Abstract
The strong biological activities of nanoparticles are an essential factor because the resistance shown by bacterial species to chemical biocide is one of the major problems. In the current study, it was hypothesized that the biological waste might have biological power to reduce the silver nitrate into silver nanoparticles (AgNPs). Therefore, the biosynthesis of AgNPs using biological waste residue and their antibacterial influence were studied. The X-ray diffraction, Fourier transform infrared spectroscopy, scanning electron microscopy, and energy-dispersive X-ray techniques were used to analyze the fabricated AgNPs. In major findings, the SEM results revealed the spherical and small-sized AgNPs. The biological waste residue synthesized nanoparticles revealed that the highest inhibition zone was 25.74 ± 0.20 mm against Sphingomonas sp., while the smallest zone of inhibition was observed 9.76 ± 0.37 mm against Massilia sp. The best results were obtained against gram-positive and gram-negative bacterial isolates. Therefore, it is suggested to use the biological waste instead of other biological sources because of less toxicity, cost-effective, and easy availability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Green nanotechnology is the present most fast-tracking field of science that offering the probability of materializing low dimension particles with no or less environmental toxicity [1]. The plants extract, microorganisms, and other biological waste can synthesize nanoparticles (NPs) in small size (1–100 nm) [2, 3]. Especially, the biological waste (fruit peel) and plant extract are more convenient than microorganisms because the presence of phytochemicals in plants and biological waste make the NPs reduce and stable [4]. There are certain drawbacks in nanoparticles fabrication using microorganisms, for instance, less abundant growth, pathogenicity, and preservation of aseptic condition [5]. Therefore, plant leaves extract can promote the effective synthesis of NPs [6]. NPs can be synthesized by physical, chemical, and hybrid systems. However, the attention of researchers has been diverted to the biological synthesis of NPs due to their eco-friendly nature, rapid synthesis, and lower cost [7].
Silver (Ag) is a highly thermal and electrical conductor with a shiny and soft physical shape. It is being used in numerous forms like coins, sutures, vessels, foils, and colloids as a solution [2]. According to the literature study, earlier in 300 BC, the Ag was used as a therapeutic and medicinal agent that is cited in the old medicinal book “Charak Samhita” [8]. Ag was mainly used as an antimicrobial agent until the discovery of antibiotics, and it was being used as a prominent tonic agent for many infectious diseases [9]. Hence, a similar role is now playing by AgNPs instead of Ag [10].
Among all kinds of nanoparticles, AgNPs have gained the significant attention of researchers due to their wide-ranging applications [11]. The AgNPs have numerous compensations over silver salts/ions despite the fact that released Ag ions are the main agent of their biocidal activities. The AgNPs have several advantages over silver ions/salts, despite released silver ions being the primary agents of their bactericidal properties. The surfaces of AgNPs act as reservoirs for the release of silver ions at a limited rate; therefore, silver ions can be provided at a desirable rate without instant overdose [12]. Presently, the AgNPs are being used in electronic, medical, and optical applications due to their large surface area to volume ratio [13]. The AgNPs are widely applicable in diverse fields, i.e., sensing application [14], biomedical applications [15], biosensor application [16], as an alternative anticancer agent [17], and application of AgNPs in the conservation of cultural heritage [18]. Therefore, AgNPs have gained the prominent attention of researchers and scientists in synthetic biology and applied science.
Plants and microorganisms have been used for the fabrication of AgNPs [19]. Due to the limitations, for instance, plants are seasonal while microorganisms may cause contamination. Waste fruit peels are safe and non-toxic. Therefore, the present study was aimed to synthesize AgNPs using biological waste and alter the waste into beneficial products. In the current study, the antibacterial influence of biological waste synthesized AgNPs was studied. Even the concentrations of AgNPs were determined, and that was most effective in controlling the growth of bacterial isolates.
2 Materials and Methods
The present study was carried out at MOE Key Laboratory of Cell Activities and Stress Adaptations, School of Life Sciences, Lanzhou University, Lanzhou, China. The materials used in the current experiment were of analytical grade.
2.1 Biological Waste Collection
The biological waste and byproducts such as peels of different fruits were collected from the fruit market near Lanzhou University, Lanzhou, Gansu, China.
2.2 Biological Waste Extract Preparation
The collected biological waste and byproducts were washed with deionized water and dried in a convection oven at 40 °C for 48 h. The dried waste was then powdered using a grinder. Ten grams of powder residue was added to 100 mL of deionized water and heated at 60 °C for 15 minutes. The solution was cooled at room temperature, then filtered, and stored for further use.
2.3 Silver Nitrate Solution
99.99% pure silver nitrate (AgNO3) in solution form was purchased from Sigma Aldrich Beijing, China. The silver nitrate was dissolved in double-distilled water (ddH2O). The ratio between the AgNO3 solution and ddH2O was maintained at 1:9 (10 ml AgNO3 in 90 ml ddH2O).
2.4 Synthesis of Silver Nanoparticles
For the biological synthesis of AgNPs, 20 mL of AgNO3 was added to 80 mL of deionized water. The reaction mixture was placed in a shaker incubator for 24 h at 37 °C. The color change of the mixture solution was monitored from time to time.
The prepared biological AgNPs were centrifuged at 12,000 rpm for 20 minutes. The biological waste extract was discarded, while the synthesized AgNPs were attached at the bottom. Further, the AgNPs were kept in a microwave oven at 37 °C for drying. After drying, the powder AgNPs were obtained. The obtained AgNPs were stored for further characterization to confirm their shape, size, and dispersity.
2.5 Characterizations of Waste Induced AgNPs
The biologically synthesized AgNPs were characterized for confirmation of their shape and size. To examine the development of B-AgNPs and exploited their optical properties, UV-Vis spectrometry was used. Plasmon bands and optical density (OD) were recorded after the synthesis of AgNPs. The phase purity of biological waste synthesized AgNPs was analyzed by X-ray diffraction (XRD) operated at 30 kV and 15 mA. Fourier transform infrared spectroscopy (FTIR) is a modern technique that is used to obtain a spectrum of absorption. The complete dried powder of AgNPs was placed onto the sample holder, and FTIR spectra were recorded. The recorded raw data was further drawn by GraphPad Prism 7 Mac version. The surface size and morphology of biological waste synthesized AgNPs were studied using a scanning electron microscopy (SEM) instrument. The prepared AgNPs were properly mixed in distilled water (dH2O) were sonicated by ultrasonication. The prepared sample was loaded into a specimen holder. The micrographs of the samples were taken. The size of the synthesized B-AgNPs was measured by ImageJ software. The complete elemental analysis was performed by energy-dispersive X-ray (EDX; EDAX X1 Analyzer). The powder sample of AgNPs was compressed before analysis with EDX.
2.6 Antibacterial Activity
All apparatuses and culture media plates were sterilized by autoclaving at 115 °C and 15 psi for 20 min. The biological activity of AgNPs was investigated against several bacterial species, e.g., Microbacterium sp., Bacillus sp., Staphylococcus sp., Sphingomonas sp., Sphingosinicella sp., Arthrobactor sp., and Massilia sp. that are environmental bacterial species. The biological activity was assessed by the well diffusion method. This was done by inoculating the pure culture of each bacterial species in 5 ml of nutrient broth and incubated at 37 °C for 24 h in the shaker. The zone of inhibition method was used to check the antibacterial activity. 20 ml of nutrient agar was poured into a petri dish and sterilized for 15 min, and the media became solid. 0.1 mL of each bacterial solution was poured. Afterward, four wells were made for the application of B-AgNPs solution in triplicates. The plates were kept at the shaker incubator for 48 h. A zone of inhibition was measured.
For antibacterial activity, the AgNP solution was made by adding 0.03 mg AgNPs in 2 mL deionized water. The ultrasonicator was used to dissolve AgNPs powder in dH2O. During the sonication, the temperature was maintained between 40 and 45 °C with 100 Hertz frequency for 45 minutes.
2.7 Statistical Analysis
All measures were conducted in triplicate, so the experimental data were analyzed by Microsoft Excel 2016 (Microsoft Inc. USA) for standard error.
3 Results and Discussion
In the current study, AgNPs were synthesized from the AgNO3 solution and 100 mL of biological waste residue. After 24 h of the treatment of AgNO3 with biological waste residue, the color was changed into dark brown.
3.1 Characterizations of Waste Induced AgNPs
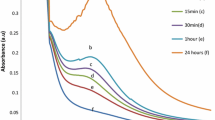
UV-vis is one of the most exciting properties of AgNPs. This technique was used to trail the synthesis of AgNPs by the reduction of AgNO3 solution with biological waste residue. The maximum absorbance of B-AgNPs was at 420 nm, which confirms the synthesis of B-AgNPs, as shown in (Fig. 1). The highest peak in our study was noted at 420 nm, but it can be increased with the passage of time and concentrations. Another conducted research has revealed that different concentrations influence on wavelength absorbance of UV-Vis spectrometry, i.e., 350-nm, 400-nm, 450-nm, 500-nm, and 550-nm wavelength absorbance was observed using different concentrations of AgNPs solutions [20]. According to Mie’s theory, the absorbance increases by increasing time and particle shape [21].
XRD is an analytical technique that is used to confirm the crystalline structure of any nanoparticles. Therefore, the XRD was carried out for the biological waste residue-induced AgNPs for the confirmation of the crystalline structure. Mostly, the XRD pattern show sharp peaks at 2θ = 38/1, 3/44, 4/64, and 4/77, which can be allocated to the (111), (200), (220), and (311) reflection of the face-centered cubic [22]. In the current study, the peaks at 111, 200, 220, and 311 are allocated to the structure of AgNPs. The lower peaks in the XRD of the present study might be due to the residue present in the biological waste. Similar results have been obtained by [23], which supports our XRD results. Our study was also supported by [24], as confirmed by JCPDS File no. 040784, which corresponds to 111, 200, and 220, as shown in (Fig. 2).
The FTIR spectra of synthesized AgNPs by biological waste residue are shown in (Fig. 3). We have summarized Table 1 from the higher to the lower frequency range. The spectrum at 3877 cm−1 indicates O=H stretching. A similar group and compound class have also been obtained by 3688 cm−1. Absorption 3283 and 2949 cm−1 appeared strong and medium sharp, indicating C-H bending and stretching, which are alkyne and alkene, respectively. The overall frequency range of the FTIR spectrum is given in Table 1.
The SEM analysis technique was carried out to know the surface topology and the known size of AgNPs. The spherical shape of AgNPs is the important factor that plays a crucial role in the synthetic pathways for the absorption of AgNPs that mainly cause microbial cell death. In the current study, the SEM results revealed the polydisperse, small size (50–90 nm), and spherical AgNPs, as given in (Fig. 4). Mostly, the plant species are used to synthesize the spherical shape AgNPs, e.g., the results of Dipankar and Murugan [25] supported our study. They have used plant species to fabricate AgNPs. Their SEM results have confirmed 44–64 nm size with polydisperse form. The exact SEM results for the AgNPs were obtained by Feroze et al. in 2019 [26]. Their results have confirmed the spherical shape and average size as 60–80 nm. Herein the current study, the AgNPs are mostly aggregated, and some of them are scattered and dispersed.
The EDX spectrum of biological waste residue fabricated AgNPs is shown in (Fig. 5), which clearly unveils the absence of certain elemental peaks and shows the highest presence of AgNPs. The sharp peak in the EDX spectrum has confirmed the reduction of AgNO3 into AgNPs. The present study is confirmed and supported by Dipankar and Murugan [25]. So, in this study, the biological waste residue synthesized AgNPs revealed a separate signal of the metallic silver region at 3 keV and representing the formation and abundance of AgNPs using biological waste residue.
3.2 Antibacterial Activity of AgNPs
The antibacterial influence of the present synthesized AgNPs was tested laboratory-scale against eight kinds of bacterial species isolated from cultural heritage. The antibacterial activity was checked by well diffusion method. On each plate, the activity was performed in triplicate for standard error.
3.3 Minimum Inhibitory Concentration
The well diffusion method was used to investigate the antibacterial influence of AgNPs. At the end of the activity, the zone of inhibition was measured. The largest zone of inhibition means the highest antibacterial activity of B-AgNPs. The antibacterial activity was noted in millimeters (mm). The highest average zone of inhibition in triplicate was noted against Sphingomonas sp. is 25.74 ± 0.20 mm, followed by Staphylococcus sp. is 24.44 ± 0.28 mm; Bacillus sp. is 19.93 ± 0.89 mm, Arthrobactor sp. is 18.36 ± 0.25 mm, Microbacterium sp. is 16.34 ± 0.29 mm, Sphingosinicella sp. is 11.89 ± 0.32 mm, and the smallest zone was noted against Massilia sp. 9.76 ± 0.37 mm, given in Table 2. The current study revealed that the antibacterial activity of AgNPs was more effective against certain types of environmental bacterial isolates.
The strong biological activities of any nanomaterials are a very important factor because the resistance shown by bacterial species to chemical biocide is one of the main problems in applied science. In this study, the antibacterial activity of biological waste residue synthesized AgNPs was checked against bacterial isolates. The microbial circle formed around the discs designated the antimicrobial activity of AgNPs. The largest zone that appeared in the activity was the strongest antibacterial activity. In the current study, the highest average zone of inhibition was noted against Sphingomonas sp. is 25.74 ± 0.20 mm, while the smallest zone was noted against Massilia sp. 9.76 ± 0.37 mm. In the current study, the antibacterial activity method and obtained results are supported by [27], as they conducted their study using plant species to synthesize AgNPs and were applied against bacterial species for antibacterial activity. A recent study has revealed that the AgNPs are very good reductive agents against biofilm. The highest biofilm reduction was 86.36% which is a great achievement in applied sciences [28]. Another conducted study results revealed that 25% of bacterial species isolated from marine were reduced using plant synthesized AgNPs [29]. Feroze et al. (2019) have conducted their study for the synthesis of AgNPs using Penicillium oxalicum and applied it against certain bacterial isolates [26]. Their results have revealed the highest zone of inhibition (17.5 ± 0.5 mm). In comparison, our study has revealed the highest zone of inhibition (25.74 ± 0.20), which is far larger than some other conducted studies. Therefore, it is concluded that the biological synthesis of AgNPs should be continued.
4 Conclusion
Environment-friendly synthesis of AgNPs has an extensive range of applications in diverse domains of science and technology, among which the application and influence against bacterial isolates have been reported. The analytical techniques have revealed the polydisperse and the small size of AgNPs. The promising results have been obtained due to their antibacterial influence. The fabrication method of AgNPs using biological waste residue might be superior to plants and microbial synthesis of AgNPs because plants are seasonal while microbes may cause contamination or skin infection during the experiment. Much of the literature has described the drawbacks of plant and microbial synthesis of AgNPs. Therefore, priority should be given to any other biological medium which is rich in proteins and other biological molecules.
References
Maheshwaran, G., Nivedhitha Bharathi, A., Malai Selvi, M., Krishna Kumar, M., Mohan Kumar, R., & Sudhahar, S. (2020). Green synthesis of Silver oxide nanoparticles using Zephyranthes rosea flower extract and evaluation of biological activities. Journal of Environmental Chemical Engineering, 8(5), 104137. https://doi.org/10.1016/j.jece.2020.104137.
Ali, I., Qiang, T. Y., Ilahi, N., Adnan, M., & Sajjad, W. (2018). Green synthesis of silver nanoparticles by using bacterial extract and its antimicrobial activity against pathogens. International Journal Bioscience, 13(5), 1–15. https://doi.org/10.12692/ijb/13.5.1-15.
Korkmaz, N. (2020). Bioreduction: the biological activity, characterization, and synthesis of silver. Turkish Journal of Chemistry, 44(2), 325–334. https://doi.org/10.3906/kim-1910-8.
Singh, J., Dutta, T., Kim, K.-H., Rawat, M., Samddar, P., & Kumar, P. (2018). ‘Green’ synthesis of metals and their oxide nanoparticles: applications for environmental remediation. Journal of Nanobiotechnology, 16(1), 84. https://doi.org/10.1186/s12951-018-0408-4.
Korkmaz, N., Ceylan, Y., Taslimi, P., Karadağ, A., Bülbül, A. S., & Şen, F. (2020). Biogenic nano silver: Synthesis, characterization, antibacterial, antibiofilms, and enzymatic activity. Advanced Powder Technology, 31(7), 2942–2950. https://doi.org/10.1016/j.apt.2020.05.020.
Sidorova, D. E., Lipasova, V. A., Nadtochenko, V. A., Baranchikov, A. E., Astafiev, A. A., Svergunenko, S. L., Koksharova, O. A., Pliuta, V. A., Popova, A. A., Gulin, A. A., & Khmel, I. A. (2018). Synthesis of silver nanoparticles with the use of herbaceous plant extracts and effect of nanoparticles on bacteria. Applied Biochemistry and Microbiology, 54(8), 816–823. https://doi.org/10.1134/S0003683818080069.
Mathivanan, K., Selva, R., Chandirika, J. U., Govindarajan, R. K., Srinivasan, R., Annadurai, G., & Duc, P. A. (2019). Biologically synthesized silver nanoparticles against pathogenic bacteria: Synthesis, calcination and characterization. Biocatalysis and Agricultural Biotechnology, 22, 101373. https://doi.org/10.1016/j.bcab.2019.101373.
Galib, B. M., Mashru, M., Jagtap, C., Patgiri, B. J., & Prajapati, P. K. (2011). Therapeutic potentials of metals in ancient India: A review through Charaka Samhita. J-AIM, 2(2), 55–63. https://doi.org/10.4103/0975-9476.82523.
Mohr, K. I. (2016). History of antibiotics research. In How to Overcome the Antibiotic Crisis (pp. 237–272). Switzerland: Springer.
Zazo, H., Colino, C. I., & Lanao, J. M. (2016). Current applications of nanoparticles in infectious diseases. Journal of Controlled Release, 224, 86–102. https://doi.org/10.1016/j.jconrel.2016.01.008.
Rashmi, B. N., Harlapur, S. F., Avinash, B., Ravikumar, C. R., Nagaswarupa, H. P., Anil Kumar, M. R., Gurushantha, K., & Santosh, M. S. (2020). Facile green synthesis of silver oxide nanoparticles and their electrochemical, photocatalytic and biological studies. Inorganic Chemistry Communications, 111, 107580. https://doi.org/10.1016/j.inoche.2019.107580.
Lim, J. K., Liu, T., Jeong, J., Shin, H., Jang, H. J., Cho, S.-P., & Park, J. S. (2020). In situ syntheses of silver nanoparticles inside silver citrate nanorods via catalytic nanoconfinement effect. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 605, 125343. https://doi.org/10.1016/j.colsurfa.2020.125343.
Roy, A., Bulut, O., Some, S., Mandal, A. K., & Yilmaz, M. D. (2019). Green synthesis of silver nanoparticles: Biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Advances, 9(5), 2673–2702. https://doi.org/10.1039/C8RA08982E.
Ali, I., Imkan, I. F., Ahmad, F., Nisar, J., Shah, M. R., Ali, S., Ullah, S., Althagafi, I. I., & Ateeq, M. (2021). Sensing applications of triazole conjugated silver nanoparticles. Journal of Molecular Structure, 1226, 129306. https://doi.org/10.1016/j.molstruc.2020.129306.
Al-Ansari, M. M., Dhasarathan, P., Ranjitsingh, A. J. A., & Al-Humaid, L. A. (2020). Ganoderma lucidum inspired silver nanoparticles and its biomedical applications with special reference to drug resistant Escherichia coli isolates from CAUTI. Saudi Journal Biology Science, 27(11), 2993–3002. https://doi.org/10.1016/j.sjbs.2020.09.008.
El Barghouti, M., Akjouj, A., & Mir, A. (2020). Design of silver nanoparticles with graphene coatings layers used for LSPR biosensor applications. Vacuum, 180, 109497. https://doi.org/10.1016/j.vacuum.2020.109497.
Nesrin, K., Yusuf, C., Ahmet, K., Ali, S. B., Muhammad, N. A., Suna, S., & Fatih, Ş. (2020). Biogenic silver nanoparticles synthesized from Rhododendron ponticum and their antibacterial, antibiofilm and cytotoxic activities. Journal of Pharmaceutical and Biomedical Analysis, 179, 112993. https://doi.org/10.1016/j.jpba.2019.112993.
Kakakhel, M. A., Wu, F., Gu, J.-D., Feng, H., Shah, K., & Wang, W. (2019). Controlling biodeterioration of cultural heritage objects with biocides: A review. International Biodeterioration & Biodegradation, 143, 104721. https://doi.org/10.1016/j.ibiod.2019.104721.
Zamarchi, F., & Vieira, I. C. (2021). Determination of paracetamol using a sensor based on green synthesis of silver nanoparticles in plant extract. Journal of Pharmaceutical and Biomedical Analysis, 196, 113912. https://doi.org/10.1016/j.jpba.2021.113912.
Roy, P., Das, B., Mohanty, A., & Mohapatra, S. (2017). Green synthesis of silver nanoparticles using Azadirachta indica leaf extract and its antimicrobial study. Applied Nanoscience, 7(8), 843–850. https://doi.org/10.1007/s13204-017-0621-8.
Pirtarighat, S., Ghannadnia, M., & Baghshahi, S. (2019). Green synthesis of silver nanoparticles using the plant extract of Salvia spinosa grown in vitro and their antibacterial activity assessment. Journal Nanostructure Chemistry, 9(1), 1–9. https://doi.org/10.1007/s40097-018-0291-4.
Jian, W., Ma, Y., Wu, H., Zhu, X., Wang, J., Xiong, H., Lin, L., & Wu, L. (2019). Fabrication of highly stable silver nanoparticles using polysaccharide-protein complexes from abalone viscera and antibacterial activity evaluation. International Journal of Biological Macromolecules, 128, 839–847. https://doi.org/10.1016/j.ijbiomac.2019.01.197.
Khan, T., Yasmin, A., & Townley, H. E. (2020). An evaluation of the activity of biologically synthesized silver nanoparticles against bacteria, fungi and mammalian cell lines. Colloids and Surfaces, B: Biointerfaces, 194, 111156. https://doi.org/10.1016/j.colsurfb.2020.111156.
Shah, M., Nawaz, S., Jan, H., Uddin, N., Ali, A., Anjum, S., Giglioli-Guivarc'h, N., Hano, C., & Abbasi, B. H. (2020). Synthesis of bio-mediated silver nanoparticles from Silybum marianum and their biological and clinical activities. Materials Science and Engineering: C, 112, 110889. https://doi.org/10.1016/j.msec.2020.110889.
Dipankar, C., & Murugan, S. (2012). The green synthesis, characterization and evaluation of the biological activities of silver nanoparticles synthesized from Iresine herbstii leaf aqueous extracts. Colloids and Surfaces, B: Biointerfaces, 98, 112–119. https://doi.org/10.1016/j.colsurfb.2012.04.006.
Feroze, N., Arshad, B., Younas, M., Afridi, M. I., Saqib, S., & Ayaz, A. (2020). Fungal mediated synthesis of silver nanoparticles and evaluation of antibacterial activity. Microscopy Research and Technique, 83(1), 72–80. https://doi.org/10.1002/jemt.23390.
Mani, M., Chang, J. H., Dhanesh Gandhi, A., Kayal Vizhi, D., Pavithra, S., Mohanraj, K., Mohanbabu, B., Babu, B., Balachandran, S., & Kumaresan, S. (2020). Environmental and biomedical applications of AgNPs synthesized using the aqueous extract of Solanum surattense leaf. Inorganic Chemistry Communications, 121, 108228. https://doi.org/10.1016/j.inoche.2020.108228.
Korkmaz, N., Ceylan, Y., Hamid, A., Karadağ, A., Bülbül, A. S., Aftab, M. N., Çevik, Ö., & Şen, F. (2020). Biogenic silver nanoparticles synthesized via Mimusops elengi fruit extract, a study on antibiofilm, antibacterial, and anticancer activities. Journal of Drug Delivery Science and Technology, 59, 101864. https://doi.org/10.1016/j.jddst.2020.101864.
Lakhan, M. N., Chen, R., Shar, A. H., Chand, K., Shah, A. H., Ahmed, M., Ali, I., Ahmed, R., Liu, J., Takahashi, K., & Wang, J. (2020). Eco-friendly green synthesis of clove buds extract functionalized silver nanoparticles and evaluation of antibacterial and antidiatom activity. Journal of Microbiological Methods, 173, 105934. https://doi.org/10.1016/j.mimet.2020.105934.
Funding
Mian Adnan Kakakhel is a recipient of Doctoral studies award at Lanzhou University by the Chinese Government Scholarship Council. Zaheer Ud Din was financially supported by the Young Doctors Cooperation Fund, Qilu University of Technology (Shandong Academy of Sciences) (Grant No. 2019BSHZ006).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Research Involving Humans and Animals Statement
None.
Informed Consent
None.
Conflict of Interest
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kakakhel, M.A., Saif, I., Ullah, N. et al. Waste Fruit Peel Mediated Synthesis of Silver Nanoparticles and Its Antibacterial Activity. BioNanoSci. 11, 469–475 (2021). https://doi.org/10.1007/s12668-021-00861-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-021-00861-2