Abstract
The Iowa Newborn Screening (NBS) Program began screening for very long-chain acyl-CoA dehydrogenase deficiency (VLCAD) in 2003. Untreated VLCAD can lead to liver failure, heart failure, and death. Current confirmatory testing recommendations by the American College of Medical Genetics (ACMG) for VLCAD list molecular and functional analysis (i.e., fibroblast fatty acid oxidation probe) as optional. This can lead to misclassification of VLCAD carriers as false positives. Iowa implemented a comprehensive VLCAD confirmatory testing algorithm at the beginning of 2016 that included both molecular and fibroblast analysis. Here, we compare the historic multi-algorithmic confirmatory testing protocol (2005–2016) to this comprehensive protocol (2016–2017). A metabolic specialist reviewed all medical records and NBS data for each out-of-range VLCAD that fell in each testing period. During the comprehensive testing period, 48,651 specimens were screened. Thirteen individuals with out-of-range C14:1 results were classified as follows after review: ten carriers, zero true positives, zero false positives, zero lost to follow-up, and four unable to assess carrier status. During the variable testing period, a total of 486,566 specimens were screened. Eighty-five individuals with out-of-range C14:1 were classified as follows: 45 carriers, two true positives, four false positives, four lost to follow-up, and 30 unable to assess carrier status. Our findings suggest that many out-of-range VLCAD cases that do not receive molecular confirmatory testing could be carriers mistakenly classified as false positives. We recommend comprehensive molecular and functional testing for all children with out-of-range VLCAD NBS results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fatty acid beta-oxidation is an important energy-producing metabolic pathway in times of catabolic stress (exercise, fasting, illness, etc.), and in infants, this process is crucial due to increased metabolic rates and very little glycogen reserve (Vishwanath 2016). Very long-chain acyl-CoA dehydrogenase deficiency (VLCAD) is an autosomal recessive disorder that interferes with fatty acid beta-oxidation, causing hypoglycemia, hepatomegaly, cardiomyopathy, and potentially death in early infancy (Leslie et al. 1993). Critically, if VLCAD is identified early by newborn screening, a low-fat, high carbohydrate diet, and an avoidance of fasting can prevent the major, irreversible health consequences historically seen in untreated infants (Saudubray et al. 2016; Scalais et al. 2015).
VLCAD was added to NBS panels in the early 2000s, as part of expanded newborn screening using tandem mass spectrometry (American College of Medical Genetics/American Society of Human Genetics and Technology Transfer Committee Working 2000; Spiekerkoetter et al. 2003). Now, a part of the Recommended Uniform Screening Panel (RUSP), all infants in the USA are screened for VLCAD (Berry 2015). State-run laboratories use different techniques to screen for VLCAD, with most using a single analyte cut-off for acylcarnitine C14:1. C14:1 is elevated in the blood of infants with pathogenic ACADVL mutations (Zytkovicz et al. 2001). VLCAD is a particularly challenging disorder for newborn screening, because the levels of abnormal biochemical markers seen in truly affected individuals overlap the levels of abnormal biochemical markers that can be seen in VLCAD carriers (individuals who are not at personal risk of disease, but who carry a single pathogenic mutation).
Infants with out-of-range C14:1 NBS results are evaluated by metabolic specialists who use diagnostic tests in order to determine the child’s true diagnosis (Ozben 2013). The current ACMG protocol for the diagnostic evaluation of out-of-range VLCAD NBS results involves the collection of a variety of metabolic labs, crucially, a quantitative plasma acylcarnitine profile (Fig. 1) (Genetics 2001). Beyond the plasma acylcarnitine, decisions regarding which follow-up tests to conduct vary by clinician (Ozben 2013). Some, but not all, specialist clinicians order comprehensive ACADVL gene sequencing. Much of the time, this confirmatory testing reveals a single ACADVL mutation, giving NBS for VLCAD a historically low positive predictive value (Merritt et al. 2014, 2015). Clinicians who are concerned that traditional exonic genetic testing will not detect all possible pathogenic mutations for VLCAD may order functional enzyme assays (fatty acid oxidation (FAO) probes), the gold standard for diagnosing VLCAD (Coughlin and Ficicioglu 2010). Both of these tests are currently optional in the ACMG algorithm—and therefore dependent upon clinician preference.
The completion of confirmatory testing with molecular and functional studies allows for three distinct outcome categories: VLCAD, carrier for VLCAD, and false positive for VLCAD. Individuals with one mutation in the ACADVL gene and normal enzyme activity (or enzyme activity consistent with carrier status) can conclusively be given the label of carrier, and those with no mutations and normal enzyme activity can be labeled false positives. Without molecular testing, these outcome classifications become inaccurate and ambiguous.
Issues in NBS case classification arise from differences in confirmatory testing algorithms state to state, institution to institution, and provider to provider. Individual newborn screening programs tend to have consistent algorithms over time and may not be aware of the practice variation between programs and between providers. For the Iowa NBS program, a programmatic change led the metabolic consultant to eliminate the optional designation of the molecular and functional confirmatory tests, and from 2016 to 2017, recommended functional and molecular studies for every out-of-range VLCAD case. We used this change in practice to compare the two confirmatory testing protocols with a specific focus on changes in NBS case classification.
Study population and methods
For this study, we examined all initial C14:1 NBS results of infants born in Iowa between January 3, 2005 and August 11, 2017 (N = 493,749). From 2005 through 2015, Iowa metabolic specialists evaluated newborns with an elevated C14:1 NBS using the traditional ACMG confirmatory testing recommendations; we will refer to this data set as the “variable testing period.” During this time, at the discretion of the metabolic specialist, these newborns received any combination of molecular and functional testing. From 2016 to 2017, molecular and functional studies were recommended to every elevated C14:1 NBS; we will refer to this data set as the “comprehensive testing period.”
A retrospective chart review was completed for infants with out-of-range C14:1 acylcarnitine values during both testing periods (n = 98, see Supplemental Fig. S1 for Iowa NBS C14:1 cut-offs). The metabolic specialist for the Iowa NBS program reviewed available medical records and NBS case files, the NBS test results, and the NBS short-term follow-up record. Data were collected for comparison using Excel. Particular attention was paid to whether or not molecular testing of the ACADVL gene was completed, as well as if functional enzyme studies (FAO probe) were assessed.
Each case was given a new study outcome classification based on the confirmatory testing completed, independent from any previous NBS case closure classification (Table 1). Specifically, cases with two mutations and/or an abnormal fatty acid oxidation probe were categorized as a “true positive.” If only one mutation was identified, the case was categorized as a “carrier.” If no mutations were detected, the case was categorized as a “false positive.” Importantly, if the mutation analysis was not performed, the case was categorized as “unable to assess carrier status.” Each case, regardless of the time period under which it fell, had the opportunity to be assigned to any of the case classification categories, allowing for direct comparison of the two protocols.
The Institutional Review Board of the University of Iowa reviewed this project and determined it did not meet the regulatory definition of human subject research, and therefore did not require IRB oversight.
Results
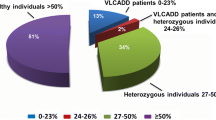
During the variable testing period, there were 85 out-of-range C14:1 cases identified by the Iowa NBS program. Upon review of the confirmatory testing evidence, the majority were determined to be carriers (n = 45), with the next largest category being unable to assess carrier status (n = 30). Four cases were found to be false positives after sequencing, having no detectable mutations in the ACADVL gene. Two cases of true VLCAD were identified, and four cases were lost to follow-up. All results are shown in (Fig. 2a).
During the comprehensive testing period, there were 13 out-of-range cases of VLCAD, and again, the majority of cases were carriers (n = 9). There were a few cases where we continued to be unable to assess carrier status (n = 4). No cases of VLCAD and no false positives were identified, and no cases were lost to follow-up during this timeframe (Fig. 2b). While all infants assessed by the University of Iowa team during the comprehensive protocol were given the opportunity to have molecular and functional testing, not all underwent testing. Two infants were evaluated out-of-state, and the provider did not recommend molecular testing. The other two were seen at the University of Iowa; one family declined molecular testing, and the other’s insurance denied coverage.
The variable testing period and the comprehensive testing period are compared in Fig. 3. A number of cases were reclassified as “unable to assess” after our evidence review. In the Iowa NBS short-term follow-up database, these cases were commonly labeled as false positive or (rarely) carrier when no molecular testing had been completed. Compared side-by-side, as percentages of total cases, the comprehensive protocol increased carrier detection and decreased the number of cases NBS would be unable to classify.
Discussion
Inconsistent and incomplete confirmatory testing after a positive NBS result leads to the misclassification of VLCAD carriers as false positives. It is technically true that any positive NBS result that is not confirmed as a case of VLCAD is a false positive with respect to screening for the condition of VLCAD. However, in some circumstances, false positive results can be further classified as carriers. Failure to do so—and to not indicate to this to the newborn (i.e., family) tested—can have implications for those tested and for the NBS program. Failure to test leaves the individual to erroneously conclude that the testing was a fluke. In reality, the newborn has not received information that is potentially valuable to their future reproductive health—as well as to their parents. For the NBS laboratory, classification has critical implications when optimizing laboratory processes and performing cut-off evaluations. Incorrectly labeling and sorting outcomes data at the laboratory and/or follow-up level can lead to skewed false positive rates, positive predictive values, and setting erroneous cut-offs.
More precise classification categories will help address these issues. While case definitions for all core screening conditions (VLCAD included) have been developed by the national NBS resource center NewSTEPs, to facilitate consistent categorization across NBS programs, the categories they suggest do not seem nuanced enough (NewSTEPs 2018). For NBS laboratories to benefit from more comprehensive confirmatory testing, NBS programs need to create case classifications that accurately and clearly delineate carriers from false positives, and faithfully exclude cases that do not meet the requirements for each of these classifications.
There are nontrivial implications of inconsistent confirmatory testing for the clinicians caring for these newborns and their families. For the provider, there is a potential for incomplete or inaccurate information to be given to parents. The subset of cases that receive complete genetic testing and functional studies would benefit from genetic and reproductive counseling, while the other cases would be left with questions.
We acknowledge several limitations to our study. At this time, we are unable to draw statistical conclusions due to the small number of cases in the comprehensive testing timeframe. While our data suggest that a change in C14:1 cut-off might be necessary, no change has been implemented in Iowa to date. However, the trends noted here exemplify an important phenomenon that needs to be addressed across NBS programs. Utilizing transparent case definitions should improve data quality for the evaluation of current laboratory processes, which could possibly translate into changes in cut-off.
Conclusion
The current ACMG protocol can lead to variable testing patterns across metabolic specialists; therefore, variable confirmatory testing results and outcomes reported back to NBS programs. Using the new VLCAD case classifications from this study, the Iowa NBS program is set to assess current VLCAD screening cut-offs knowing the strengths and limitations of the data collected by NBS confirmatory testing and the short-term follow-up program.
From our experience, we recommend that fatty acid oxidation probe and molecular testing be completed for all patients with out-of-range VLCAD NBS results, as this leads to a complete confirmatory testing picture for both the clinic and laboratory. However, as we experienced, barriers persist that can hinder the collection of these more complicated tests—denial of coverage by insurance, parental refusal, and instances of cases being followed up at outside facilities that do not have comprehensive testing protocols. This last issue could be addressed by more comprehensive national recommendations, and we feel that the benefits of requiring these tests be completed outweigh the challenges of implementation.
NBS short-term follow-up programs would be responsible for integrating this comprehensive data into their case classifications. With consistent testing behind case classifications, state NBS programs could begin to compare data in order to optimize testing protocols and cut-offs. Prior to comparing data, it is critical for programs to understand the limitations of their data and the differences in the data collected by each individual program.
References
American College of Medical Genetics/American Society of Human Genetics T, Technology Transfer Committee Working G (2000) Tandem mass spectrometry in newborn screening. Am Coll Med Genet/Am Soc Hum Genet Test Technol Transf Comm Work Group Genet Med 2:267–269. https://doi.org/10.1097/00125817-200007000-00011
Berry SA (2015) Newborn screening. Clin Perinatol 42:441–453. https://doi.org/10.1016/j.clp.2015.03.002
Coughlin CR 2nd, Ficicioglu C (2010) Genotype-phenotype correlations: sudden death in an infant with very-long-chain acyl-CoA dehydrogenase deficiency. J Inherit Metab Dis 33(Suppl 3):S129–S131. https://doi.org/10.1007/s10545-009-9041-6
Genetics ACoM (2001) ACMG ACT Sheets and Confirmatory Algorithms. In: Bethesda (MD)
Leslie ND, Valencia CA, Strauss AW, Zhang K (1993) Very long-chain acyl-coenzyme A dehydrogenase deficiency. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A (eds) GeneReviews((R)). Seattle (WA)
Merritt JL 2nd et al (2014) Infants suspected to have very-long chain acyl-CoA dehydrogenase deficiency from newborn screening. Mol Genet Metab 111:484–492. https://doi.org/10.1016/j.ymgme.2014.01.009
Miller MJ, Burrage LC, Gibson JB, Strenk ME, Lose EJ, Bick DP, Elsea SH, Sutton VR, Sun Q, Graham BH, Craigen WJ, Zhang VW, Wong LJC (2015) Recurrent ACADVL molecular findings in individuals with a positive newborn screen for very long chain acyl-coA dehydrogenase (VLCAD) deficiency in the United States. Mol Genet Metab 116:139–145. https://doi.org/10.1016/j.ymgme.2015.08.011
NewSTEPs (2018) NewSTEPs case definitions. Association of Public Health Laboratories. https://wwwnewstepsorg/case-definitions. Accessed 25 July 2018
Ozben T (2013) Expanded newborn screening and confirmatory follow-up testing for inborn errors of metabolism detected by tandem mass spectrometry. Clin Chem Lab Med 51:157–176. https://doi.org/10.1515/cclm-2012-0472
Saudubray J-M, van den Berghe G, Walter JH (2016) Inborn metabolic diseases: diagnosis and treatment, 6th edn. Springer-Verlag, Berlin Heidelberg. https://doi.org/10.1007/978-3-662-49771-5
Scalais E, Bottu J, Wanders RJ, Ferdinandusse S, Waterham HR, De Meirleir L (2015) Familial very long chain acyl-CoA dehydrogenase deficiency as a cause of neonatal sudden infant death: improved survival by prompt diagnosis. Am J Med Genet A 167A:211–214. https://doi.org/10.1002/ajmg.a.36803
Spiekerkoetter U, Sun B, Zytkovicz T, Wanders R, Strauss AW, Wendel U (2003) MS/MS-based newborn and family screening detects asymptomatic patients with very-long-chain acyl-CoA dehydrogenase deficiency. J Pediatr 143:335–342. https://doi.org/10.1067/S0022-3476(03)00292-0
Vishwanath VA (2016) Fatty acid beta-oxidation disorders: a brief review. Ann Neurosci 23:51–55. https://doi.org/10.1159/000443556
Zytkovicz TH, Fitzgerald EF, Marsden D, Larson CA, Shih VE, Johnson DM, Strauss AW, Comeau AM, Eaton RB, Grady GF (2001) Tandem mass spectrometric analysis for amino, organic, and fatty acid disorders in newborn dried blood spots: a two-year summary from the New England Newborn Screening Program. Clin Chem 47:1945–1955
Acknowledgements
The authors thank the Iowa Ladies Football Academy and the Stead Family Department of Pediatrics University of Iowa Hospital and Clinics for providing the support that made this study possible.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 49 kb)
Rights and permissions
About this article
Cite this article
Atkins, A.E., Tarini, B.A., Phillips, E.K. et al. Misclassification of VLCAD carriers due to variable confirmatory testing after a positive NBS result. J Community Genet 10, 447–451 (2019). https://doi.org/10.1007/s12687-019-00409-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12687-019-00409-8