Abstract
This randomized, active-controlled, double-blind study assessed the pharmacodynamics, pharmacokinetics and safety of ticagrelor in Japanese patients and a smaller cohort of non-Japanese Asian patients. The study recruited patients aged 20–80 years who had received aspirin 75–100 mg/day for ≥2 weeks and had percutaneous coronary intervention or acute coronary syndrome >3 months previously. Patients received 4 weeks’ treatment with ticagrelor 45 mg bid, ticagrelor 90 mg bid or clopidogrel 75 mg qd (all with aspirin). The inhibition of platelet aggregation (IPA, final-extent) and pharmacokinetics of ticagrelor were assessed on days 1 and 28. Overall, 139 Asian patients were randomized (ticagrelor 45 mg bid, n = 50; ticagrelor 90 mg bid, n = 43; clopidogrel, n = 46) of whom 118 were Japanese. Mean final-extent IPA was greater with ticagrelor 90 mg bid versus ticagrelor 45 mg bid and with both ticagrelor doses versus clopidogrel. At the end of the dosing interval on day 28, mean final-extent IPA was 10.0 % higher (95 % confidence interval 0.5–19.5 %) for ticagrelor 90 mg bid versus ticagrelor 45 mg bid, 15.1 % higher (5.8–24.4 %) for ticagrelor 45 mg bid versus clopidogrel, and 25.1 % higher (15.5–34.7 %) for ticagrelor 90 mg bid versus clopidogrel. In Japanese patients, exposure to ticagrelor and its active metabolite AR-C124910XX increased dose-proportionally. The safety profile of ticagrelor was consistent with previous studies. Ticagrelor was associated with enhanced IPA versus clopidogrel in Japanese patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ticagrelor is an orally active P2Y12 receptor antagonist [1, 2] used for the prevention of atherothrombotic events in patients with acute coronary syndrome (ACS) [3]. The efficacy and safety of ticagrelor in patients with ACS was investigated in the PLATelet inhibition and patient Outcomes (PLATO) trial [4]; ticagrelor plus aspirin reduced the rate of the primary composite endpoint of myocardial infarction, stroke or death from vascular causes relative to clopidogrel plus aspirin. Although the rate of major bleeding not related to coronary artery bypass grafting was higher in the ticagrelor group than the clopidogrel group, no treatment-group difference in the rate of overall major bleeding was observed in PLATO.
After oral administration, ticagrelor is rapidly absorbed and displays a linear and predictable pharmacokinetic (PK) profile [5–8]. Although ticagrelor does not require metabolic activation to inhibit platelet aggregation, an approximately equipotent active metabolite, AR-C124910XX, is generated via cytochrome P450 (CYP) 3A enzymes [9]. This metabolite is present in a concentration approximately 40 % that of the parent compound [8]. Unlike the thienopyridine compounds, clopidogrel and prasugrel, the active metabolites of which bind irreversibly to the P2Y12 receptor, ticagrelor and AR-C124910XX bind reversibly to the P2Y12 receptor [2]. Combined with the short half-lives of ticagrelor and AR-C124910XX, the reversible nature of ticagrelor’s binding to P2Y12 leads to a level of platelet inhibition in standardized ex vivo testing that is closely related to drug plasma concentration [3, 8, 10].
Data from PLATO and other studies have led to the approval of ticagrelor in many countries worldwide, including several in Asia (although not currently in Japan). Notably, the efficacy and safety of antiplatelet agents may differ between Asian and Caucasian patients, a phenomenon that may in part be due to differences in drug-metabolizing enzymes, e.g. loss-of-function alleles in the CYP2C19 gene, for example, are highly prevalent in the Asian population [11]. CYP2C19 loss-of-function alleles confer a higher risk of cardiovascular events during clopidogrel treatment in Caucasian populations [12], but the clinical impact in Asian populations is not yet known [13]. Data from the J-Cypher registry in Japan indicate that Japanese patients receiving clopidogrel after drug-eluting stent implantation are at low risk of stent thrombosis [14], but the prevalence or impact of CYP2C19 allele frequency was not assessed in the J-Cypher study. As ticagrelor does not require metabolic activation, it may be a particularly suitable candidate for use as an antiplatelet agent in patients of Asian descent. Accordingly, a number of studies and trial analyses have been performed to describe the properties of ticagrelor in Asian populations. For example, in Phase I studies, the tolerability and PK profiles of ticagrelor were broadly similar in Japanese and Caucasian healthy volunteers, although exposure to the parent compound and AR-C124910XX was slightly higher in Japanese volunteers [15]. In addition, a subgroup analysis of PLATO showed that the benefits of ticagrelor relative to clopidogrel were similar in Asian (defined as ‘of oriental race’) and Caucasian patients with ACS, although the number of Asian patients in this trial was relatively small (1096 versus 17077 Caucasian patients) [4].
The current study was performed to characterize the pharmacodynamic (PD) profile of ticagrelor in Japanese patients with stable coronary artery disease (CAD). The primary objective was to investigate, in Japanese patients, the effect of two doses of ticagrelor [45 and 90 mg twice daily (bid)] on adenosine diphosphate (ADP)-induced platelet aggregation. Secondary objectives included assessment of the PK and safety of ticagrelor in Japanese patients and a smaller cohort of non-Japanese Asian patients.
Methods
Patients
Eligible patients were men or women aged 20–80 years who had received aspirin 75–100 mg/day for ≥2 weeks before randomization and had either any percutaneous coronary intervention (PCI) or previously documented ACS >3 months before randomization. Exclusion criteria included: ACS, transient ischemic attack (TIA) or stroke within 3 months before randomization; anticoagulant therapy or antiplatelet therapy other than aspirin within 2 weeks before randomization; percutaneous intervention, or surgical procedure planned during study; chronic atrial fibrillation, condition(s) associated with increased bleeding risk; increased risk of bradycardiac events; platelet count <10 × 104/µL; hemoglobin <10 g/dL; renal failure requiring dialysis; and moderate or severe liver disease. All patients provided written informed consent.
Study design and treatments
This multicenter, randomized, double-blind, parallel-group study (study code: D5130C00065; ClinicalTrials.gov Identifier: NCT01118325) was performed in 15 centers in Japan and the Philippines from April 2010 to March 2011. The study protocol was approved by the relevant institutional review board/ethics committees and the study was conducted in accordance with the Declaration of Helsinki, Good Clinical Practice guidelines and the AstraZeneca policy on bioethics. An independent data safety monitoring board monitored all aspects of the study.
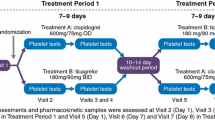
Patient eligibility for the study was assessed at an enrolment visit held 3–14 days before the start of a 4-week treatment period. On day 1 of the treatment period, a randomization visit was held, at which eligibility was re-confirmed and enrolled patients were randomized to receive ticagrelor 45 mg bid, ticagrelor 90 mg bid or clopidogrel 75 mg once daily (qd). Randomization codes were allocated sequentially from a list generated in blocks of a predefined size to ensure all three treatment groups were equally populated. Investigators and patients were blinded to treatment assignment and all study treatments were identical in appearance, packaging and labeling. Ticagrelor or clopidogrel was first administered at the randomization visit. Throughout the treatment period, all patients received aspirin 75–100 mg qd, maintained at a stable dose. In addition to the enrolment and randomization visits, study visits were held at days 7 and 14, at the end of treatment (day 28), and at the end of a 4-week follow-up period. Use of the following medications was prohibited: anticoagulants; ticlopidine; dipyridamole; cilostazol; fibrinolytic therapy; strong CYP3A inhibitors; simvastatin or lovastatin at doses >40 mg; CYP3A substrates with a narrow therapeutic index; and potent inducers of CYP3A.
Assessments
Pharmacodynamics
The primary PD endpoint was final-extent inhibition of platelet aggregation (IPA), as measured by optical aggregometry of platelet-rich plasma (PRP) stimulated with 20 µM ADP. Venous blood samples (10 mL) were collected via a cannula pre-dose (within 30 min of dosing) and at 1, 2, 4, 8 and 12 h on day 1, and pre-dose and at 2, 4, 8, 12 and 24 h on day 28. Upon collection, 9 mL of each sample was centrifuged with 1 mL of 3.13 % w/v trisodium citrate to separate and obtain platelet poor plasma (PPP) and PRP. PPP was used to set the baseline for the aggregation tests, which were started 1 h (±10 min) after sampling. Final-extent IPA, the most appropriate measure of IPA for P2Y12 receptor antagonists [16], was measured 6 min after the addition of ADP to PRP samples (adjusted to 250000 platelets/µL).
Pharmacokinetics
Blood samples (3 mL) for the determination of ticagrelor and AR-C124910XX in plasma were collected at the same time-points on days 1 and 28 as the samples for pharmacodynamic analyses. The plasma concentrations of ticagrelor and AR-C124910XX were determined with a validated bioanalytical method using high performance liquid chromatography with tandem mass spectroscopic detection (LC–MS/MS) after protein precipitation. The validated calibration range was 1–2000 ng/mL for ticagrelor and 2.5–1000 ng/mL for AR-C124910XX [17].
Safety and tolerability
Adverse events (AEs) were monitored throughout. Bleeding events were assessed using the PLATO trial classification scheme [18], which is a modification of that used in the Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) trial [19]. In summary, fatal/life-threatening major bleeding comprised fatal bleeding, intracranial bleeding, intrapericardial bleeding with cardiac tamponade, hypovolaemic shock or severe hypotension due to bleeding and requiring pressors or surgery, clinically overt or apparent bleeding associated with a >5 g/dL decrease in hemoglobin (Hb), or transfusion of ≥4 units of whole blood or packed red blood cells (PRBCs) for bleeding. Other major bleeding involved significantly disabling bleeding, clinically overt or apparent bleeding associated with a decrease in Hb of 3–5 g/dL, or transfusion of 2–3 units of whole blood or PRBCs for bleeding. Minor bleeding required medical intervention to stop or treat bleeding. Minimal bleeding included all other bleeding events not requiring intervention or treatment. Bleeding events were centrally adjudicated by an independent clinical endpoint committee. Other safety variables included standard clinical laboratory values (hematology, clinical chemistry and urinalysis), physical examination, vital signs, and 12-lead electrocardiogram (ECG). Holter ECG was also performed in Japanese patients only.
Statistics
The sample size was not calculated using formal statistical methods. Assuming 34 Japanese patients were included in each group and taking into account data from a previous PD study [1], the expected two-sided 95 % confidence interval (CI) of the difference in final-extent IPA between the two ticagrelor groups was approximately 12 %. To visually compare PK parameters between Japanese and non-Japanese Asian patients, seven non-Japanese Asian patients per group were required. Assuming a drop-out rate of 10 %, 45 patients per group were to be randomized.
Final-extent IPA was calculated as the percentage change from the baseline (pre-dose on day 1) aggregation value. Percentage inhibition was restricted to the range 0–100 % and data falling outside this range were truncated to the appropriate limit. Differences in final-extent IPA at 2, 4, 8, 12 and 24 h post-dose on day 28 were analyzed using an analysis of covariance (ANCOVA) model including treatment group. All Japanese patients who received ≥1 dose of study drug, had post-dose PD measurements, and no protocol deviations/violations that might affect ticagrelor PD, were included in the PD analysis.
For ticagrelor and AR-C124910XX, the following PK parameters were calculated using standard non-compartmental methods: maximum plasma concentration (C max), time to C max (t max), minimum plasma concentration (C min), average plasma concentration during the dosing internal (C av), half-life (t 1/2), area under the plasma–concentration time curve from time zero to end of the dosing interval (AUC0–τ ,), accumulation ratio (R ac) and oral clearance (CL/F). PK parameters were summarized by treatment group and ethnic population (Japanese/non-Japanese Asian) using descriptive statistics. An exploratory analysis was performed using a non-linear sigmoid maximum observed plateau effect (E max) model to investigate the relationship between IPA and drug concentrations in Japanese patients. Final extent individual peak IPA (IPAmax) was estimated as the highest IPA within a dosing interval and area under the effect curve (AUEC) for IPA was calculated over the dosing interval from IPA–time curves using the linear trapezoidal rule. Individual IPA at the end of the dosing interval was termed IPAmin. Safety and tolerability data for all patients who received ≥1 dose of study drug were summarized by treatment group using descriptive statistics.
Results
Patients
A total of 139 patients were randomized to ticagrelor 45 mg bid (n = 50), ticagrelor 90 mg bid (n = 43) or clopidogrel 75 mg qd (n = 46) (Fig. 1). Of the 118 randomized patients recruited in Japan, 117 were Japanese. The study was completed by 137 patients. Two Japanese patients discontinued due to AEs (gastrointestinal hemorrhage in one patient in the ticagrelor 45 mg bid group and urticaria in one patient in the clopidogrel 75 mg qd group). All Japanese patients were included in the PD analysis; PD data are not presented for non-Japanese Asian patients due to technical problems with IPA measurement. All patients in the two ticagrelor groups were included in the PK analysis, with the exception of seven Japanese patients (four in the 45 mg bid group and three in the 90 mg bid group) who were excluded because of technical difficulties at the central laboratory. Safety and tolerability data were collected from all randomized patients.
Demographics and baseline clinical characteristics were comparable across treatment groups (Table 1). Most study patients were male (89 %) and all were Asian. In all groups, the majority of patients (>80 %) were Japanese. Use of concomitant medications was similar in the three treatment groups. Separate analysis showed that demographics, baseline characteristics (Table 1) and concomitant medication use were also well balanced across treatment groups in Japanese patients only.
Pharmacodynamics: inhibition of platelet aggregation in Japanese patients
At every time-point on both day 1 and day 28, mean final-extent IPA was greater with ticagrelor 90 mg than with ticagrelor 45 mg (Fig. 2). Additionally, both doses of ticagrelor were associated with greater final-extent IPA than clopidogrel 75 mg at all time-points on these days (Fig. 2). For example, at the end of the dosing interval on day 28, mean final-extent IPA was 57 and 67 % with ticagrelor 45 mg and 90 mg bid, respectively, and 42 % with clopidogrel 75 mg qd. In the clopidogrel 75 mg group, mean final-extent IPA on day 1 was much lower than at day 28 (Fig. 2).
Mean between-group differences in final-extent IPA on day 28 are shown in Table 2. At the end of the dosing interval on day 28 (12 h time-point), the final-extent IPA was 10.0 % higher for ticagrelor 90 mg bid versus ticagrelor 45 mg bid, 15.1 % higher for ticagrelor 45 mg bid versus clopidogrel 75 mg qd, and 25.1 % higher for ticagrelor 90 mg bid versus clopidogrel 75 mg qd.
Pharmacokinetics of ticagrelor in Japanese and non-Japanese Asian patients
PK parameters for ticagrelor and AR-C124910XX are summarized in Table 3. Ticagrelor was rapidly absorbed in both Japanese and non-Japanese Asian patients, with a median t max of 2–4 h. In both ethnic populations, median t max of AR-C124910XX was similar to that of ticagrelor. C max and AUC0–τ of ticagrelor and AR-C124910XX increased in a dose-proportional manner with the two doses of ticagrelor in Japanese and non-Japanese Asian patients. C min, C av, R ac and t 1/2, were broadly similar across the two ethnic populations.
Pharmacokinetic/pharmacodynamic relationship in Japanese patients
PK/PD analyses showed that, in Japanese patients treated with ticagrelor, final-extent IPA increased as the plasma concentration of ticagrelor increased (Fig. 3). Final-extent AUEC, IPAmax and IPAmin also increased in line with ticagrelor concentration (data not shown).
Safety and tolerability in Japanese and non-Japanese Asian patients
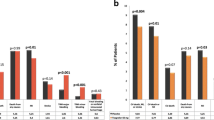
More patients experienced AEs in the ticagrelor 90 mg bid group than the ticagrelor 45 mg bid and clopidogrel 75 mg qd groups (Table 4). The gastrointestinal hemorrhage that led to discontinuation of one ticagrelor-treated patient was classed as a serious AE (SAE) and considered related to treatment; this event was also the only PLATO-defined major bleeding event reported during the study. No PLATO-defined minor bleeding events were reported. The frequency of PLATO-defined minimal bleeding was numerically higher in the ticagrelor 90 mg bid group than the other treatment groups (Table 4). In addition to the case of gastrointestinal hemorrhage, two other SAEs were reported (pneumonia and lip/oral cavity cancer in patients in the ticagrelor 45 and 90 mg bid groups, respectively). Both of these events occurred during the follow-up period and were not considered related to study treatment. There were no deaths during the study. The most common non-bleeding AEs included nasopharyngitis and abdominal discomfort (Table 4). In all three treatment groups, there were no clinically important changes in hematology, clinical chemistry, urinalysis, physical examination findings, vital signs, or 12-lead ECG. No clinically relevant changes in Holter ECG were observed in Japanese patients. Paroxysmal atrial fibrillation (AF) was observed by Holter ECG in two patients (one each in the ticagrelor 45 mg bid and clopidogrel 75 mg qd groups) but no ventricular pause longer than 3 s was observed during the study.
Discussion
In this multicenter, active-controlled study, ticagrelor 90 mg bid was associated with enhanced final-extent IPA compared with ticagrelor 45 mg bid or clopidogrel 75 mg qd in Japanese patients with stable CAD. In addition, the lower dose of ticagrelor was associated with greater final-extent IPA versus clopidogrel 75 mg qd. PK analyses showed that ticagrelor was rapidly absorbed in Japanese and non-Japanese Asian patients and exposure to ticagrelor and AR-C124910XX increased in a dose-proportional manner in both populations.
The current study was designed to mirror the previously reported phase II DISPERSE study, which included only Caucasian patients [1]. In DISPERSE, ticagrelor 100 mg bid, 200 mg bid and 400 mg qd caused rapid and nearly complete IPA and were associated with greater steady-state IPA than ticagrelor 50 mg bid and clopidogrel 75 mg qd. The two ticagrelor doses used in the current study (45 and 90 mg bid) were equivalent to the 50 and 100 mg bid doses of an early formulation of ticagrelor used in the DISPERSE study. Higher doses of ticagrelor were not investigated in the current study because 90 mg bid is the recommended clinical dose; moreover, studies in healthy volunteers have shown that exposure to ticagrelor and its active metabolite is higher in Japanese than Caucasian subjects [15].
Mean final-extent IPA for ticagrelor 90 mg bid and clopidogrel 75 mg qd in the current study appeared to be lower than in the DISPERSE study [1]. At 12 h post-dose on day 28 of the current study, mean final-extent IPA was 67 % with ticagrelor 90 mg bid and 42 % with clopidogrel 75 mg qd. At steady state in the DISPERSE study, mean final-extent IPA at 12 h post-dose was 82 % with ticagrelor 100 mg bid (equivalent to 90 mg bid ticagrelor in the current study) and approximately 60 % with clopidogrel [1]. Although resistance to clopidogrel, and to some extent prasugrel, has been reported in some Japanese patients because of CYP2C19 polymorphisms [20, 21], this enzyme is not involved in the metabolism of ticagrelor [9]. Therefore, we would not expect CYP2C19 variants to account for the IPA differences between the current study and DISPERSE, although this possibility cannot be excluded in the absence of genotype data. The observed differences may be partly attributable to differences in the equipment used to measure IPA in the two studies.
In phase I studies, exposure to ticagrelor and AR-C124910XX was slightly higher in Japanese than Caucasian volunteers [15]. The PK results of the current study were consistent with those observations; AUC0–τ and C max of ticagrelor and AR-C124910XX on day 28 were 1.3–1.5-fold higher in Japanese patients in the current study than in the Caucasian patients in the DISPERSE study. Exposure to ticagrelor and AR-C124910XX has also been shown to be higher in Chinese volunteers than in those of Caucasian ethnicity [22]. Although there were relatively few non-Japanese Asian patients recruited to the current study, there were no notable differences in the PK profiles of ticagrelor and AR-C124910XX between such patients and the larger cohort of Japanese patients.
Phase I studies also showed that IPA with ticagrelor was slightly higher in Japanese than Caucasian subjects, consistent with the higher exposure to ticagrelor and AR-C124910XX observed in Japanese subjects [15]. Although final-extent IPA was lower in the current study than in DISPERSE for equivalent doses of ticagrelor and clopidogrel, relative differences in IPA between ticagrelor 90 mg bid and clopidogrel 75 mg qd were similar across the two studies, indicating that the superior antiplatelet efficacy of ticagrelor (with respect to IPA) previously reported in Caucasian patients is also observed in Japanese patients.
The safety profile of ticagrelor in the current study was consistent with that observed in other studies performed in Asian and non-Asian populations [1, 4, 15, 22]. The incidence of AEs and bleeding events was higher with ticagrelor 90 mg bid than ticagrelor 45 mg bid or clopidogrel 75 mg qd. However, most AEs were mild in intensity and most bleeding events were classified as minimal. Only one major bleeding event—gastrointestinal bleeding in a patient receiving ticagrelor 45 mg bid—occurred during the study. No bradyarrhythmias were seen on Holter monitoring of Japanese patients, events which occurred at a higher frequency in the ticagrelor vs clopidogrel group of Caucasian patients in the PLATO study [4].
In conclusion, ticagrelor 45 mg bid and 90 mg bid were associated with enhanced IPA compared with clopidogrel 75 mg qd in Japanese patients with stable CAD. The PK profiles of ticagrelor and its active metabolite in Japanese and non-Japanese Asian patients were similar to those observed in Caucasian or Chinese populations. No significant safety concerns arose from the current study.
References
Husted S, Emanuelsson H, Heptinstall S, Sandset PM, Wickens M, Peters G. Pharmacodynamics, pharmacokinetics, and safety of the oral reversible P2Y12 antagonist AZD6140 with aspirin in patients with atherosclerosis: a double-blind comparison to clopidogrel with aspirin. Eur Heart J. 2006;27:1038–47.
van Giezen JJ, Nilsson L, Berntsson P, Wissing BM, Giordanetto F, Tomlinson W, et al. Ticagrelor binds to human P2Y12 independently from ADP but antagonizes ADP-induced receptor signaling and platelet aggregation. J Thromb Haemostas. 2009;7:1556–65.
Husted S. Evaluating the risk-benefit profile of the direct-acting P2Y(12) inhibitor ticagrelor in acute coronary syndromes. Postgrad Med. 2011;123:79–90.
Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–57.
Butler K, Teng R. Pharmacokinetics, pharmacodynamics, safety and tolerability of multiple ascending doses of ticagrelor in healthy volunteers. Br J Clin Pharmacol. 2010;70:65–77.
Teng R, Butler K. AZD6140, the first reversible oral platelet P2Y12 receptor antagonist, has linear pharmacokinetics and provides near complete inhibition of platelet aggregation, with reversibility of effect in healthy subjects. Can J Clin Pharmacol. 2010;15:e426.
Teng R, Butler K. Pharmacokinetics, pharmacodynamics, tolerability and safety of single ascending doses of ticagrelor, a reversibly binding oral P2Y(12) receptor antagonist, in healthy subjects. Eur J Clin Pharmacol. 2010;66:487–96.
Teng R, Oliver S, Hayes MA, Butler K. Absorption, distribution, metabolism, and excretion of ticagrelor in healthy subjects. Drug Metab Dispos. 2010;38:1514–21.
Zhou D, Andersson TB, Grimm SW. In vitro evaluation of potential drug–drug interactions with ticagrelor: cytochrome P450 reaction phenotyping, inhibition, induction and differential kinetics. Drug Metab Dispos. 2011;39:703–10.
Gurbel PA, Bliden KP, Butler K, Tantry US, Gesheff T, Wei C, et al. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation. 2009;120:2577–85.
Hasan MS, Basri HB, Hin LP, Stanslas J. Genetic polymorphisms and drug interactions leading to clopidogrel resistance: why the Asian population requires special attention. Int J Neurosci. 2013;123:143–54.
Simon T, Verstuyft C, Mary-Krause M, Quteineh L, Drouet E, Méneveau N, et al. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009;360:363–75.
Roden DM, Stein CM. Clopidogrel and the concept of high-risk pharmacokinetics. Circulation. 2009;119:2127–30.
Kimura T, Morimoto T, Nakagawa Y, Kawai K, Miyazaki S, Muramatsu T, et al. Very late stent thrombosis and late target lesion revascularization after sirolimus-eluting stent implantation: five-year outcome of the j-Cypher Registry. Circulation. 2012;125:584–91.
Teng R, Butler K. Comparison of the pharmacokinetics, pharmacodynamics and tolerability of single and multiple doses of ticagrelor in healthy Japanese and Caucasian volunteers. Int J Clin Pharmacol Ther. 2013 [Epub ahead of print].
Labarthe J, Théroux P, Angioï M, Ghitescu M. Matching the evaluation of the clinical efficacy of clopidogrel to platelet function tests relevant to the biological properties of the drug. J Am Coll Cardiol. 2005;46:638–45.
Sillén H, Cook M, Davis P. Determination of ticagrelor and two metabolites in plasma samples by liquid chromatography and mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:2299–306.
James S, Åkerblom A, Cannon CP, Emanuelsson H, Husted S, Katus H, et al. Comparison of ticagrelor, the first reversible oral P2Y12 receptor antagonist, with clopidogrel in patients with acute coronary syndromes: rationale, design, and baseline characteristics of the PLATelet Inhibition and patient Outcomes (PLATO) trial. Am Heart J. 2009;157:599–605.
Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502.
Hoshino K, Horiuchi H, Tada T, Tazaki J, Nishi E, Kawato M, et al. Clopidogrel resistance in Japanese patients scheduled for percutaneous coronary intervention. Circ J. 2009;73:336–42.
Yokoi H, Kimura T, Isshiki T, Ogawa H, Ikeda Y. Pharmacodynamic assessment of a novel P2Y12 receptor antagonist in Japanese patients with coronary artery disease undergoing elective percutaneous coronary intervention. Thromb Res. 2012;129:623–8.
Li H, Butler K, Yang L, Yang Z, Teng R. Pharmacokinetics and tolerability of single- and multiple-doses of ticagrelor in healthy Chinese volunteers. Clin Drug Investig. 2012;32:87–97.
Acknowledgments
Medical writing support was provided by Rick Flemming and David Evans at Gardiner Caldwell Communications and was funded by AstraZeneca. The authors acknowledge Takaaki Isshiki (Teikyo University School of Medicine), Hiroyuki Daida (Juntendo University Faculty of Medicine), Yasuo Ikeda (Waseda University School of Advanced Science and Engineering), Hisao Ogawa (Kumamoto University School of Medicine) and Makoto Takagi (Saiseikai Central Hospital), who were members of the data safety monitoring board. Technical training in the use of platelet aggregation equipment and the surveillance of aggregation curve accuracy was provided to staff from all sites by Laura West of the University of Sheffield. The authors would also like to thank all medical staff and patients involved in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hiasa, Y., Teng, R. & Emanuelsson, H. Pharmacodynamics, pharmacokinetics and safety of ticagrelor in Asian patients with stable coronary artery disease. Cardiovasc Interv and Ther 29, 324–333 (2014). https://doi.org/10.1007/s12928-014-0277-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12928-014-0277-1