal occlusion of smaller-sized intracranial vessel segments. This was not investigated in the present work, but we intend to do so in the near future. At the current developmental stage, the model does not acknowledge an arterial bifurcation as a preferred location for thrombo-embolic events since the clot placement was done in the common carotid artery. Lastly, by using the common carotid artery as the target vessel, the model does not consider morphological differences between extra- and intracranial arteries.
In conclusion, the use of a rabbit carotid artery model for sonothrombolysis research is feasible and provides features which might mimic an arterial thrombotic-occlusive event in humans more closely. It has the potential to provide a more profound insight into the interplay between pro-thrombolytic activities and counterbalancing platelet activation induced by ultrasound. The ability to study the flow mechanics during pre-/post- and during sonothrombolysis might be an additional option to study the impact of blood flow on sonothrombolysis. Further investigations are needed, and planned, to confirm reproducibility and reliability of this model.
References
Alexandrov AV, Mikulik R, Ribo M, et al. A pilot randomized clinical safety study of sonothrombolysis augmentation with ultrasound-activated perflutren-lipid microspheres for acute ischemic stroke. Stroke. 2008;39(5):1464–9.
Alexandrov AV, Molina CA, Grotta JC, et al. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004;351(21):2170–8.
Molina CA, Barreto AD, Tsivgoulis G, et al. Transcranial ultrasound in clinical sonothrombolysis (TUCSON) trial. Ann Neurol. 2009;66(1):28–38.
Eggers J, Konig IR, Koch B, Handler G, Seidel G. Sonothrombolysis with transcranial color-coded sonography and recombinant tissue-type plasminogen activator in acute middle cerebral artery main stem occlusion: results from a randomized study. Stroke. 2008;39(5):1470–5.
Eggers J, Seidel G, Koch B, Konig IR. Sonothrombolysis in acute ischemic stroke for patients ineligible for rt-PA. Neurol. 2005;64(6):1052–4.
Braaten JV, Goss RA, Francis CW. Ultrasound reversibly disaggregates fibrin fibers. Thromb Haemost. 1997;78(3):1063–8.
Devcic-Kuhar B, Pfaffenberger S, Gherardini L, et al. Ultrasound affects distribution of plasminogen and tissue-type plasminogen activator in whole blood clots in vitro. Thromb Haemost. 2004;92(5):980–5.
Pfaffenberger S, Devcic-Kuhar B, El-Rabadi K, et al. 2 MHz ultrasound enhances t-PA-mediated thrombolysis: comparison of continuous versus pulsed ultrasound and standing versus travelling acoustic waves. Thromb Haemost. 2003;89(3):583–9.
Datta S, Coussios CC, Ammi AY, Mast TD, de Courten-Myers GM, Holland CK. Ultrasound-enhanced thrombolysis using Definity((R)) as a cavitation nucleation agent. Ultrasound Med Biol. 2008;34(9):1421–33.
Schafer S, Kliner S, Klinghammer L, et al. Influence of ultrasound operating parameters on ultrasound-induced thrombolysis in vitro. Ultrasound Med Biol. 2005;31(6):841–7.
Datta S, Coussios CC, McAdory LE, et al. Correlation of cavitation with ultrasound enhancement of thrombolysis. Ultrasound Med Biol. 2006;32(8):1257–67.
Behrens S, Daffertshofer M, Spiegel D, Hennerici M. Low-frequency, low-intensity ultrasound accelerates thrombolysis through the skull. Ultrasound Med Biol. 1999;25(2):269–73.
Holscher T, Raman R, Ernstrom K, et al. In vitro sonothrombolysis with duplex ultrasound: first results using a simplified model. Cerebrovasc Dis. 2009;28(4):365–70.
Alonso A, Dempfle CE, Della Martina A, et al. In vivo clot lysis of human thrombus with intravenous abciximab immunobubbles and ultrasound. Thromb Res. 2009;124(1):70–4.
Dijkmans PA, Juffermans LJ, Musters RJ, et al. Microbubbles and ultrasound: from diagnosis to therapy. Eur J Echocardiogr. 2004;5(4):245–56.
Daffertshofer M, Gass A, Ringleb P, et al. Transcranial low-frequency ultrasound-mediated thrombolysis in brain ischemia: increased risk of hemorrhage with combined ultrasound and tissue plasminogen activator: results of a phase II clinical trial. Stroke. 2005;36(7):1441–6.
Siegel RJ, Atar S, Fishbein MC, et al. Noninvasive transcutaneous low frequency ultrasound enhances thrombolysis in peripheral and coronary arteries. Echocardiogr. 2001;18(3):247–57.
Frenkel V, Oberoi J, Stone MJ, et al. Pulsed high-intensity focused ultrasound enhances thrombolysis in an in vitro model. Radiol. 2006;239(1):86–93.
Rosenschein U, Furman V, Kerner E, Fabian I, Bernheim J, Eshel Y. Ultrasound imaging-guided noninvasive ultrasound thrombolysis: preclinical results. Circ. 2000;102(2):238–45.
Daffertshofer M, Huang Z, Fatar M, et al. Efficacy of sonothrombolysis in a rat model of embolic ischemic stroke. Neurosci Lett. 2004;361(1–3):115–9.
Saguchi T, Onoue H, Urashima M, Ishibashi T, Abe T, Furuhata H. Effective and safe conditions of low-frequency transcranial ultrasonic thrombolysis for acute ischemic stroke: neurologic and histologic evaluation in a rat middle cerebral artery stroke model. Stroke. 2008;39(3):1007–11.
Culp WC, Flores R, Brown AT, et al. Successful microbubble sonothrombolysis without tissue-type plasminogen activator in a rabbit model of acute ischemic stroke. Stroke. 2011;42(8):2280–5.
Flores R, Hennings LJ, Lowery JD, Brown AT, Culp WC. Microbubble-augmented ultrasound sonothrombolysis decreases intracranial hemorrhage in a rabbit model of acute ischemic stroke. Invest Radiol. 2011;46(7):419–24.
Culp WC, Erdem E, Roberson PK, Husain MM. Microbubble potentiated ultrasound as a method of stroke therapy in a pig model: preliminary findings. J Vasc Interv Radiol. 2003;14(11):1433–6.
Marder VJ, Chute DJ, Starkman S, et al. Analysis of thrombi retrieved from cerebral arteries of patients with acute ischemic stroke. Stroke. 2006;37(8):2086–93.
Rasmussen LE, Vanhoutte PM, Jensen BL, Skott O. Continuous flow augments reactivity of rabbit carotid artery by reducing bioavailability of NO despite an increase in release of EDHF. Am J Physiol Heart Circ Physiol. 2006;291(4):H1521–8.
Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke. 2000;31(7):1672–8.
Holscher T, Wilkening WG, Molkenstruck S, Voit H, Koch C. Transcranial sound field characterization. Ultrasound Med Biol. 2008;34(6):973–80.
Hoelscher T FD, Raman R, Ernstrom K, Zadicario E, Bradley WG, Voie A. Noninvasive transcranial clot lysis using high intensity focused ultrasound. Journal of Neurology and Neurophysiology 2011;S1-002.
Poliachik SL, Chandler WL, Mourad PD, et al. Effect of high-intensity focused ultrasound on whole blood with and without microbubble contrast agent. Ultrasound Med Biol. 1999;25(6):991–8.
Abramowicz JS, Miller MW, Battaglia LF, Mazza S. Comparative hemolytic effectiveness of 1 MHz ultrasound on human and rabbit blood in vitro. Ultrasound Med Biol. 2003;29(6):867–73.
Williams AR, Chater BV, Allen KA, Sherwood MR, Sanderson JH. Release of beta-thromboglobulin from human platelets by therapeutic intensities of ultrasound. Br J Haematol. 1978;40(1):133–42.
Kornowski R, Meltzer RS, Chernine A, Vered Z, Battler A. Does external ultrasound accelerate thrombolysis? Results from a rabbit model. Circ. 1994;89(1):339–44.
Acknowledgments
This work has been supported by the National Institute of Health (R01HL091043-01A2) and InSightec, Inc., Tirat Carmel, Israel. We would like to thank very much Cheryl Schendel and Daniel Lotz who contributed significantly to the development of the model.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hölscher, T., Fisher, D.J., Ahadi, G. et al. Introduction of a Rabbit Carotid Artery Model for Sonothrombolysis Research. Transl. Stroke Res. 3, 397–407 (2012). https://doi.org/10.1007/s12975-012-0194-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-012-0194-5