Abstract
This review provides a synthesis of the work done by our laboratory that demonstrates the presence of cellular immune responses directed towards brain antigens in animals following experimental stroke as well as in patients following ischemic stroke. These responses include both antigen-specific Th1(+) responses, which are associated with worse stroke outcome, and antigen-specific Treg responses, which are associated with better stroke outcome. The likelihood of developing a detrimental Th1(+) response to brain antigens is increased by administration of a systemic inflammatory stimulus in experimental stroke and by systemic infection in patients with stroke. We propose that the microenvironment within the lymph nodes and brain is altered by systemic inflammation and allows for bystander activation of lymphocytes and the development of autoimmune responses to brain antigens following cerebral ischemic injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Few therapies are proven to improve outcome after stroke, and the time frame for effective intervention is short. Identification of processes that contribute to disability and are amenable to delayed intervention is needed. It is clear that stroke induces alterations in the immune response, and there is a robust inflammatory response that occurs immediately after stroke which appears to contribute to ischemic brain injury [1–3]. This response includes the infiltration of leukocytes from the peripheral circulation into the ischemic brain as well as the activation of resident inflammatory cells [4]. For the purposes of this review, we distinguish between the early or innate immune response, which is mediated by neutrophils, monocytes, and lymphocytes not constrained by antigen specificity, and the delayed or adaptive immune response, which is mediated by lymphocytes activated to a specific antigen.
Because the peripheral immune system gains access to the brain following stroke, lymphocytes encounter antigens that are unique to the central nervous system (CNS) [5–8]. These antigens include intracellular/cryptic antigens from injured neurons and glia. And given that stroke induces compromise of the blood–brain barrier (BBB), dying cells “release” antigens into the systemic circulation, which means that CNS antigens can also be presented to lymphocytes in peripheral lymph nodes as well as brain [9–11]. The possibility of an autoimmune response developing to brain unique antigens thus exists after stroke. Indeed, the presence of a cellular immune response to myelin basic protein (MBP) and “sensitivity to nervous-tissue antigens” in patients with stroke was documented in the early 1970s [12–15]. More recently, it has been shown that individuals who experience stroke have higher titers of antibodies to CNS antigens such as neurofilaments and portions of the N-methyl-d-aspartate (NMDA) receptor [16, 17]. These immune responses are largely considered to occur as an epiphenomenon of cerebral tissue injury; the possibility that such responses have pathological consequences has previously not been addressed. The focus of our research has been to determine if an adaptive (or antigen specific) cellular immune response to brain antigens influences stroke outcome.
One of the most commons ways to measure the cellular response to a given antigen is by ELISPOT assay. Briefly, ELISPOTs are cell-based ELISA assays optimized to detect the secretion of a particular cytokine. For our experiments, the number of lymphocytes secreting interferon (IFN)-γ specifically in response to stimulation with a given antigen (primarily MBP) is used as an indicator of a Th1 response, and the number of lymphocytes secreting transforming growth factor (TGF)-β1 specifically in response to stimulation with the same antigen (MBP) is used as an indicator of a Treg response. The convention that we have adopted is to use the term “Th1” to refer to the ratio between the antigen-specific IFN-γ response and the TGF-β1 response, and the term “Th1(+)” to indicate that a given animal/person has a Th1 response that exceeds a defined threshold. Our initial assumption was that a robust Th1 response to brain antigens would occur following stroke by virtue of the fact that novel brain antigens are exposed/presented to the immune system. To test this assumption, we used a model of severe stroke (3 h of middle cerebral artery occlusion [MCAO]) in Lewis rats, followed by prolonged periods of survival (up to 3 months). Not only was a Th1 type immune response to MBP not seen in these animals following MCAO, the predominant response was more consistent with that of a Treg response characterized by the antigen-specific secretion of TGF-β1 [18]. (Of note, clinical studies also demonstrate an increase in circulating regulatory T cells, as detected by flow cytometry and the detection of CD4+CD25hifoxp3+ cells, after stroke [19].)
Accumulated data demonstrate that the type of immune response that develops after antigen presentation, an effector response (i.e., Th1, Th2, Th17) or a regulatory response (Treg), depends upon the microenvironment at the site of antigen presentation. The brain is enriched with cytokines and neuropeptides that suppress lymphocyte activation [20, 21]. This “immunosuppressive milieu” contributes to the relative immune privilege of the brain. In addition, microglia, the primary antigen presenting cells (APCs) in brain, do not generally express the major histocompatibility complex (MHC) II molecule or the costimulatory molecules needed to activate lymphocytes [22, 23]. Expression of both MHC II and costimulatory molecules, however, can be induced by systemic administration of lipopolysaccharide (LPS) [18, 24]. Capitalizing upon these observations, we found that Th1(+) responses to MBP could be induced in our experimental model by perturbing the system with an intraperitoneal injection of LPS at the time of stroke [18]. LPS is a component of the Gram negative bacterial cell wall and also represents a common pathogen associated molecular pattern (PAMP) that is able to initiate the innate immune response (with upregulation of MHC II and costimulatory molecules on APCs) through activation of toll-like receptors (TLRs) [25]. Endogenous molecules known as alarmins are also able to activate the innate immune response through stimulation of TLRs [26]. Common alarmins include heat shock proteins (hsps), uric acid, S100B, and high-mobility box protein (HMGB)-1. PAMPs and alarmins are collectively referred to as danger associated molecular patterns (DAMPs), and the initiation of the innate immune response by DAMPs can create an environment which facilitates the development of the immune response, including immune responses to self [27, 28].
Because antigen presentation, lymphocyte activation, and antigen driven lymphocyte proliferation take time (akin to the development of a protective immune response following vaccination with a pathogen), the effects of an antigen-specific Th1(+) response on stroke outcome would not likely be realized for many days after the onset of cerebral ischemia. Additionally, clinical signs of experimental allergic encephalomyelitis (EAE) are generally not seen for 9–10 days after immunization with myelin associated proteins, suggesting that even under conditions optimized for the development of an immune response, adequate amplification of that response to the point where it is of clinical consequence takes many days [29]. In animals with a pre-existing pool of MBP-specific Th1(+) cells, it is possible that the cells could contribute to early post-ischemic inflammatory response and affect short-term outcome. Indeed, we previously showed that animals “sensitized” to MBP prior to MCAO had increased short-term mortality [30]. Adoptive transfer of cells specific for myelin oligodendrocyte glycoprotein (MOG) into mice with severe combined immunodeficiency at the time of stroke is also seen to worse stroke outcome [31].
Our experimental studies have focused on the effect of the adaptive immune response on long-term outcomes from stroke. When examined 1 month after MCAO, animals with a Th1(+) response to MBP display worse outcome than animals without a Th1(+) response [18, 32]. A similar association between Th1(+) responses to MBP and poor outcome is also seen at 3 months after MCAO [33]. In addition to the effects of the Th1(+) response on long-term outcome from the index stroke, the development of CNS autoreactive T cells following stroke could predispose stroke survivors to worse outcome from recurrent strokes. Further, dementia is common after stroke, and it is intriguing to consider the possibility that stroke-induced autoimmune responses lead to chronic low-grade inflammation and predispose to dementia [34–39]. Another common clinical occurrence after stroke is that of the “anamnestic recall” of stroke-related deficits. The term “anamnestic recall” describes the transient re-emergence of stroke-related symptoms, usually during a systemic infection, in patients who had recovered from their stroke. It is possible that the inflammatory state associated with systemic infection could activate autoreactive T cells which mediate this transient worsening of neurological symptoms. In an attempt to model the “anamnestic recall” of stroke-related deficits, we found that animals with a Th1(+) response to MBP were more likely to evidence a decline in neurologic function when exposed to either a non-specific inflammatory stimulus (LPS) or the relevant autoantigen (MBP) 1 month after MCAO [40]. The ways in which post-stroke autoimmunity could contribute to the chronic sequelae of stroke are still quite speculative, but our data suggest that the possibility should be considered.
In contrast to the detrimental effects of the Th1(+) response on stroke outcome, Treg responses to these same antigens appear to be neuroprotective. Firstly, deletion of Treg cells prior to stroke is associated with increased CNS inflammation, increased infarct volume, and worse outcome [41]. Secondly, enhancing the endogenous pool of Treg cells that recognize brain antigens as well as vascular antigens, on the other hand, decreases infarct volume and improves stroke outcome [30, 42, 43]. These antigen-specific Treg cells can be induced using the paradigm of mucosal tolerance [30, 42, 43]. Adoptive transfer of lymphocytes from animals “tolerized” to antigens like MBP or myelin oligodendrocyte protein (MOG) to naïve animals at the time of stroke results in better outcome, demonstrating a role for these cells in modulating the response to stroke [42, 44]. The early benefit of antigen-specific Treg cells in experimental stroke is probably due to the fact that the antigen to which these Treg cells react is present at the site of ischemic brain injury and activates local Treg cells to secrete cytokines like interleukin (IL)-10 and TGF-β1[42, 43]. While these cytokines are secreted in response to antigen-specific stimulation, the cytokines themselves have antigen non-specific effects that modulate the local immune response (“bystander tolerance”) [45]. In addition to their immunomodulatory effects, both IL-10 and TGF-β1 appear to be directly neuroprotective, suggesting another potential mechanism for improving stroke outcome [46–48]. Recent data also highlight an important protective role of IL-10 secreting regulatory B cells following stroke [49]. Of potential therapeutic interest, we showed that pre-ischemic induction of MBP-specific Treg cells could prevent the LPS medicated induction of MBP-specific Th1 cells [33, 50].
Infection is common in the immediate post-stroke period and occurs in up to 20% of patients with stroke [51–58]. The administration of LPS to animals during MCAO is thus a clinically relevant model in that it mimics post-stroke infection. The risk of infection is greatest among patients with the most severe strokes, which is in part related to the fact that ischemic brain injury induces a systemic “immunodepression” that is most profound in patients with severe stroke [59–66]. Post-stroke infection also portends poor outcome from stroke, and its effect on outcome is independent of both stroke severity and age [51, 67, 68]. Urinary tract infections (UTIs) and pneumonias (PNAs) are the most common infections in the post-stroke period, but the association between PNA and increased disability and mortality is more robust than that for UTI [51, 67, 68]. The mechanisms by which infection confers worse outcome are unclear, but our data demonstrating that LPS (a component of the Gram-negative bacterial cell wall and thus an infection “mimic”) increases the likelihood of developing a Th1(+) response to MBP, which is associated with worse outcome, may provide a clue.
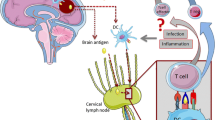
In an attempt to validate our laboratory findings in the clinical setting, we evaluated whether patients who developed infection in the immediate post-stroke period would be more likely to develop a Th1(+) response to brain antigens. In an observational study where 25% of patients developed infection in first 15 days after ischemic stroke, we found that PNA (but not UTI) was associated with an increased likelihood of developing a Th1(+) response to both MBP and glial fibrillary acidic protein (GFAP) [69]. In fact, patients with PNA were nearly five times more likely to develop a Th1(+) response to MBP than patients who remained infection free. Increasing stroke severity was also predictive of developing a Th1(+) response to MBP [69]. Because stroke severity was the most important predictor of infection in this cohort of patients, it is difficult to untangle the relative contributions of each to the observed autoimmune response to MBP [70]. More robust Th1 responses to MBP were associated with worse outcome from stroke independent of initial stroke severity, however, thus arguing for a uniquely detrimental role of the immune response [69]. Whether the immune response to MBP is responsible for the worse outcome or is merely a marker for an immune response to another antigen is unknown. Even if the immune response was initially directed towards MBP, it is possible that an immune response to an entirely different antigen is responsible for the effect on stroke outcome. Ongoing tissue injury associated with inflammation continually exposes new antigens to the immune system, allowing for recognition of these new antigens by lymphocytes, and the activation of these lymphocytes is facilitated by the inflammatory milieu in the local environment. The sequential change in the target of the immune response is known as “epitope spreading,” and this phenomenon can result in different peptides within a protein or an entirely different protein being recognized by the immune system. Figure 1 depicts our model to explain lymphocyte activation in the brain following stroke; a similar scenario likely plays out in peripheral lymphoid organs.
Model to explain the development of CNS autoimmune response in the brain following stroke. Under usual circumstances (a), lymphocytes may transit within the cerebral vasculature but do not infiltrate into tissue. Microglia may express a limited number of TLRs and low levels of MHC II. Following stroke (b), there is an increase in the number of lymphocytes within the CNS vasculature, and these lymphocytes infiltrate the ischemic brain tissue. Microglia are activated and express increased amounts of MHC II and costimulatory molecules. Antigens released from dying cells can thus be presented to lymphocytes by the microglia, allowing for an immune response to develop to these antigens (the type of response depends upon the local microenvironment, but the bulk of data suggests that a Treg response predominates following stroke). Alarmins (such as HMGB-1 and S100B) released from injured cells also have the ability to activate microglia through TLR signaling. In the setting of systemic infection (c), there may be increased numbers of lymphocytes present within the circulation. PAMPs associated with the infectious agent(s) further activate microglia to express more TLRs as well as more MHC II and costimulatory molecules. This increase in TLR and MHC II expression allows for more successful antigen presentation by the microglia, and the inflammatory cytokines within the local microenvironment favor the development of Th1 responses. Additional cell damage from the immune response leads to increased release of alarmins and further activation of the immune system. This cycle of cell damage and antigen presentation leads to the possibility of “epitope spreading” and generation of immune responses to previously unrecognized antigens
In the experimental stroke model used in our laboratory, we find little overlap in the immune response to CNS antigens between the mononuclear cells isolated from brain and those isolated from spleen 1 month after MCAO [32]. Recent data highlight the fact that the immune response is locally regulated and compartmentalized, and this regulation is dynamic and changes over time [71–80]. In an observational study of patients with ischemic stroke, we found that the immune response to MBP at 90 days after stroke onset was associated with worse outcome at 90 days after stroke onset [69]. It is important to note, however, that the response to MBP in these individuals was not static. Figure 2 shows the Th1 response to MBP over time for those patients who had a Th1(+) response at 90 days (a) and those who did not (b); the increased Th1 response to MBP at 90 days does not persist indefinitely. The fact that catastrophic autoimmune injury is not seen in patients with a Th1 response to MBP and that the degree of the Th1 response to MBP decreases over time suggests that endogenous immunomodulatory mechanisms are at play. This hypothesis is supported by the fact that the standard models of experimental allergic encephalomyelitis usually cause a monophasic illness and that the numbers of myelin-specific Th1 type cells in these animals decline over the course of time [77, 81].
Th1 responses to MBP in patients with a Th1(+) response to MBP at 90 days (a) differ significantly over the course of time (P < 0.001 by Kruskal–Wallis H test; pairwise comparisons were not done). The Th1 responses to MBP in those patients without a Th1(+) response at 90 days are similar at all time points (b)
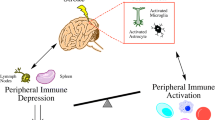
As mentioned, cerebral infarction induces a “depression” in the systemic immune response that appears to be sympathetically mediated [82, 83]. A teleological explanation for this immunodepression is that it prevents the development of autoimmune responses to brain following stroke. Stroke severity is the greatest driver of this immunodepression [61, 62]. Indeed, we found that animals exposed to a shorter duration of ischemia (2-h MCAO) had more robust Th1 responses to brain antigens than animals exposed to a longer duration of ischemia (3-h MCAO; Fig. 3). As a consequence of this post-stroke immunodepression, however, there appears to be an increased risk of infection [62, 82, 84]. We believe that infections occurring in the setting of immunodepression lead to an inflammatory response that is sufficient to allow for “bystander activation” of lymphocytes to brain antigens (Fig. 4). In addition, the death of astrocytes, oligodendrocytes, and neurons exposes new and cryptic (intracellular) antigens to the immune system. With ongoing tissue injury related to these immune responses, new antigens are constantly exposed, leading to the possibility of “epitope spreading”.
Our re-interpretation of the model originally proposed by Dirnagl and colleagues [85]. Stroke-induced changes in the immune response associated with severe stroke prevent the development of autoimmune responses to brain antigens but predispose to infection. The inflammatory response associated with infection overrides the systemic immunodepression and creates an environment that can support the successful activation of the immune response to self-antigens
In summary, our findings demonstrate that development of a Th1 response to brain antigens (particularly MBP) is associated with worse outcome from both experimental and clinical stroke and that the likelihood of developing such a response is increased by systemic administration of LPS or systemic infection (primarily PNA). The development of Th1 responses to brain antigens provides a plausible explanation why individuals who develop infection after stroke are at increased risk of disability and death. More importantly, the recognition of this phenomenon suggests that modulation of the post-ischemic immune response might provide an attractive target for delayed stroke therapy.
References
Chapman KZ, Dale VQ, Denes A, Bennett G, Rothwell NJ, Allan SM, et al. A rapid and transient peripheral inflammatory response precedes brain inflammation after experimental stroke. J Cereb Blood Flow Metab. 2009;29(11):1764–8.
Kleinig TJ, Vink R. Suppression of inflammation in ischemic and hemorrhagic stroke: therapeutic options. Curr Opin Neurol. 2009;22(3):294–301.
Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184(1–2):53–68.
Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17(7):796–808.
Jander S, Kraemer M, Schroeter M, Witte OW, Stoll G. Lymphocytic infiltration and expression of intercellular adhesion molecule-1 in photochemically induced ischemia of the rat cortex. J Cereb Blood Flow Metab. 1995;15(1):42–51.
Schroeter M, Jander S, Witte OW, Stoll G. Local immune responses in the rat cerebral cortex after middle cerebral artery occlusion. J Neuroimmunol. 1994;55(2):195–203.
Braun JS, Jander S, Schroeter M, Witte OW, Stoll G. Spatiotemporal relationship of apoptotic cell death to lymphomonocytic infiltration in photochemically induced focal ischemia of the rat cerebral cortex. Acta Neuropathol (Berl). 1996;92(3):255–63.
Campanella M, Sciorati C, Tarozzo G, Beltramo M. Flow cytometric analysis of inflammatory cells in ischemic rat brain. Stroke. 2002;33(2):586–92.
Jauch EC, Lindsell C, Broderick J, Fagan SC, Tilley BC, Levine SR. Association of serial biochemical markers with acute ischemic stroke: the National Institute of Neurological Disorders and Stroke recombinant tissue plasminogen activator stroke study. Stroke. 2006;37(10):2508–13.
van Zwam M, Huizinga R, Melief MJ, Wierenga-Wolf AF, van Meurs M, Voerman JS, et al. Brain antigens in functionally distinct antigen-presenting cell populations in cervical lymph nodes in MS and EAE. J Mol Med. 2009;87(3):273–86.
Planas AM, Gomez-Choco M, Urra X, Gorina R, Caballero M, Chamorro A. Brain-derived antigens in lymphoid tissue of patients with acute stroke. J Immunol. 2012;188(5):2156–63.
Youngchaiyud U, Coates AS, Whittingham S, Mackay IR. Cellular-immune response to myelin protein: absence in multiple sclerosis and presence in cerebrovascular accidents. Aust N Z J Med. 1974;4(6):535–8.
Wang WZ, Olsson T, Kostulas V, Hojeberg B, Ekre HP, Link H. Myelin antigen reactive T cells in cerebrovascular diseases. Clin Exp Immunol. 1992;88(1):157–62.
Kallen B, Nilsson O, Thelin C. Effect of encephalitogenic protein on migration in agarose of leukoytes from patients with multiple sclerosis. A longitudinal study of patients with relapsing multiple sclerosis or with cerebral infarction. Acta Neurol Scand. 1977;55(1):47–56.
Rocklin RE, Sheremata WA, Feldman RG, Kies MW, David JR. The Guillain–Barre syndrome and multiple sclerosis. In vitro cellular responses to nervous-tissue antigens. N Engl J Med. 1971;284(15):803–8.
Bornstein NM, Aronovich B, Korczyn AD, Shavit S, Michaelson DM, Chapman J. Antibodies to brain antigens following stroke. Neurology. 2001;56(4):529–30.
Dambinova SA, Khounteev GA, Izykenova GA, Zavolokov IG, Ilyukhina AY, Skoromets AA. Blood test detecting autoantibodies to N-methyl-d-aspartate neuroreceptors for evaluation of patients with transient ischemic attack and stroke. Clin Chem. 2003;49(10):1752–62.
Becker KJ, Kindrick DL, Lester MP, Shea C, Ye ZC. Sensitization to brain antigens after stroke is augmented by lipopolysaccharide. J Cereb Blood Flow Metab. 2005;25(12):1634–44.
Yan J, Greer JM, Etherington K, Cadigan GP, Cavanagh H, Henderson RD, et al. Immune activation in the peripheral blood of patients with acute ischemic stroke. J Neuroimmunol. 2009;206(1–2):112–7.
Fontana A, Constam DB, Frei K, Malipiero U, Pfister HW. Modulation of the immune response by transforming growth factor beta. Int Arch Allergy Immunol. 1992;99(1):1–7.
Suter T, Biollaz G, Gatto D, Bernasconi L, Herren T, Reith W, et al. The brain as an immune privileged site: dendritic cells of the central nervous system inhibit T cell activation. Eur J Immunol. 2003;33(11):2998–3006.
Bailey SL, Carpentier PA, McMahon EJ, Begolka WS, Miller SD. Innate and adaptive immune responses of the central nervous system. Crit Rev Immunol. 2006;26(2):149–88.
Perry VH. A revised view of the central nervous system microenvironment and major histocompatibility complex class II antigen presentation. J Neuroimmunol. 1998;90(2):113–21.
Buttini M, Limonta S, Boddeke HW. Peripheral administration of lipopolysaccharide induces activation of microglial cells in rat brain. Neurochem Int. 1996;29(1):25–35.
Janeway Jr CA, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216.
Oppenheim JJ, Tewary P, de la Rosa G, Yang D. Alarmins initiate host defense. Adv Exp Med Biol. 2007;601:185–94.
Yang D, de la Rosa G, Tewary P, Oppenheim JJ. Alarmins link neutrophils and dendritic cells. Trends Immunol. 2009;30(11):531–7.
Bianchi ME, Manfredi AA. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev. 2007;220:35–46.
Mannie M, Swanborg RH, Stepaniak JA. Experimental autoimmune encephalomyelitis in the rat. Curr Protoc Immunol. 2009;Chapter 15:Unit 15 2.
Becker KJ, McCarron RM, Ruetzler C, Laban O, Sternberg E, Flanders KC, et al. Immunologic tolerance to myelin basic protein decreases stroke size after transient focal cerebral ischemia. Proc Natl Acad Sci U S A. 1997;94(20):10873–8.
Ren X, Akiyoshi K, Grafe MR, Vandenbark AA, Hurn PD, Herson PS et al. Myelin specific cells infiltrate MCAO lesions and exacerbate stroke severity. Metab Brain Dis. 27(1):7–15.
Zierath D, Thullbery M, Hadwin J, Gee JM, Savos A, Kalil A, et al. CNS immune responses following experimental stroke. Neurocrit Care. 2010;12:274–84.
Gee JM, Zierath D, Hadwin J, Savos A, Kalil A, Thullbery M, et al. Long term immunologic consequences of experimental stroke and mucosal tolerance. Exp Transl Stroke Med. 2009;1:3.
Pohjasvaara T, Erkinjuntti T, Ylikoski R, Hietanen M, Vataja R, Kaste M. Clinical determinants of poststroke dementia. Stroke. 1998;29(1):75–81.
Leys D, Henon H, Mackowiak-Cordoliani MA, Pasquier F. Poststroke dementia. Lancet Neurol. 2005;4(11):752–9.
Fillit HM, Kemeny E, Luine V, Weksler ME, Zabriskie JB. Antivascular antibodies in the sera of patients with senile dementia of the Alzheimer’s type. J Gerontol. 1987;42(2):180–4.
Ishida K, Kaneko K, Kubota T, Itoh Y, Miyatake T, Matsushita M, et al. Identification and characterization of an anti-glial fibrillary acidic protein antibody with a unique specificity in a demented patient with an autoimmune disorder. J Neurol Sci. 1997;151(1):41–8.
Karczewski P, Hempel P, Kunze R, Bimmler M. Agonistic autoantibodies to the alpha(1)-adrenergic receptor and the beta(2)-adrenergic receptor in Alzheimer’s and vascular dementia. Scand J Immunol. 2012; epub ahead of print.
Rosenmann H, Meiner Z, Geylis V, Abramsky O, Steinitz M. Detection of circulating antibodies against tau protein in its unphosphorylated and in its neurofibrillary tangles-related phosphorylated state in Alzheimer’s disease and healthy subjects. Neurosci Lett. 2006;410(2):90–3.
Zierath D, Hadwin J, Savos A, Carter KT, Kunze A, Becker KJ. Anamnestic recall of stroke-related deficits: an animal model. Stroke. 2010;41(11):2653–60.
Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15(2):192–9.
Frenkel D, Huang Z, Maron R, Koldzic DN, Moskowitz MA, Weiner HL. Neuroprotection by IL-10-producing MOG CD4+ T cells following ischemic stroke. J Neurol Sci. 2005;233(1–2):125–32.
Chen Y, Ruetzler C, Pandipati S, Spatz M, McCarron RM, Becker K, et al. Mucosal tolerance to E-selectin provides cell-mediated protection against ischemic brain injury. Proc Natl Acad Sci U S A. 2003;100(25):15107–12.
Becker K, Kindrick D, McCarron R, Hallenbeck J, Winn R. Adoptive transfer of myelin basic protein-tolerized splenocytes to naive animals reduces infarct size: a role for lymphocytes in ischemic brain injury? Stroke. 2003;34(7):1809–15.
Miller A, Lider O, Weiner HL. Antigen-driven bystander suppression after oral administration of antigens. J Exp Med. 1991;174(4):791–8.
Sharma S, Yang B, Xi X, Grotta JC, Aronowski J, Savitz SI. IL-10 directly protects cortical neurons by activating PI-3 kinase and STAT-3 pathways. Brain Res.1373:189–94.
Zhou Z, Peng X, Insolera R, Fink DJ, Mata M. Interleukin-10 provides direct trophic support to neurons. J Neurochem. 2009;110(5):1617–27.
Buisson A, Lesne S, Docagne F, Ali C, Nicole O, MacKenzie ET, et al. Transforming growth factor-beta and ischemic brain injury. Cell Mol Neurobiol. 2003;23(4–5):539–50.
Ren X, Akiyoshi K, Dziennis S, Vandenbark AA, Herson PS, Hurn PD et al. Regulatory B cells limit CNS inflammation and neurologic deficits in murine experimental stroke. J Neurosci. 31(23):8556–63.
Gee JM, Kalil A, Thullbery M, Becker KJ. Induction of immunologic tolerance to myelin basic protein prevents central nervous system autoimmunity and improves outcome after stroke. Stroke. 2008;39(5):1575–82.
Aslanyan S, Weir CJ, Diener HC, Kaste M, Lees KR. Pneumonia and urinary tract infection after acute ischaemic stroke: a tertiary analysis of the GAIN International trial. Eur J Neurol. 2004;11(1):49–53.
Davenport RJ, Dennis MS, Wellwood I, Warlow CP. Complications after acute stroke. Stroke. 1996;27(3):415–20.
Grau AJ, Buggle F, Schnitzler P, Spiel M, Lichy C, Hacke W. Fever and infection early after ischemic stroke. J Neurol Sci. 1999;171(2):115–20.
Langhorne P, Stott DJ, Robertson L, MacDonald J, Jones L, McAlpine C, et al. Medical complications after stroke: a multicenter study. Stroke. 2000;31(6):1223–9.
Indredavik B, Rohweder G, Naalsund E, Lydersen S. Medical complications in a comprehensive stroke unit and an early supported discharge service. Stroke. 2008;39(2):414–20.
Hong KS, Kang DW, Koo JS, Yu KH, Han MK, Cho YJ, et al. Impact of neurological and medical complications on 3-month outcomes in acute ischaemic stroke. Eur J Neurol. 2008;15(12):1324–31.
Minnerup J, Wersching H, Browinkel B, Dziewas R, Heuschmann PU, Nabavi D, et al. The impact of lesion location and lesion size on post-stroke infection frequency. J Neurol Neurosurg Psychiatry. 2010;81:198–202.
Vermeij FH, Scholte op Reimer WJ, de Man P, van Oostenbrugge RJ, Franke CL, de Jong G, et al. Stroke-associated infection is an independent risk factor for poor outcome after acute ischemic stroke: data from the Netherlands Stroke Survey. Cerebrovasc Dis. 2009;27(5):465–71.
Chamorro A, Urra X, Planas AM. Infection after acute ischemic stroke: a manifestation of brain-induced immunodepression. Stroke. 2007;38(3):1097–103.
Chamorro A, Amaro S, Vargas M, Obach V, Cervera A, Torres F, et al. Interleukin 10, monocytes and increased risk of early infection in ischaemic stroke. J Neurol Neurosurg Psychiatry. 2006;77(11):1279–81.
Haeusler KG, Schmidt WU, Fohring F, Meisel C, Helms T, Jungehulsing GJ, et al. Cellular immunodepression preceding infectious complications after acute ischemic stroke in humans. Cerebrovasc Dis. 2008;25(1–2):50–8.
Hug A, Dalpke A, Wieczorek N, Giese T, Lorenz A, Auffarth G, et al. Infarct volume is a major determiner of post-stroke immune cell function and susceptibility to infection. Stroke. 2009;40(10):3226–32.
Offner H, Vandenbark AA, Hurn PD. Effect of experimental stroke on peripheral immunity: CNS ischemia induces profound immunosuppression. Neuroscience. 2009;158(3):1098–111.
Prass K, Braun JS, Dirnagl U, Meisel C, Meisel A. Stroke propagates bacterial aspiration to pneumonia in a model of cerebral ischemia. Stroke. 2006;37(10):2607–12.
Schulte-Herbruggen O, Klehmet J, Quarcoo D, Meisel C, Meisel A. Mouse strains differ in their susceptibility to poststroke infections. Neuroimmunomodulation. 2006;13(1):13–8.
Vogelgesang A, Grunwald U, Langner S, Jack R, Broker BM, Kessler C, et al. Analysis of lymphocyte subsets in patients with stroke and their influence on infection after stroke. Stroke. 2008;39(1):237–41.
Johnston KC, Li JY, Lyden PD, Hanson SK, Feasby TE, Adams RJ, et al. Medical and neurological complications of ischemic stroke: experience from the RANTTAS trial. RANTTAS Investigators. Stroke. 1998;29(2):447–53.
Katzan IL, Cebul RD, Husak SH, Dawson NV, Baker DW. The effect of pneumonia on mortality among patients hospitalized for acute stroke. Neurology. 2003;60(4):620–5.
Becker KJ, Kalil AJ, Tanzi P, Zierath DK, Savos AV, Gee JM, et al. Autoimmune responses to the brain after stroke are associated with worse outcome. Stroke. 2011;42:2763–9.
Tanzi P, Cain K, Kalil A, Zierath D, Savos A, Gee JM, et al. Post-stroke infection: a role for IL-1ra? Neurocrit Care. 2011;14(2):244–52.
Kovacevic-Jovanovic V, Laban O, Stanojevic S, Miletic T, Dimitrijevic M, Radulovic J. Changes in immunological and neuronal conditions markedly altered antibody response to intracerebroventricularly injected ovalbumin in the rat. Neuroimmunomodulation. 1997;4(4):181–7.
Siegmund K, Feuerer M, Siewert C, Ghani S, Haubold U, Dankof A, et al. Migration matters: regulatory T-cell compartmentalization determines suppressive activity in vivo. Blood. 2005;106(9):3097–104.
Hofstetter HH, Grau C, Buttmann M, Forsthuber TG, Gaupp S, Toyka KV, et al. The PLPp-specific T-cell population promoted by pertussis toxin is characterized by high frequencies of IL-17-producing cells. Cytokine. 2007;40(1):35–43.
Attinger A, MacDonald HR, Acha-Orbea H. Lymphoid environment limits superantigen and antigen-induced T cell proliferation at high precursor frequency. Eur J Immunol. 2001;31(3):884–93.
Chow LH, Feurer C, Borel JF. Chronic relapsing experimental allergic encephalomyelitis in the Lewis rat: studies on immune regulation. J Neuroimmunol. 1988;19(4):329–38.
Zhang X, Reddy J, Ochi H, Frenkel D, Kuchroo VK, Weiner HL. Recovery from experimental allergic encephalomyelitis is TGF-beta dependent and associated with increases in CD4 + LAP + and CD4 + CD25+ T cells. Int Immunol. 2006;18(4):495–503.
Hofstetter HH, Targoni OS, Karulin AY, Forsthuber TG, Tary-Lehmann M, Lehmann PV. Does the frequency and avidity spectrum of the neuroantigen-specific T cells in the blood mirror the autoimmune process in the central nervous system of mice undergoing experimental allergic encephalomyelitis? J Immunol. 2005;174(8):4598–605.
`Bischof F, Hofmann M, Schumacher TN, Vyth-Dreese FA, Weissert R, Schild H, et al. Analysis of autoreactive CD4 T cells in experimental autoimmune encephalomyelitis after primary and secondary challenge using MHC class II tetramers. J Immunol. 2004;172(5):2878–84.
Gordon FL, Nguyen KB, White CA, Pender MP. Rapid entry and downregulation of T cells in the central nervous system during the reinduction of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2001;112(1–2):15–27.
Tan LJ, Vanderlugt CL, McRae BL, Miller SD. Regulation of the effector stages of experimental autoimmune encephalomyelitis via neuroantigen-specific tolerance induction. III. A role for anergy/deletion. Autoimmunity. 1998;27(1):13–28.
Targoni OS, Baus J, Hofstetter HH, Hesse MD, Karulin AY, Boehm BO, et al. Frequencies of neuroantigen-specific T cells in the central nervous system versus the immune periphery during the course of experimental allergic encephalomyelitis. J Immunol. 2001;166(7):4757–64.
Prass K, Meisel C, Hoflich C, Braun J, Halle E, Wolf T, et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med. 2003;198(5):725–36.
Wong CH, Jenne CN, Lee WY, Leger C, Kubes P. Functional innervation of hepatic iNKT cells is immunosuppressive following stroke. Science. 334(6052):101–5.
Meisel C, Schwab JM, Prass K, Meisel A, Dirnagl U. Central nervous system injury-induced immune deficiency syndrome. Nat Rev Neurosci. 2005;6(10):775–86.
Dirnagl U, Klehmet J, Braun JS, Harms H, Meisel C, Ziemssen T, et al. Stroke-induced immunodepression: experimental evidence and clinical relevance. Stroke. 2007;38(2 Suppl):770–3.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Becker, K. Autoimmune Responses to Brain Following Stroke. Transl. Stroke Res. 3, 310–317 (2012). https://doi.org/10.1007/s12975-012-0154-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-012-0154-0