Abstract
Counselees from different countries may differ in demographic and medical characteristics and this could affect their pre-counselling cognitions and psychosocial variables. Research outcomes may therefore not be easily transferable between countries. To examine this, a cross-national comparison of UK (West Midlands: WM) and Dutch (Middle Netherlands: MN) counselees in breast cancer genetic counselling was conducted. Two hundred thirty-eight WM and 156 MN proband counselees were compared on demographics, breast cancer history and referral pathways. Multivariate logistic regression analyses were performed to check whether national differences in knowledge of breast cancer and heredity, risk perception, worry and information needs persisted when corrected for the background characteristics. About half of the Dutch compared to 8% of UK counselees were affected by breast cancer. More UK than Dutch counselees were at high risk from hereditary breast cancer. UK counselees had higher risk perceptions and more knowledge about breast cancer prevalence, but these differences lost significance when corrected for counselees' risk levels and other background characteristics. Counselees from the UK might report higher levels of worry than Dutch counselees and this could not be explained by their background characteristics. Comparisons of findings between the UK and the Netherlands show that the UK seems to have a higher percentage of high-risk referrals and these counselees seem to have higher risk perceptions. Irrespective of their actual risk level, UK counselees might be more worried. Comparing findings between the different countries raises questions about how transferable research findings are from one culture to another.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer genetic counselling has been widely introduced in many countries, with differences in genetic service provision which are partly due to major international differences in health care systems (Meiser et al. 2006; Hopwood et al. 2003). There is often an assumption that countries can benefit from international research findings of psychosocial consequences of cancer genetic counselling. However, findings from one country may not be easily transferable to another due to the differences in genetic service provision. Therefore, we compared breast cancer genetic referrals, i.e. counselees' demographics, disease status, referral pathway, knowledge of breast cancer and heredity, risk perception, worry and information needs of an English and a Dutch genetics unit: the West Midlands Regional Genetics Unit (WMRGU) in the West Midlands (WM), UK and the Department of Medical Genetics of the University Medical Center Utrecht (UMCU), serving the Middle Netherlands (MN), the central region of the Netherlands (NL).
In both the UK and the NL, public awareness of hereditary breast cancer has continued to increase in the last decade, leading to a rise in demand for breast cancer genetic counselling. Both breast cancer patients and unaffected women with a positive family history can be referred by their General Practitioner (GP) or specialist consultant, e.g. surgeon, medical oncologist, radiation oncologists and radiologist. A primary goal of breast cancer genetic counselling is to carry out a risk assessment, educate the counselee about their risk, prevention and early detection with the aim of reducing morbidity and mortality (Biesecker et al. 1993; Biesecker 2001). Since both UK and Dutch physicians cannot directly order a DNA test for BRCA testing, all BRCA testing is conducted through the genetics units. Counselees are invited to attend the genetics unit for one to three visits. Counselees assessed as not needing DNA testing to be performed on a blood sample from them or from an affected family member, will generally attend for one visit. Counselees who require DNA testing based on their risk assessment will attend for a second and sometimes a third visit in which the results of their DNA test will be disclosed. The aims and setting of breast cancer genetic counselling in the UK and the NL are thus similar.
Cancer genetics services and broader health care systems differ substantially between the UK and the NL (Godard et al. 2003). In the UK, the National Health Service (NHS) is funded by means of general taxation. At the point of provision, health care including genetic counselling is free of charge. Hospitals are state-owned and GPs have contracts with the NHS. (Private insurance schemes fund only a small proportion of healthcare). Health care is organised in geographic subdivisions, commissioned via Strategic Health Authorities (Van der Zee and Kroneman 2007). In contrast, the Dutch Social Security (based) Health care system is funded by means of earmarked premiums. All residents have a legal obligation to take out health insurance, for which they have to pay. The obligatory basic health insurance covers genetic counselling. The Dutch system is strongly influenced by health care providers and insurers. Care is provided by non-profit hospitals and individual GPs, subject to national legislation and policies (Van der Zee and Kroneman 2007).
The WMRGU provides cancer genetic counselling for the WMs and is one of the 24 cancer genetic centres in the UK. The models of care in operation at the WMRGU broadly reflect genetics service provision in the UK. Similar to most UK genetics units, the WMRGU has set up a triage system as a means of regulating access to cancer genetics services, see for examples of triage systems in other NHS regions (Holloway et al. 2004; Elwyn et al. 2002; Wilson et al. 2005). Counselees fill in a family history form. GPs or specialist nurses in some areas have received training by the WMRGU to draw up a pedigree based on this form and identify those at population risk so that they can be reassured. Where increased risk is identified based on the referral guidelines in Table 1 (WMRGU 2004), these patients are referred to WMRGU for a genetic counsellor to assess the inherited genetic predisposition to cancer. The counsellor classifies a counselee as being at population (<17%), moderate (≥17 and ≤30%) or high (>30%) lifetime risk of developing breast cancer (NICE 2006). The assessment includes careful checking of the counselees' family history of cancer with national cancer registries, once consent has been obtained from family members. The WMRGU offers an appointment to all counselees at high risk and a small number from other risk categories where clarification on family history is required or high levels of worry or anxiety are reported by the referring clinician. Most counselees who are at low to moderate risk are informed about their risk and advice for surveillance by a letter (see Phelps et al. 2004 for an example).
The Department of Medical Genetics of the UMCU roughly serves the MN, which is somewhat larger than the province of Utrecht. In the NL, cancer genetic counselling is provided by eight departments of Medical Genetics of university medical centres and by the familial cancer clinic of the Netherlands Cancer Institute, without geographic subdivision. The Department of Medical Genetics of the UMCU provides breast cancer genetic counselling according to the Dutch guidelines (STOET et al. 2005), as shown in Table 1, and services are therefore similar to those of the other centres and to that of the familial cancer clinic of the Netherlands Cancer Institute. Criteria for referral to breast cancer genetic counselling are part of the Dutch guidelines (STOET et al. 2005), and these are accessible for all health care providers (CBO Dutch Institute for Healthcare Improvement 2005a, 2005b). Training to GPs and specialist consultants about the national guidelines for referral is not obligatory and covers only a small percentage of all physicians in a catchment area. Like other Dutch genetics units, the Department of Medical Genetics of the UMCU performs no triage, but offers all counselees a consultation. Counselees are sent a family history form, which they are requested to complete and return prior to their appointment. The counsellor prepares the pedigree from the family history and after checking relatives' medical files following their consent. From this information, an assessment of cancer predisposition can be made to classify counselees to be at population (<20%), moderate (≥20% and <30%) or high (≥30%) risk
Despite the differences between countries based on the health care delivery system, some similarities have been described in cancer genetic counselees in various countries and within different health care systems, who have been reported to be more highly educated than the general population and to primarily be Caucasian (Ellington et al. 2005; Meiser et al. 2001; Pieterse et al. 2005a). A possible explanation for this finding is that better-educated individuals are more knowledgeable about the possibilities of genetic counselling and genetic risks in general (Culver et al. 2001). They would therefore be more likely to seek genetic counselling or ask their GP for a referral. Additionally, different cultural values concerning genetics might explain fewer requests for counselling and physicians might assume that patients from other than Caucasian ethnicities would not be interested (Shields et al. 2008).
Also, similarities in counselees' information needs have been found. Both UK and Dutch counselees who are the first in their family to request cancer genetic counselling (probands) have unrealistic expectations or do not know what to expect (Metcalfe et al. 2007; Bernhardt et al. 2000; Hallowell et al. 1997; Pieterse et al. 2005b). Many expect to be offered a DNA-test independent of their disease status and risk profile (Metcalfe et al. 2007; Hallowell et al. 1997; Pieterse et al. 2005c). Also, many people in the UK and the NL lack basic genetic knowledge (Henneman et al. 2004; Calsbeek et al. 2007; Morren et al. 2007; Mesters et al. 2005; Walter et al. 2004). Thus, both UK and Dutch counselees have important information needs and seem to lack genetic knowledge upon entering breast cancer genetic counselling.
Breast cancer genetic counselling referrals have never been compared internationally, though cross reference to international studies are frequently used to support and develop further research and clinical activity. Two studies recently carried out independently of each other, at the WMRGU in the UK and at the Department of Medical Genetics of the UMCU in the NL, investigated cancer genetic counselling referrals and counselees' knowledge of breast cancer and heredity, risk perception, worry and information needs with regard to breast cancer genetic predisposition. We explore whether patients referred to these centres are comparable in terms of age, parity, ethnicity, education, personal and cancer family history and referral pathway. Additionally, we assess the impact of these demographic and medical variables on knowledge, risk perception, worry and information needs. The findings will provide important insights as to whether study findings from different countries are likely to be transferable.
Method
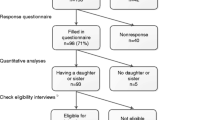
This study involved secondary analyses of data of female adult probands in breast cancer genetic counselling from a UK and a Dutch study. The WMRGU included counselees from August 2005 to December 2006. Although at the WMRGU counselees at population and moderate risk of breast cancer often are not invited for a consultation, but receive a letter of reassurance, all individuals referred to the genetics unit were included in this study. Research ethics committee approval was obtained for the study from the West Birmingham Research Ethics Committee and the study was carried out in accordance with the UK's NHS research governance guidelines. The Department of Medical Genetics of the UMCU included from February 2008 to July 2009. Ethical approval was obtained from the medical ethical committee of the UMCU. Counselees in both countries completed a questionnaire at home, after their referral. Within this period of 17 months, 238 probands for breast cancer genetic counselling of the WMRGU completed a pre-visit questionnaire, with a response rate of 48%. Of the Department of Medical Genetics at the UMCU, 156 probands completed a pre-visit questionnaire, with a response rate of 56%. There were no significant differences between participants and decliners of the WMRGU in breast cancer risk (p = 0.64). For counselees of the UMCU, this risk was not available for decliners. However, there were no significant differences between participants and decliners in breast cancer status (p = 0.66) and age (p = 0.50) (see Table I in Supplementary materials).
Counselee demographics
Counselees' age, whether they had children, and the children's sex was collected in the questionnaires. Ethnicity was assessed in accordance with national definitions. The WMRGU questionnaire assessed ethnicity by asking counselees to indicate the cultural background that best described them (National 2004a). The UMCU questionnaire assessed where counselee's parents were born. In the NL, individuals where at least one parent is born abroad, are defined as having a foreign background (Statistics 2009a). Both questionnaires assessed the highest educational attainment. However, the education systems between the two countries are markedly different. Therefore, we categorized the educational attainment into four levels that broadly correspond to the International Standard Classification of Education (ISCED) (Unesco 1997): First, less than high school (GCSE)/secondary education (corresponds to ISCED level 1). Second, high school (GCSE)/secondary education (completing education at 16–17 years; comparable to ISCED level 2 or 3). Third, further or vocational education, e.g. a training or an apprenticeship qualification (comparable to ISCED level 3). And fourth, university or higher vocational education (degree or above; comparable to ISCED level 5).
It was also ascertained whether the counselee was affected with breast cancer and/or had first and/or second-degree family members affected with breast cancer. Additionally, the referring physician (GP or consultant) and initiator of referral (patient or physician) were assessed. For the Dutch counselees, the questionnaire data of counselees' disease status was checked with the medical files and high reliability was found; only one counselee was not affected with breast cancer according to her medical file, while she had reported to be affected in the questionnaire.
Breast cancer risk
Counselees' lifetime risk of developing breast cancer was based on the counsellors' estimation. In the WM, through assessment genetic counsellors classified a counselee as being at population (<17%), moderate (17%–30%) or high risk (>30%) of developing breast cancer during their lifetime (NICE 2006). During this study, in Utrecht, a genetic counsellor indicated the counselees' lifetime risk of developing breast cancer post-visit on a continuous scale from 0% to 100%, based on the pedigree that was drawn up prior to or during the first consultation. We categorized this risk based on the UK's definition of risk categories. The counsellors' risk estimation was based on the Claus tables (Claus et al. 1994) in both the WM and the MN. In the WM, calculation was performed with the Cyrillic system, in which the Claus tables are integrated (Cyril Chapman personal communication). In the MN, the calculation was based on the Claus tables and the Claus-extended formula as integrated in the Dutch national guidelines (Van Asperen et al. 2004). These systems are not likely to result in systematic bias in the calculation of lifetime risks.
Breast cancer knowledge, risk perception and worry
The questionnaires of the WMRGU and the UMCU both assessed knowledge about breast cancer and heredity with two similar statements based on the Breast Cancer Knowledge Scale (Claes et al. 2003; Pieterse et al. 2005a). Respondents indicated whether each statement was correct, incorrect or whether they did not know. Perceptions of breast cancer risk were assessed using a 3-item Likert scale. The counselee indicated her perceived breast cancer risk as lower, the same as or higher than the average risk for a woman of her age (Metcalfe et al. 2009). Counselee's worry about developing breast cancer was assessed with one question: ‘how worried are you about getting breast cancer’. This was assessed using a 4-item scale, ranging from not at all worried to very worried (Glanz et al. 1999), the Dutch version of this question was used earlier by Van Oostrom et al. (2003).
Information needs
Counselees' information needs were assessed in both questionnaires. Both centres have independently developed a set of questions to assess information needs from the counselee perspective. The Birmingham scale for information needs consisted of 25 items. Importance was assessed with a 5-point scale anchored by ‘not important’ and ‘extremely important’ and included a middle category for ‘not sure’ (Metcalfe et al. 2009). In the Dutch questionnaire, counselees' needs were assessed using a counselee-centred instrument, called QUOTE-geneca (Pieterse et al. 2005b). The QUOTE-geneca consisted of 25 general and 19 cancer genetic counselling specific items. Respondents indicated importance on a 4-point scale anchored by ‘not important’ and ‘extremely important’. We dichotomized the data to not important and important. We considered ten items from the Birmingham scale and the QUOTE-geneca to be similar. Table 6 displays both the English and Dutch wording of these items.
Analysis
SPSS software, version 17 was used for the analyses. Data from both countries were combined into one dataset. Descriptive statistics were used to describe the counselees' characteristics. Chi-square and t tests were used to explore for differences in counselees' demographic characteristics, type of referral, cancer worry, risk perception, knowledge and information needs between the two countries. Logistic regression analyses were conducted to check whether national differences in cancer knowledge, risk perception, worry and information needs persisted when corrected for demographic and medical characteristics. Independent variables in these logistic regression analyses were the country and counselees' age, having children, ethnicity, education, disease status and being referred by a GP or consultant. A correlation table was used to check for multicollinearity. The fit of the model for each dependent variable was tested with a Hosmer–Lemeshow test. The pseudo percentage of explained variance of each model was estimated by use of the Nagelkerke R 2. Additionally, we produced crosstabs with chi-square to check for associations between type of referral and risk category, between initiator of referral and educational attainment and between educational attainment and risk category in both countries.
Factor analyses were undertaken on the information needs from Birmingham scale for information needs and the QUOTE-geneca. Principal Component Analyses (PCA) with varimax rotation on the needs questionnaires showed three similar factors: risk of counselee and family, genetic counselling procedures and hereditary breast cancer. For the Birmingham questionnaire, PCA identified four needs with satisfactory internal consistencies (alpha 0.89–0.52). Except for the three shared factors, the factor screening and prevention was identified. The appropriateness of conducting PCA was shown by KMO (0.91) and Barlett's test of sphericity (χ² = 3,079.98; p < 0.00). For the QUOTE-geneca two factor analyses; one for the generic and one for the cancer-specific items, identified four and five needs, respectively, with satisfactory internal consistencies (alpha = 0.90–0.58). The six additional factors of the QUOTE-geneca constitute emotional consequences of the counselling, determination of being a carrier of a breast cancer gene mutation, meaning of being a carrier of a breast cancer gene mutation, sensitive communication, emotional support and assessment of susceptibility to breast cancer. The appropriateness of conducting PCA was proved by KMO (0.83) and Barlett (χ² = 1,756.10; p = 0.00).
Results
Demographics
As shown in Table 2, the mean age of the WM counselees and the MN counselees did not differ significantly. About three quarters of the counselees had children. Dutch counselees had higher educational attainments (p < 0.001). The Dutch counselees were more ethnically diverse than their British counterparts. Few of the WM counselees (2.1%) considered themselves to have another cultural background than white British or white Irish. Almost one fifth (17.3%) of the MN counselees had at least one parent who was born abroad and were therefore considered to have a foreign background. For six of them (3.8%), at least one of their parents was born in a non-Western country. MN counselees with a foreign background did not significantly differ in knowledge, risk perception or worry with MN counselees with a Dutch background (not in table; 82% vs. 76% has correct knowledge, p = 0.52; 70% vs. 71% perceives their risk as higher than average, p = 0.87; 37% vs. 32% very worried, p = 0.58).
Personal and family cancer history and risk
While almost half of the counselees in the MN (43.6%) were affected with breast cancer, this applied to only 8% of the counselees in the WM (Table 2). More of the WM counselees had first-degree family members who were affected with breast cancer, as compared to the MN counselees. There was also a large difference in the risk of breast cancer. Almost a third of the counselees in the WM (29%) versus 17.5% of the MN counselees, were considered to have a high lifetime risk for breast cancer. MN counselees were more often at population risk than WM counselees (36.4% vs. 29.4%). In both countries, affected counselees were more often at high risk than unaffected counselees (χ 2 = 9.2; df = 2; p = 0.01; see Table 3).
Referral and initiator of referral
Most WM counselees were referred by their GP as opposed to the MN counselees who were mainly referred by their consultant (Table 2). In both countries, counselees unaffected with breast cancer were more likely to be referred by a GP and affected counselees were more likely to be referred by a consultant (χ 2 = 105.8; df = 2; p < 0.001; Table 3).
In both the WM and the MN, unaffected counselees, as compared to affected counselees, were more likely to report that they had raised the cause for concern about an inherited breast cancer predisposition (χ 2 = 82.7; df = 5; p < 0.001;Table 3). Also, counselees referred by their GP had more often requested a referral to breast cancer genetic counselling, compared to those referred by a consultant (χ 2 = 55.0; df = 10; p < 0.001). For counselees from the WM, there was no statistically significant association between their educational level and who raised cause for concern. However, higher-educated Dutch counselees had raised the cause for concern more often than those less educated (χ 2 = 18.1; df = 3; p < 0.001).
Crosstab analyses showed that the GPs in the WM referred more women who were at high breast cancer risk (26.3%) compared to their colleagues in the MN (10.8%) (χ 2 = 10.8; df = 2; p < 0.01). Of WM consultants' referrals, 36.8% were at high risk, and of Dutch consultants' referrals, this was 21.9%. In both WM and MN, consultants' referrals were more often assigned to the high-risk category than GP's referrals (χ 2 = 7.1; df = 2; p < 0.05).
Knowledge about breast cancer and heredity
With regard to knowledge about breast cancer and heredity, more counselees from the WM (62.8%) than from the MN (42.9%) were aware that having a breast cancer gene mutation will not necessarily lead to the development of breast cancer (Table 4). This difference remained significant after correcting for demographic variables, risk and referral pathway (OR = 3.05; p < 0.001; Table 5). Less WM (64.5%) than MN (76.8%) counselees were aware that a person without a breast cancer gene mutation can still develop breast cancer. However, this difference lost significance after correcting for the demographic variables, risk and referral pathway (Table 5). Outcomes of multivariate analysis further showed that higher-educated counselees had more accurate knowledge. Also, counselees at high risk more often knew that a person who does not have a breast cancer gene mutation can still develop breast cancer (OR = 2.06; p < 05). Self-referred counselees were less often aware of this fact (OR = 0.29; p < 0.05).
Risk perception and worry
Of the unaffected counselees, most British (80%) and Dutch (79.3%) rated themselves to be at higher risk of developing breast cancer than the average woman (Table 4). British unaffected counselees had higher risk perceptions than Dutch unaffected counselees. However, this difference lost significance when corrected for disease status, educational level and risk category (Table 5). Being affected with breast cancer was associated with a lower risk perception (OR = 0.28, p < 0.05).
British unaffected counselees were also more worried about developing breast cancer than their Dutch counterparts (WM, 59.2%; MN, 33%; Table 4). The results of multivariate analysis showed that being from the WM was indeed associated with higher worry (OR = 2.8; p < 0.001; Table 5). Additionally, being younger and being at low risk was associated to increased worry.
Information needs
Both the counselees from the WM and those from the MN considered the factor ‘risk of counselee and family’ as the most important information need (Table 6). The factor ‘genetic counselling procedures’, about what to expect of breast cancer genetic counselling and how long procedures take, was the second most important need. The factor ‘hereditary breast cancer’ was considered least important by both WM and MN counselees. This last factor included information about the prevalence, inheritance patterns and traits of hereditary cancer and thus constituted general education about genetics. There were no significant differences between the WM and MN counselees in the importance they attached to these factors of information needs, either in univariate or in multivariate analyses. Younger counselees and those who had children valued the factor ‘genetic counselling procedures’ more than older counselees and those without children (age OR = 0.97; CI 0.94–1.00; p < 0.05; children OR = 2.19; CI 1.16–4.15; p = 0.01).
We compared the importance scores of WM and MN counselees on ten items from the information needs questionnaires that were considered to be similar. Both WM and MN counselees considered most items important or very important (Table 6). Information about screening and prevention was rated as important by the highest percentage of counselees from both the WM and the MN. The second most important item was the counselee's risk of breast cancer for WM counselees and the family members' risk of cancer for the Dutch counselees. Information about the procedure of family history analysis was considered to be important by the lowest percentage of counselees. The item about family members' risk of developing cancer was rated as important by significantly more Dutch than WM counselees.
Discussion
In comparing characteristics of women referred to breast cancer genetic counselling at the WMRGU in West Midlands, UK and the UMCU in the Middle Netherlands similarities were observed in counselees' information needs. However, important differences emerged between the two groups of women relating to educational attainment, ethnicity, breast cancer diagnoses, referral pathway and counselees' breast cancer risk. These differences partly explained variation in knowledge and risk perception.
In the Dutch cohort, the number of counselees affected by breast cancer was much higher than in the UK group. Similar percentages of breast cancer-affected probands were reported in other Dutch studies (Van Asperen et al. 2002; Van Dijk et al. 2004) and a European comparative study of cancer genetic counselling also found that UK (cancer genetics centre Manchester) had few (10%) affected counselees, compared to 33% in the NL (genetics centre Leiden) (Hopwood et al. 2003). Most Dutch affected counselees were referred by their consultant; most unaffected counselees were referred by their GP. The WMRGU received fewer referrals from consultants; counselees were more likely to be referred by their GP and this was often at the counselee's request. A referral pattern which is reflected across the UK: A comparative study of UK cancer genetic centres reported that on average 67% of referrals were from a GP (Hopwood et al. 2004), compared to 66% in the WM sample of the current study. Consistent with the high percentage of Dutch affected patients referred by their consultant, Dutch consultants' knowledge of breast cancer and heredity was recently found to be satisfactorily (Van Riel et al. 2010). However, in the UK, very few women affected by breast cancer were being referred for cancer genetic counselling, and this requires further work to elucidate the reasons. Possibly, UK consultants are not aware of referral criteria for breast cancer patients or they may be carrying out the familial risk assessment without the advice from the geneticists.
A lower percentage of women in the population risk category were referred to the WMRGU than to the UMCU, suggesting better referral accuracy in the lower risk spectrum in the WM. At the WMRGU, 29% versus 36% of the counselees at the UMCU were not at increased risk for breast cancer based on their pedigree. This relatively low percentage in the WM is in line with national findings in the UK (Wonderling et al. 2001). The higher percentage of Dutch counselees at population risk cannot be explained by differences between the referral guidelines, as Table 1 has shown that these are quite comparable.
The difference in referral accuracy might partly be due to the training that the WMRGU provides to GPs to draw up a pedigree and assess genetic predisposition to cancer. Dutch GP's have generally not received training from a genetics unit. Studies of GP genetics knowledge show they have poor knowledge (Watson et al. 2001) and that breast cancer genetics education (Watson et al. 2002; Bethea et al. 2008) and communication of referral guidelines (Lucassen et al. 2001) could indeed improve GPs' management of familial breast cancer. The lower percentage of counselees at population risk might indicate that UK GPs prefer to refer patients to a cancer diagnostic clinic (Al Habsi et al. 2008) or more often reassure population-risk individuals, without referring them for breast cancer genetic counselling. This would be in accordance with the NICE guideline recommending that low-risk individuals are dealt within primary care and moderate-risk individuals in cancer units (NICE 2006). Possibly, Dutch GPs might be more tempted to refer patients who request it even if the patient is not appropriate for referral based on guidelines, as was found in a US study (White et al. 2008). However, genetics education might be more easily organised within the geographic subdivisions of the NHS than within the more loosely organised Dutch Social Security Health care system, which lacks clear geographical subdivisions. Dutch GPs could refer to more than one genetics unit and are therefore less tied to one unit. Also, it might be easier to reach British GPs who are employed by the NHS than Dutch GPs who are independent practitioners. Referral accuracy might thus depend on the health care system, however, this study is too limited in scope to draw conclusions.
The WM counselees came from more educationally diverse backgrounds, whereas the MN counselees tended to have higher levels of educational attainment. Consistent with earlier findings of the UMCU (Pieterse et al. 2005c) and with studies of other Dutch genetics centres (Van Asperen et al. 2002; Van Dijk et al. 2004), almost half of the counselees in Utrecht was higher educated (MSc/BSc). Thirty-eight percent of the population in the province of Utrecht is higher educated (Statistics 2003) and 28% of the Dutch population (Statistics 2009a). Of the MN counselees, 2.6% was educated at the lowest level (less than high school), compared to 8.4% in the Dutch population. In contrast, WMRGU breast cancer genetic counselees were more representative of the WM population, which was observed previously for cancer genetic counselling in general (Metcalfe et al. 2009). Of the WM counselees, 11% had less than high school qualifications, compared to 13% of the English population (National 2009), to which WM educational attainments are comparable (Williams and Botterill 2006). Less-educated British counselees therefore seem to access breast cancer genetic counselling better than their Dutch counterparts.
Since most counselees in both the WM and MN had raised the cause for concern about hereditary breast cancer themselves, the role of the physician does not explain the participation of less-educated WM counselees. Additionally, British GPs have been found to adopt a reactive rather than proactive role in the referral of asymptomatic patients with a family history of cancer (Al Habsi et al. 2008). Our findings suggest that less-educated British counselees raise the cause of concern for hereditary breast cancer more often than their Dutch counterparts and this might explain the genetic counselling uptake of a more educationally diverse group in the WM. We currently lack explanations for this finding, and suggested further study is required.
In relation to ethnicity, the Dutch breast cancer genetic counselling seems to be more inclusive, as counselees in MN were from a somewhat more ethnically diverse group than the counselees in the WM. Consistent with earlier findings (Metcalfe et al. 2009), few WM counselees are likely to describe themselves as being from a different cultural background than white British or white Irish (2%). This does not represent the West Midlands population (14% from a black or minority ethnic group (National 2004a), nor the UK population (8% from a black or minority ethnic group) (National 2004b). These findings from the West Midlands are in line with the national study of UK cancer genetic counselling; only 3% of the cancer genetic counselees were individuals from ethnic minorities (Wonderling et al. 2001). In contrast, the 17% of Dutch counselees had a foreign background, compared to one fifth of the population of both the province of Utrecht (Statistics 2008) and the NL (Statistics 2009a). However, only 4% of the counselees had a non-western foreign background, compared with 10% of the Dutch population and 11% of the province of Utrecht (Statistics 2009b). This low percentage of counselees with a non-western background is similar to that of a later study of cancer genetic counselees at the UMCU (Van Riel et al. 2011). Differences in the UK and Dutch definition of ethnicity may have affected the findings. However, both the UK and NL individuals, with a non-western ethnic background, are underrepresented in breast cancer genetic counselling, as has been reported for other countries (Ellington et al. 2005; Meiser et al. 2001). No differences were found in the knowledge, risk perception and worry of Dutch counselees with a foreign background compared to counselees with a Dutch background. However, we expect that this is due to the fact that in most cases, counselees had a western instead of a non-western background. Increased inclusion of women with non-western background in breast cancer genetic counselling is expected to lead to larger health literacy problems and these women might also differ in risk perception and breast cancer worry (Glanz et al. 1999; Culver et al. 2001).
British unaffected counselees might be more worried about developing breast cancer and might have more accurate knowledge about the penetrance of BRCA mutations than their Dutch counterparts. These differences in cancer worry and knowledge between counselees from the WM and MN remained when corrected for counselees' age, disease status and their risk. The larger percentage of unaffected counselees in the WM did thus not explain the higher worry of WM counselees. Indeed, studies within Dutch centres reported similar pre-visit cancer worry and knowledge for affected and unaffected counselees (Van Dijk et al. 2006; Pieterse et al. 2011). Importantly, a comparative study of UK cancer genetic centres found no significant differences in counselees' cancer worry between centres pre-counselling (Hopwood et al. 2004). Our finding might thus reflect a national difference in the attitudes towards breast cancer. There are possibly national factors that predict these outcomes, for instance the attention for hereditary cancer in the media might differ between the UK and NL.
Unaffected counselees from the WM had higher risk perceptions than their counterparts from the MN. Also, they were less aware that a person without a breast cancer gene mutation can still develop breast cancer than the counselees in the MN. These differences were explained by counselees' educational attainment, breast cancer status and risk and referral pathway, not the country where the genetic counselling was being delivered. Therefore, differences in counselees' characteristics can be important predictors.
Where similarities existed, information needs were consistent between counselees in both the WM and MN, with the same order of priority. Counselees wanted to know more about their personal risk and that of their family, to understand what was involved with regard to genetic counselling (genetic counselling procedures) and they wanted to understand the inheritance pattern of cancer (hereditary breast cancer). In accordance with earlier findings, the information needs were independent of risk levels (Metcalfe et al. 2009). These information needs were not influenced by other counselee characteristics, e.g. educational level, either and thus seem to be robust findings. Since these three factors appeared in two independently developed questionnaires, these might constitute core information needs for probands in breast cancer genetic counselling.
Implications for clinical practice
The juxtaposition of the outcomes from the two research studies has implications for clinicians, policy-makers and researchers. We have shown that between the UK and the NL, there are important differences that need careful consideration in translating findings from one country's research to another's, in relation to breast cancer genetic counselling. However, comparison of the two countries has provided important insights for each other's practice and offers opportunities to learn how breast cancer genetic counselling might be improved to reach its target audience. Either through more appropriate referral by education of GPs (NL) and consultants (UK) or improved inclusivity of ethnic minorities (UK) and less well educated (NL). Despite both countries being members of the European Union, there were large inconsistencies in the way demographic information, i.e. ethnicity and educational attainment was recorded in the two countries and standardisation of reporting should possibly be considered.
Limitations
The inclusion of counselees at the WMRGU was from 2005 to 2006, whereas the Department of Medical Genetics at the UMCU included counselees from 2008 to 2009. Consequently, there might be differences in findings due to the time lag. However, the guidelines regarding the referral to the WMRGU for breast cancer genetic counselling have not changed between 2005 and 2009 (WMRGU 2004) and nor have the Dutch guidelines for referral to breast cancer genetic counselling during this period (STOET et al. 2005). Moreover, comparisons of counselee characteristics in the current study with one that included female probands who requested breast cancer genetic counselling from 2001 to 2003 at the UMCU (Pieterse et al. 2011) shows that counselees' characteristics of have been stable. The mean age of counselees was 44 (SD = 9.3) in the prior study, compared to 43 (SD = 11.5) in the current Dutch dataset. Also comparable to the current Dutch findings, in the prior study, about half of all counselees (53%) was breast cancer affected and a large percentage (36%) was higher educated (BSc/MSc). Moreover, the correct pre-visit knowledge of breast cancer and heredity was 4.8 (CI 4.5–5.1) in the prior study and was 4.7 (CI 4.4–4.9) in the dataset of the current study at the UMCU (Albada 2011). This finding is consistent with studies of cancer patients' reporting that their genetic knowledge did not increase over time (Calsbeek et al. 2007). Thus, a comparison of two studies from one genetics centre indicates that characteristics of the population of counselees have not changed much from 2005 to 2009.
The information needs items that we compared were similar but not literally translated. Additionally, the item about cancer worry (Glanz et al. 1999) has been translated (Van Oostrom et al. 2003) and is widely used. However, the English and Dutch answer categories might result in different answer tendencies because of slightly differing emphases on meanings.
This study involved only two cancer genetic counselling units, and there may be variations even within the same country. Although we have tried to connect our findings to other studies of breast cancer genetic counselling in the UK and the NL, this might still show an incomplete picture. For example, data about the percentage of counselees from ethnic minorities was not reported for other Dutch cancer genetics centres and we thus lacked a comparison. Further work is required to examine the findings in a wider international context. If studies included two to four centres for each country involved, differences at national and international level could be unravelled more easily. Nonetheless, this article begins the debate about the transferability of findings between settings internationally, where research from different countries is often used to inform policy and practice.
Conclusion
Researchers and clinicians often use research carried out in a wide variety of countries interchangeably, as if the findings are all comparable despite differences in health service provisions or cultures. By taking two studies of breast cancer genetic counselling, we have shown substantial variations exist in counselee characteristics, including educational background, ethnicity, referral pathway, cancer diagnosis and risk level. Importantly, these differences influenced counselees' pre-visit levels of knowledge, risk perception and breast cancer worry. More specifically, UK counselees had higher risk perception, but were also at higher risk of developing breast cancer. UK counselees were also more worried and this could not be explained by their background characteristics. Differences in culture or attention for hereditary cancer in the media that could cause this worry need to be studied further. These findings indicate that international publications have to be interpreted with caution if applied to another setting. However, core components in information needs were remarkably similar between UK and Dutch counselees.
References
Al Habsi H, Lim JN, Chu CE, Hewison J (2008) Factors influencing the referrals in primary care of asymptomatic patients with a family history of cancer. Genet Med 10:751–757
Albada A (2011) Preparing for breast cancer genetic counselling: web-based education for counselees. PhD thesis Utrecht University
Bernhardt BA, Biesecker BB, Mastromarino CL (2000) Goals, benefits, and outcomes of genetic counseling: client and genetic counselor assessment. Am J Med Genet 94:189–197
Bethea J, Qureshi N, Drury N, Guilbert P (2008) The impact of genetic outreach education and support to primary care on practitioner's confidence and competence in dealing with familial cancers. Community Genet 11:289–294
Biesecker BB (2001) Goals of genetic counseling. Clin Genet 60:323–330
Biesecker BB, Boehnke M, Calzone K, Markel DS, Garber JE, Collins FS, Weber BL (1993) Genetic counseling for families with inherited susceptibility to breast and ovarian cancer. JAMA 269:1970–1974
Calsbeek H, Morren M, Bensing J, Rijken M (2007) Knowledge and attitudes towards genetic testing: a two year follow-up study in patients with asthma, diabetes mellitus and cardiovascular disease. J Genet Couns 16:493–504
CBO Dutch Institute for Healthcare Improvement (2005a) Guideline hereditary tumours: Familial mamma/ovarian carcinoma. (Richtlijn erfelijke tumoren: Familiair Mamma/Ovarium carcinoom). www.oncoline.nl
CBO Dutch Institute for Healthcare Improvement (2005b) Guideline hereditary tumours: Hereditary mamma/ovarian carcinoma. (Richtlijn erfelijke tumoren: Hereditair Mamma/Ovarium carcinoom). www.oncoline.nl
Claes E, Evers-Kiebooms G, Boogaerts A, Decruyenaere M, Denayer L, Legius E (2003) Communication with close and distant relatives in the context of genetic testing for hereditary breast and ovarian cancer in cancer patients. Am J Med Genet 116A:11–19
Claus EB, Risch N, Thompson WD (1994) Autosomal dominant inheritance of early onset breast cancer. Implications for risk prediction. Cancer 73:643–651
Culver J, Burke W, Yasui Y, Durfy S, Press N (2001) Participation in breast cancer genetic counseling: the influence of educational level, ethnic background and risk perception. J Genet Couns 10:215–231
Ellington L, Roter D, Dudley WN, Baty BJ, Upchurch R, Larson S, Wylie JE, Smith KR, Botkin JR (2005) Communication analysis of BRCA1 genetic counseling. J Genet Couns 14:377–386
Elwyn G, Iredale R, Gray J (2002) Reactions of GPs to a triage-controlled referral system for cancer genetics. Fam Pract 19:65–71
Glanz K, Grove J, Lerman C, Gotay C, Le Marchand L (1999) Correlates of intentions to obtain genetic counseling and colorectal cancer gene testing among at-risk relatives from three ethnic groups. Cancer Epidemiol Biomarkers Prev 8:329–336
Godard B, Kääriänen H, Kristoffersson U, Tranebjaerg L, Coviello D, Aymé S (2003) Provision of genetic services in Europe: current practices and issues. Eur J Hum Genet 11:S13–S48
Hallowell N, Murton F, Statham H, Green JM, Richards MPM (1997) Women's need for information before attending genetic counselling for familial breast or ovarian cancer: a questionnaire, interview, and observational study. Br Med J 314:281–283
Henneman L, Timmermans DR, Van der Wal G (2004) Public experiences, knowledge and expectations about medical genetics and the use of genetic information. Community Genet 7:33–43
Holloway S, Porteous M, Cetnarskyj R, Anderson E, Rush R, Fry A, Gorman D, Steel M, Campbell H (2004) Patient satisfaction with two different models of cancer genetic services in south-east Scotland. Br J Cancer 90:582–589
Hopwood P, Van Asperen CJ, Borreani G, Bourret P, Decruyenaere M, Dishon S, Eisinger F, Evans DG, Evers-Kiebooms G, Gangeri L, Hagoel L, Legius E, Nippert I, Rennert G, Schlegelberger B, Sevilla C, Sobol H, Tibben A, Welkenhuysen M, Julian-Reynier C (2003) Cancer genetics service provision: a comparison of seven European centres. Community Genet 6:192–205
Hopwood P, Wonderling D, Watson M, Cull A, Douglas F, Cole T, Eccles D, Gray J, Murday V, Steel M, Burn J, McPherson K (2004) A randomised comparison of UK genetic risk counselling services for familial cancer: psychosocial outcomes. Br J Cancer 91:884–892
Lucassen A, Watson E, Harcourt J, Rose P, O'Grady J (2001) Guidelines for referral to a regional genetics service: GPs respond by referring more appropriate cases. Fam Pract 18:135–140
Meiser B, Butow P, Baratt A, Gattas M, Gaff C, Haan E, Gleeson M, Dudding T, Tucker K, The Psychological Impact Collaborative Group (2001) Risk perceptions and knowledge of breast cancer genetics in women at increased risk of developing hereditary breast cancer. Psychol Health 16:297–311
Meiser B, Gaff C, Julian-Reynier C, Biesecker BB, Esplen MJ, Vodermaier A, Tibben A (2006) International perspectives on genetic counseling and testing for breast cancer risk. Breast Dis 27:109–125
Mesters I, Ausems A, De Vries H (2005) General public's knowledge, interest and information needs related to genetic cancer: an exploratory study. Eur J Cancer Prev 14:69–75
Metcalfe A, Werrett J, Burgess L, Clifford C (2007) Psychosocial impact of the lack of information given at referral about familial risk for cancer. Psychooncology 16:458–465
Metcalfe A, Werrett J, Burgess L, Chapman C, Clifford C (2009) Cancer genetic predisposition: information needs of patients irrespective of risk level. Fam Cancer 8:403–412
Morren M, Rijken M, Baanders AN, Bensing J (2007) Perceived genetic knowledge, attitudes towards genetic testing, and the relationship between these among patients with a chronic disease. Patient Educ Couns 65:197–204
National Statistics (2004a) People & migration, Ethnicity. http://www.statistics.gov.uk/CCI/nugget.asp?ID=764&Pos=4&ColRank=1&Rank=176
National Statistics (2004b) People & migration, Ethnicity: regional distribution. http://www.statistics.gov.uk/cci/nugget.asp
National Statistics (2009) Regional trends in education. http://www.statistics.gov.uk/articles/RegionalTrends/RT41-Article5.pdf
NICE (2006) Clinical guideline 14. Familial breast cancer. The classification and care of women at risk of familial breast cancer in primary, secondary and tertiary care. http://guidance.nice.org.uk/CG41/Guidance/pdf/English
Phelps C, Platt K, France L, Gray J, Iredale R (2004) Delivering information about cancer genetics via letter to patients at low and moderate risk of familial cancer: a pilot study in Wales. Fam Cancer 3:55–59
Pieterse A, Van Dulmen S, Ausems M, Schoemaker A, Beemer F, Bensing J (2005a) QUOTE-geneca: development of a counselee-centered instrument to measure needs and preferences in genetic counseling for hereditary cancer. Psychooncology 14:361–375
Pieterse AH, Ausems MGEM, Van Dulmen AM, Beemer FA, Bensing JM (2005b) Initial cancer genetic counseling consultation: change in counselees' cognitions and anxiety, and association with addressing their needs and preferences. Am J Med Genet A 137:27–35
Pieterse AH, Van Dulmen AM, Ausems MGEM, Beemer FA, Bensing JM (2005c) Communication in cancer genetic counselling: does it reflect counselees' pre-visit needs and preferences? Br J Cancer 92:1671–1678
Pieterse A, Ausems M, Spreeuwenberg P, Van Dulmen S (2011) Affected versus unaffected women seeking breast cancer genetic counselling: long-term influence on cognitions and distress. Patient Educ Couns (in press)
Shields AE, Burke W, Levy DE (2008) Differential use of available genetic tests among primary care physicians in the United States: results of a national survey. Genet Med 10:404–414
Statistics Netherlands (2003) Utrecht has the most highly educated population. http://www.cbs.nl/en-GB/menu/themas/onderwijs/publicaties/artikelen/archief/2008/2008-2436-wm
Statistics Netherlands (2008) Annual report integration 2008. http://www.cbs.nl/nl-NL/menu/themas/dossiers/allochtonen/publicaties/publicaties/archief/2008/2008-b61-pub.htm
Statistics Netherlands (2009a) Almost as many higher as lower educated Dutch. http://www.cbs.nl/en-GB/menu/themas/onderwijs/publicaties/artikelen/archief/2008/2008-2436-wm
Statistics Netherlands (2009b) People with a foreign background. http://www.cbs.nl/en-GB/menu/themas/dossiers/allochtonen/methoden/begrippen
STOET, Vereniging Klinische Genetica Nederland, Werkgroep Klinische Oncogenetica (2005) Erfelijke tumoren: Richtlijnen voor diagnostiek en preventie. www.stoet.nl
Unesco (1997) International Standard Classification of Education ISCED 1997 http://www.unesco.org/education/information/nfsunesco/doc/isced_1997.htm
Van Asperen CJ, Van Dijk S, Zoeteweij MW, Timmermans DR, De Bock GH, Meijers-Heijboer EJ, Niermeijer MF, Breuning MH, Kievit J, Otten W (2002) What do women really want to know? Motives for attending familial breast cancer clinics. J Med Genet 39:410–414
Van Asperen CJ, Jonker MA, Jacobi CE, Van Diemen-Homan JE, Bakker E, Breuning MH, Van Houwelingen JC, de Bock GH (2004) Risk estimation for healthy women from breast cancer families: new insights and new strategies. Cancer Epidemiol Biomarkers Prev 13:87–93
Van der Zee J, Kroneman MW (2007) Bismarck or Beveridge: a beauty contest between dinosaurs. BMC Health Services Research 7
Van Dijk S, Otten W, Van Asperen CJ, Timmermans DR, Tibben A, Zoeteweij MW, Silberg S, Breuning MH, Kievit J (2004) Feeling at risk: how women interpret their familial breast cancer risk. Am J Med Genet 131A:42–49
Van Dijk S, Timmermans DRM, Meijers-Heijboer EJ, Tibben A, Van Asperen CJ, Otten W (2006) Clinical characteristics affect the impact of an uninformative DNA-test result: the course of worry and distress experienced by women who apply for genetic testing for breast cancer. J Clin Oncol 24:3672–3677
Van Oostrom I, Meijers-Heijboer H, Lodder LN, Duivenvoorden HJ, Van Gool AR, Seynaeve C, Van der Meer CA, Klijn JG, Van Geel BN, Burger CW, Wladimiroff JW, Tibben A (2003) Long-term psychological impact of carrying a BRCA1/2 mutation and prophylactic surgery: a 5-year follow-up study. J Clin Oncol 21:3867–3874
Van Riel E, Warlam-Rodenhuis CC, Verhoef S, Rutgers EJ, Ausems MG (2010) BRCA testing of breast cancer patients: medical specialists' referral patterns, knowledge and attitudes to genetic testing. Eur J Cancer Care 19:369–376
Van Riel E, Van Dulmen S, Ausems MGEM (2011) Access to cancer genetic counselling: who is being referred and why? Characteristics of counselees and their referral. Submitted
Walter FM, Emery J, Braithwaite D, Marteau TM (2004) Lay understanding of familial risk of common chronic diseases: a systematic review and synthesis of qualitative research. Ann Fam Med 2:583–594
Watson E, Austoker J, Lucassen A (2001) A study of GP referrals to a family cancer clinic for breast/ovarian cancer. Fam Pract 18:131–134
Watson E, Clements A, Lucassen A, Yudkin P, Mackay J, Austoker J (2002) Education improves general practitioner (GP) management of familial breast/ovarian cancer: findings from a cluster randomised controlled trial. J Med Genet 39:779–781
White DB, Bonham VL, Jenkins J, Stevens N, McBride CM (2008) Too many referrals of low-risk women for BRCA1/2 genetic services by family physicians. Cancer Epidemiol Biomarkers Prev 17:2980–2986
Williams S, Botterill A (2006) Profiling Areas using the Output Area Classification, Regional Trends. National Statistics
Wilson BJ, Torrance N, Mollison J, Wordsworth S, Gray JR, Haites NE, Grant A, Campbell MK, Miedyzbrodzka Z, Clarke A, Watson MS, Douglas A (2005) Improving the referral process for familial breast cancer genetic counselling: findings of three randomised controlled trials of two interventions. Health Technol Assess 9:1–126
WMRGU (2004) Referral guidelines West Midlands Regional Genetics Unit (WMRGU). http://www.bwhct.nhs.uk/genetics-index/genetics-wmfacs-service/genetics-wmfacs-service-guidelines.htm
Wonderling D, Hopwood P, Cull A, Douglas F, Watson M, Burn J, McPherson K (2001) A descriptive study of UK cancer genetics services: an emerging clinical response to the new genetics. Br J Cancer 85:166–170
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table I
Breast cancer risk, breast cancer status and age of participants versus decliners (DOC 23.5 kb)
Rights and permissions
About this article
Cite this article
Albada, A., Werrett, J., Van Dulmen, S. et al. Breast cancer genetic counselling referrals: how comparable are the findings between the UK and the Netherlands?. J Community Genet 2, 233–247 (2011). https://doi.org/10.1007/s12687-011-0061-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12687-011-0061-1