Abstract
In recent years, the recovery of the valuable metals from iron-bearing solid waste from steel plant has been one of the most intensive research areas. Dumping of electric arc furnace dust is an environmental concern, and recovery of valuable metals like iron, zinc, lead from EAFD and safe disposal of residue has got enough attention. Evolution of improved and new processes has motivated industries to engage actively and targeting the new and efficient methods to recycle EAFD. The presence of valuable elements and increasing cost of waste incorporation are the motivational factors for the recycling of EAFD. In this article, the technologies that are in use to process EAFD have been discussed, and their advantages and disadvantages are also highlighted.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Electric arc furnaces (EAF) are widely used for the production of carbon and alloy steels, which is an alternative to dominant basic oxygen furnace (BOF) production route. Around 30% of crude steel is produced through EAF by melting scrap [1]. Dust formation during EAF operation has been reported by various authors [2, 3]. During the EAF operation, liquid turbulence and bursting of CO bubbles at the slag–gas interface has been identified as the primary reason for the emission of EAF dust, henceforth will be termed as EAFD [4]. Besides, volatile elements join the dust in the form of their oxides particulates. The produced fine dust particulates are carried out with the exhaust gases and are collected by the de-dusting units.
The composition of the EAFD depends on the type of scrap and other process raw materials used. Due to the presence of different scrap types with varying alloying elements, the EAFD composition becomes quite complex and extremely variant. The major elements in the EAFD are iron, zinc and calcium and other less prevalent elements are potassium, manganese, chlorine, cadmium, silicon, lead. Based on the zinc content, the EAF dust has been divided into three categories: low Zn dust (< 4 wt%), medium Zn dust (4%-20 wt%) and high Zn dust (> 20 wt%) [5]. The other major element in the EAFD is calcium [6]. Its content remains constant as it enters into the furnace as a fluxing agent, and in practice, it can be precisely controlled [7]. Due to the high operating temperature of EAF, the minor elements having low boiling points enter into the dust. As these minor elements are not beneficial and may interfere during recovery processing, and therefore, certain pre-treatments are required to enrich the content of beneficial elements [8, 9].
According to Nyirenda, the exact composition of the EAFD is extremely variable from time to time and charge to charge as it depends on the type of scrap being charged, the electric power supplied to the EAF, nature of steel being produced and addition of alloying elements [10]. The zinc content from the EAFD from around the world has a wide range of variation, reflecting the composition of scrap being used in the EAF steel production [11]. And also wide range of alloying elements (Ni, Cr, Mn and Cu) is present in EAFD, which reflects the different kinds of alloy steel scrap being used. Christopher A. Pickles cites the presence of various elements in scrap, and the dust produced is mineralogically complex [12]. The major (Fe, Zn, Ca) and minor (Cl, Pb, Si, Al, Mn, Cr, Mg, Ni, Cu & Cd) elements are found mostly in oxide form but they can also be present as fluorites, sulphides, sulphates and chlorides. Approximately 15–25 kg of fine EAF dust is generated per tonne of steel produced in electric arc furnace [13]. EAFD is considered as the hazardous waste due to the significant presence of leachable compounds of heavy metals such as Pb and Cd. According to Environmental Protection Agency (EPA), EAFD is listed as the hazardous waste, and it needs to be carefully incorporated [14].
Nowadays, there has been a significant rise in the levels of zinc and chlorine in the EAFD coming from scrap due to increase in the usage of galvanized steel, polymer coatings and paints. Paints and polymers are the primary sources of chlorine, which when exposed to high temperatures, disintegrate and vapourizes into gases (Cl2 and HCl) and volatile organic compounds. A minor amount of chlorine can also form furans and dioxins [15]. Due to this reason, chorine is considered as one of the most abundant elements in EAFD. Around 5 wt% of chlorine in the EAFD has been reported [16]. The chlorine behaviour is extremely important when it comes to recycling, as it interferes with processing in both hydrometallurgical and pyro-metallurgical processes as well as in waste incorporation process [17].
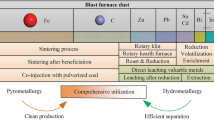
Recycling reduces the consumption of the energy in industrial production. As a result, the greenhouse emissions and particulates emitted from the industrial plants is lessened and the fuels that emit harmful gases through extraction is also minimized. However, recycling of the dust causes the volatile compounds to recirculate as level of impurities in the melt increases, and also, the operating costs increase. So, the dust requires appropriate treatment to separate the impurities from iron. Currently, there are mainly three main methods for processing of EAFD, namely hydrometallurgical, pyro-metallurgical and hybrid process.
Both hydro-metallurgical and pyro-metallurgical routes have been tried to recover the valuable non-ferrous metal value from EAFD. Hydro-metallurgical route mainly involves caustic processing, but could not be commercialized due to difficulty in solid–liquid separation (due to mixing of powdery fines to the leached solution), more rigorous thermodynamic condition to leach out zinc-ferrite, leaving the toxic waste solution to environment, and finally lower kinetics, productivity and higher specific energy consumption.
Several pyro-metallurgical processes have either been commercialized, or tested in pilot scale. The product obtained from the pyro-metallurgical process is usually impure ZnO, which has minimal commercial value unless it is purified further by post-processing using hydrometallurgical route to increase its commercial value [18]. The form of the ZnO obtained in a process decides its commercial value and acts as indicator of the efficiency of the process. So hybrid processes (pyro-metallurgical process in conjunction with post-hydro-metallurgical processing) have also been recognized.
Most of the pyro-metallurgical processes are based on carbothermic reduction. During pyro-metallurgical processing of EAFD, the exhaust hot gas contains vapours of metal chlorides. When this hot gas passes through dust collecting and gas cooling equipment, vapours of metal chlorides easily condense and adhere to the walls of equipment and also contaminate the non-ferrous metal. This has also been the major problem for many EAF dust processing plants.
Following section describes some of the pyro-metallurgical processes with their advantages and disadvantages highlighted.
2 Various Pyro-Metallurgical Process of EAF Dust Processing
2.1 Waelz Kiln Process
The Waelz kiln is the most popular process to treat high zinc EAFD and commercially exploited in Europe, America and Japan. It is used to recover zinc and other low boiling point elements from EAFD, metallurgical feed stocks and other recycled materials using a rotary kiln (Waelz kiln) [19]. Around 75% of the EAF dust is processed through coal fired Waelz kiln.
Figure 1 shows a simple flow chart of Waelz kiln process. A Waelz plant normally consists of raw material preparation, kiln line and flue gas treatment units. In the first stage, the raw materials (EAF dust, lime and coke breeze) are mixed and consolidated into composite pellets for feeding to the Waelz kiln. Three different zones can be identified in a Waelz rotary kiln. In first zone, the moist pellets get dried and preheated by hot kiln gas at 650 °C flowing in a counter-current direction. In the second zone, temperature reaches to 1100 °C where chlorides start to volatize and Zn, Pb and iron oxides start to reduce. In the final zone, temperature rises to 1200 °C, and reduction comes to completion. The reduced volatile elements like zinc and lead get evaporated and later oxidized in oxidizing furnace atmosphere to metallic oxides (PbO, ZnO) and collected in bag filter. In the kiln line, the feed is transformed into two products: Waelz zinc oxide (WZO) that accumulates the non-ferrous elements (55%–65% of Zn) of the feed, as mentioned above, and Waelz iron product (WIP) also known as Waelz Slag, which is rich in iron and contaminated with zinc and other slag constituents. WIP is a waste and find some applications in cement industry, back filling and road construction [20]. Depending upon the materials charged and operating conditions, two types of slags could be produced: silica-rich and lime-rich slags. Chlorine and alkalis volatilize with the heavy metals and are collected/separated from exhaust gas system [21]. Cooling of flue dust gas is done step by step to collect metal oxides and halides separately. WZO is contaminated with lead and should be post-processed to enrich zinc. It could be done by caustic digestion in hydrometallurgy route, or in imperial smelting process (ISP) to separate lead in lead flash condenser.
Flow chart of Waelz kiln process [19]
Advantages: (i) reliability and robustness of the process, (ii) easy installation, (iii) well-known metallurgical process, and (iv) wide range of feed materials (cheap coal).
Disadvantages: (i) accretion formation on the wall, (ii) complete loss of iron value in EAFD through WIP, and (iii) poor energy efficiency. Accretion formation on the wall of the kiln effectively reduces the movement of material through the kiln. Some zones of the kiln are easily prone to particle accumulation due to adverse thermal and flow conditions, leading to generation of low melting compounds like sulphides, sulphates, halides, resulting in a deposition of solid agglomeration along the periphery of the kiln, popularly known as rings. Porosity of kiln brick can reduce the ring formation but at the loss of heat. Ring formation can be controlled but cannot be avoided. Stable, strong and homogeneous pellet feed as well as stable suction of flue gases should be maintained for high efficient and stable operation of Waelz kiln. Instability will cause poor zinc recovery, accretion on the side wall, blockage in the flow of gases and charge, product of lower quality, high slag rate, while it recovers only one valuable product. High energy consumption is another major drawback, and its strict offsite requirements or post-processing of WZO also adds to the operating cost [22]. The process becomes only economic when its capacity is higher (> 50,000 tonnes/year) and zinc content in the EAFD is greater than 15%.
2.2 Rotary Hearth Furnace Process
EAF dust has been processed by Waelz kiln for many years, but this does not recover iron, so the residue from the Waelz kiln process is still a waste [23]. Moreover, the cost of landfilling in the developed countries is especially high. In this regard, RHF emerges as the technology to recover iron along with zinc and lead.
A rotary hearth furnace is a rotating torus ring furnace minimizing the layout space. Figure 2 shows the configuration of RHF process. RHF technology uses pulverized non-coking coal as reducing agent. The EAF dust is pelletized or briquetted with carbon reactant [24]. The pellets are fed into RHF, which consists of three different zones maintained at different temperatures by the overhead burners separated by layers/curtains of air: heating, reducing and discharging zones. The top of the furnace has oxidizing atmosphere; while atmosphere near the pellets is reducing due to the presence of carbon. The pellets are carried away by the moving floor to the preheating zone where temperature reaches up to 1000 °C. Subsequently, charge is moved into the reduction zone where temperature rises above 1100 °C, where reduction of zinc oxide and iron oxide takes place by carbon. The post-combustion of the carbon monoxide provides major thermal input to the process. The balance heat requirement is supplied by fuel gases. Combustion gas flow cross-currently to the floor motion. During the reaction, the metal oxides are reduced to their corresponding metallic forms. The oxidizing furnace atmosphere re-oxidizes zinc vapour to solid zinc oxide. Gases containing ZnO particles at 850–950 °C are cooled down rapidly by water quenching, and the zinc oxide is collected in bag filters. The fast cooling also ensures no formation of furans and dioxins. The resultant zinc oxide product quality range could be 70–90% of zinc. This zinc oxide can be directly used as feed for zinc smelters. The iron oxides are reduced to direct reduced iron under a strong reducing atmosphere, and the per cent of metallization goes up to 90% in 15–20 min of time [25].
Configuration of RHF process [20]
Advantages: (i) Reductant (carbon) and fuel (coal/coke/natural gas) for heating are different and could be controlled independently, (ii) since in the process, the material does not move physically, there are no chances of accretion formation, and therefore, comparatively low strength burden could be accepted and no flux is required. (iii) combustion system with regenerative burners can conveniently be accommodated in rotary hearth furnace to decrease the specific energy consumption. (iv) It recovers two products ZnO and DRI in one production cycle; in other words, production rate is higher compared to other processes where other secondary processes are involved.
Disadvantages: (i) Heat transfer limitation to the charge from top furnace atmosphere that restricts the number of pellet/briquette layers over the hearth thus limiting productivity. (ii) High capital cost per unit production due to low hearth area efficiency and maintenance of wall. (iii) This process is suitable to treat low zinc content dust (< 5 wt%) only [26,27,28].
2.3 PRIMUS Process
Paul Wurth developed PRIMUS process, which uses multiple hearth furnace for processing of EAFD, which contains more than 5 wt% Zn. When it is fed with EAF dust, it produces highly metalized sponge iron at comparatively lower temperature and along with ZnO product.
PRIMUS is of a multi-hearth design with cylindrical column consisting of number of superimposed compartments as shown in Fig. 3 [29]. The hearth floors are made of refractory bricks, which form self-supporting levels. These levels are opened with small holes to enable easy flow of charge from one compartment to another. Here, iron ore fines and coal fines are charged from the top. Coal fines are used both as a reductant as well as source of energy. Due to continuous and active stirring of charged materials, they pass each hearth with uniform heating avoiding any formation of agglomerates. The raw materials can be directly charged without any prior mixing due to active stirring. These raw materials undergo a sequence of steps with different temperatures; drying (150 °C), calcining (450 °C) and heating (1050 °C). The processing temperature can rise upto 1100 °C by combustion of coal and post-combustion of CO by supplying secondary air above the solid material. Inside the furnace, formation of two zones takes place with different reducing potentials, one is with higher reducing potential in the material area and another with higher oxidizing potential in the gas area. The volatilization of non-ferrous elements takes place in reducing atmosphere as they evaporate and leave as metallic fumes, whereas the re-oxidation of volatilized elements takes place in oxidizing atmosphere. ZnO and PbO get into the fume as dust particles and are separated in a bag filter. Separation of volatile elements from dust leads to the generation of high degree iron concentrate. PRIMUS process is mostly supported by the heat generated during the post-combustion of CO to sustain the reduction, and partial coal combustion is done to supplement the heat requirement. High degree of post-combustion and relatively low temperature (1100 °C) makes it a very energy-efficient process.
Flowchart of PRIMUS process [29]
Advantages: (i) The major advantage is energy efficiency due to comparatively low-temperature operation [30]. The intense mixing of the ore and coal fines through moving baffle avoids temperature stratification, which along with intimacy of iron and ore particles favours kinetics even at lower temperature, 1100 °C. Recovery of zinc in zinc oxide is as high as 95%, and also, DRI with 95% metallization is obtained. (ii) Another notable advantage of the primus process is that different steel waste residues, like EAFD, sludges, scales could be processed.
Disadvantage: The downside of this process is corrosion of furnace refractory due to intensive stirring and evaporation of alkali metals.
2.4 Pig Iron and Zinc Oxide (PIZO) Process
PIZO process uses electrical energy to produce three products in a single reactor, which are crude zinc oxide, hot metal of BF quality and slag.
The PIZO process uses a continuous channel induction furnace as the primary processing unit for the recycling of steel-making dust [31]. The channel induction furnace contains a bath of molten iron to serve as a heat sink to ensure rapid heating of the feed that contains a mixture of EAF dust and reductants in the form of briquettes, under elevated temperatures (1300–1500 °C). PIZO process is fed continuously. Formation of crude zinc oxides contaminated with oxides of cadmium and lead takes place by reduction of their oxides and vapourization at operating furnace temperature and subsequent re-oxidation through air dilution during gas cleaning and cooling outside the furnace. Such contaminated ZnO acts a crude source for zinc for pure zinc production. The liquid products are the liquid iron and slag, which are collected from bottom of the furnace. The iron produced by the process goes through the molten slag to combine with the molten iron bath in the furnace. The produced iron product is similar to pig iron which can directly be used in BOF or EAF steel making. The end product of PIZO process is a slag which contains refractory materials like silica, magnesium and alumina [31]. The produced slag can be used for road construction or back filling. The continuous channel induction furnace serves as an ideal type for continuous feeding of materials to produce iron, zinc and slag. The products have to be removed on a continuous or semi-continuous basis.
Advantages: (i) It produces three distinct saleable products—iron, zinc and slag—and no waste. The slag does not contain any leachable heavy metal like lead and cadmium (< 0.1 ppm) and zinc (max 1 ppm), which makes it saleable.
Disadvantages: Its present capacity is around 50,000 kilo-tons/yr. Upscaling has still not been realized.
2.5 Submerged Plasma Process
The process uses plasma source for heating/reducing/melting and volatilization of elements. A plasma generator is a device which converts electrical energy into heat energy carried by an ionized gas; in other words, inside the plasma torches, the cold air is transformed into high enthalpy plasma gas.
The plasma gas is mixed with natural gas (as foaming gas) and pumped into the slag bath [32] as shown in Fig. 4. Due to the high enthalpy of the plasma gas, less volume of the gas is needed to generate the same amount of heat through conventional burners. The pelletized EAF dust, mixed with reductant and other additives, is directly dumped into the furnace (slag bath) continuously. The solid feed dissolves into the reducing slag bath, resulting fuming of zinc and other volatile elements. Above the bath, the zinc gets re-oxidized and collected in a conventional bag house system. The collected dust is sent into the electrostatic separator to remove the solid ZnO particles Fig. (4). The products of the process are slag phase, metal phase, ZnO powder and off-gas [33].
Schematic of SP process [34]
Advantages: (i) Since the reductant and source of energy are independent, the process can generate high temperature even at low oxygen potential/level. (ii) Plasma torch also generates very strong agitation in the bath that results in good mixing. Injection of high enthalpy gas under high pressure conditions also creates a vigorously foaming slag. The presence of high temperature, high bath agitation, slag foaming enhances the reaction kinetics, which attain equilibrium in a short span of time.
Disadvantages: (i) Since the reactor is water cooled, it creates a lining of frozen melt inside the reactor called the freeze lining [34], which protects the refractory lining from corrosion and abrupts mechanical shocks. However, freeze linings unless optimized, there is a trade-off between productivity and enthalpy loss.
2.6 OXYCUP Process
The central part of the “OXYCUP” plant is a cupola shaft furnace which combines the function of melting in a cupola furnace as shown in fig. 5 with function of reduction in the blast furnace. In contrast to BF, such furnace can process high zinc content in the iron-bearing solid waste.
Flowchart of OXYCUP process [37]
Self-reducing carbon bricks, made by pressing EAFD, along with carbonaceous material and cement as binder, are charged along with coke and fluxes into the furnace [35]. The basicity (CaO/SiO2) of the composite brick is maintained greater than 1 to avoid formation of fayalite, which wet the brick pores and hinder the reduction process. Oxygen enriched preheated air blast (750 °C) is injected via tuyeres to rise flame temperature over 2200 °C. The direct reduction of iron oxide starts at 900 °C and is completed at 1400 °C producing liquid pig iron with 4 wt% carbon [30]. The top dust contains the components of zinc and other alkali materials that are volatilized during the reduction process and enriches in the off-gas. Subsequently, top dust is briquetted and sold as zinc product containing around 30% zinc. Molten metal and slag are continuously tapped at 1500 °C.
Advantages: (i) Overall, this process produces zero wastes as it produces pig iron of blast furnace quality, saleable slag without contamination with heavy metals, and gases rich in CO that can be used as a fuel. and (ii) Use of cheap raw material makes the process economic.
Disadvantages: Reducibility of self-reducing pellets is subjected to further research. High coal rate is another issue for high CO2 emission.
2.7 Coke-Packed Bed Process
Kawasaki steel corporation has developed a new smelting reduction furnace that consists of a shaft furnace with a coke-packed bed for processing of steel-making dust [36] as shown in Fig. 6. The shaft furnace is filled with low-grade coke. A shaft ensures a good exchange of heat between the ascending gas and descending coke. The temperature of the exhaust gas system would be relatively low because the temperature produced is transferred to the coke. In this process, tuyeres are used at two locations: through upper tuyeres, iron ore fines are injected, while lower tuyeres are used to inject preheated air blast to burn coke and produce heat. A fine mixture of EAF dust, coke and flux without agglomeration is injected through upper tuyeres, which fuses immediately after entry and reduces as it comes down through the hot coke-packed bed. Reduced molten metal and slag drip to the hearth, whereas volatile metals like zinc and lead are vapourized and extracted through the exhaust gas system. In addition, this process utilizes a fluidized bed to preheat, pre-reduce the raw fines to enhance flowability through tubes and reduce the heat load of the furnace and reduce reduction time. In order to prevent the zinc adhesion on the wall of furnace top, it is important to operate the furnace continuously. It is also necessary to keep the furnace top gas temperature and CO2/CO ratio where zinc oxide is stable, in order to prevent the accretion of zinc on furnace walls [37].
Flowchart of Coke packed bed process [39]
Advantages: (i) Solution loss reaction and coke deterioration under burden are not an issue here; hence, this process can operate with low-grade coke made from weakly coking coal. (ii) Injection of pulverized coal through the lower tuyeres also decreases the coke consumption. (iii) Efficient separation of metal and slag. (iv) Direct use of fines without agglomeration.
Disadvantages: Smelting reduction has a high carbon rate and consumes a great a deal of energy. In practice, it is necessary to keep the temperature above 1550 °C to completely reduce the metal oxides in liquid state. The off-gas credit is high and has to be utilized by power generation.
2.8 Ausmelt Process
The Ausmelt process based on top submerged lance (TSL) smelting technology is divided into three stages (i) smelting, (ii) reduction and (iii) fuming. Depending the scale/production rate, the entire 3 stages can be carried out in a single furnace or a multiple sequential furnaces. To achieve higher output in less time, a two-stage Ausmelt furnace is used for the efficient processing of EAF dust. In two-stage Ausmelt system, furnace 1 is of smelting stage and furnace 2 is of reduction and fuming stage. In practice, a blended mixture of EAF dust, fuel and reducing agent (carbon) and fluxes are fed into the first Ausmelt furnace [38, 39]. The smelting furnace is primarily used to melt the EAF dust and operates at a temperature between 1250 and 1350 °C. Oxygen/fuel is sent into the liquid slag bath through water cooled top lance, which produces intense stirring, heating and melting. Oxygen partial pressure and slag chemistry are controlled such that only zinc gets reduced and fume off leaving a slag containing 5–6% zinc, which is subsequently sent to furnace 2 for further reduction. Furnace 2, which is packed with granulated coke, ensures the reduction of slag inside the bed. The volatized non-ferrous metals oxide leaves the furnace through gas streams and is collected in the bag house. This process operates with very high levels of oxygen enrichment to minimize fuel consumption and off-gas volume.
Advantages: (i) Use of less expensive coke as reducing agent and can be operated with low cost fuel energy source. (ii) Off-gas recovery is possible to generate electricity. (iii) It has excellent environment performance due to low emissions of fugitive gases due to sealed reactor and can recover SO2 in the form sulphuric acid. (iv) High degree of process flexibility makes it suitable to treat high and low Zn EAF dust. (v) Precise control of partial pressure of oxygen and slag chemistry makes it suitable for selective removal of impurities.
Disadvantages: Low-quality zinc soot obtained through this process and complex bed operations restrain its flexible operation.
2.9 Electric Smelting Reduction Furnace Process
The electric smelting reduction furnace (ESRF) uses electrical energy for material heating and oxide reduction [40]. Figure 7 shows the flow chart of the ESRF process for processing of EAF dust. In this process, a mixture of coke, limestone and agglomerated EAF dust is charged into the furnace. The temperature in the smelting zone reaches above 1400 °C, where zinc and lead get volatilized, oxidized and leave with exhaust gas, which is cooled through the multi-tube gas cooler and sent to the bag-house to recover the non-ferrous oxide particles like ZnO, PbO, contaminated with some chloride particles. Most of the iron present in the EAF dust is recovered as molten pig iron from the bottom of the furnace, which can be directly used in BOF or EAF steel making. The crystallized slag produced under slow pot cooling can be used as an aggregate in the construction materials.
Flowchart of ESRF process [42]
Advantages: (i) The high gas temperature over 1250 °C completely decomposes the dioxins and other organic substances in EAF dust. No hazardous materials remain in the slag making it saleable.
Disadvantages: (i) The ESRF is very costly to operate and low price of recovered metals makes it difficult to commercialize. (ii) Thermal energy from exhaust gases is not actively used; only a portion of it is used for pre-heating of charged materials.
2.10 Microwave Processing
Recently, researchers have shown interest towards microwave heating as an alternate process because of its unique characteristics like fast, uniform and direct internal heating. The microwave heating of materials is characterized by volumetric, and selective heating and it overcomes the barrier of mode of transmission of heat from external to internal. It is an advanced alternative to conventional heating because microwaves are absorbed at the molecular level and heat could be generated inside the materials without increasing its surrounding temperatures to the same level [41]. Therefore, high energy efficiency can be obtained when the heat transfer is a rate-determining step. This microwave irradiation of rapid heating, higher iron metallization and higher zinc removal ratio receive larger attention towards this process. In the absence of fuel combustion and oxidizing atmosphere, the products both iron and zinc are obtained in pure metallic form [42]. Complete metal reduction process can be finished in 15 min. This is advantageous compared to traditional Waelz kiln process which takes normally 4–6 h.
Advantages: (i) Microwave process is very efficient due to selective heating, reduction and extraction. High recovery of iron and zinc is possible leaving others in dormant state [43]. (ii) Process facilities are much smaller than the conventional ones. (iii) Less harmful to environment in the absence of any fuel use. Since no fuel is used, also metals are recovered in metallic state directly [44]. (iv) No pre- or post-treatments like pelletizing and crushing are required, (v) easy separation of the recovered metals and remaining residue.
Disadvantages: The process remains in the laboratory scale and is yet to be realized in pilot scale.
3 Concluding Remarks
Cost-effective management of EAF dust economically drives for the recovery of precious metals with environmental benefits. Producing value-added products and safe landfilling/waste incorporation are the primary goals for the present industries. There are also possibilities for EAFD incorporation into other materials and products, with adequate attention to process control and end-of-life product disposal [45]. Several EAFD technologies to process these hazardous wastes have come forth over the last decades, and many of these processes have been commercialized. Most of the major processes are based on the pyro-metallurgical, or hybrid route including pre- or post-processing by the hydro-metallurgical route. Some of the commercialized and pilot-scale technologies are presented in Table 1.
However, several challenges still exist, and processes require up-gradation considering toxic emission, energy, and emission points of view. Optimizing thermodynamic and kinetic parameters is not an easy task due to the complex occurrence of ferrous and non-ferrous metals in the dust. Contamination of ZnO product with sodium, potassium, and fluorides causes toxic gas evolution during refining crude ZnO product both by pyro- and hydro-metallurgical route.
Reducing specific energy consumption, producing contamination-free products, metal adhesion on the walls, difficulty in eliminating secondary hazardous pollutants like chlorine, fluorine, contamination of solid residue with heavy metals for unsafe disposal are some of the challenging aspects for the enrichment of the valuable products from EAF dust. Finally, higher steel scrap prices and stricter environmental protection will strive the industries to focus on the processing of EAF dust.
Many of the well-established technologies like Waelz kiln are required to reassess their technologies for energy efficiency. The thermodynamics of the Waelz process has to be optimized for selective reduction of zinc and lead oxides and restrict unnecessary iron ore reduction and toxic metal incorporation to their solid residue, called the Waelz iron product, or Waelz slag.
RHF technology has emerged as the optimum technology to recover both iron and zinc from EAF dust. However, RHF is not yet ready for treating high zinc EAF dust. Some smelting reduction processes like coal bed process, PIZO, Ausmelt, Oxycup processes exist for recovery of liquid iron and zinc oxide simultaneously from high zinc EAFD. However, those are crippled with high energy requirements and off-gas credit. Recently, Wang et al. [46] reviewed the pyro-metallurgical treatment of EAFD. They opined that coal-based DRI production followed by smelting reduction could be an energy-efficient route. The off gas from the SR units could be utilized in the DRI production. However, such processes are carbon-intensive and not sustainable in the near future, which steers in low carbon technology to restrict global warming.
The microwave process is unique in selective heating, reduction, and extraction of iron and zinc, leaving others dormant. Other positive features include a smaller reactor, no fossil fuel, less emission, and purer products. More importantly, the microwave allows speedy charge processing (15 mins compared to 4-6 hours for the Waltz Kiln). However, two significant problems with microwaves are non-uniform heating and depth of penetration that depend on the material properties. Therefore, some further research is required in these directions. Besides, it may be combined with fuel heating for initial drying and preheating to make the process cost-effective because the heating core is easier with the microwave. Besides, if cheap electricity can be generated from renewable sources, such a process can become really cost-effective and carbon-free. A future microwave furnace with improved design and operated with green electricity could be a future sustainable reactor of EAFD treatment without any CO2 emission.
References
World steel Association, 2019. World Steel Association, Steel Statistical Yearbook 2019; Available on Internet: http://www.worldsteel.org/statistics/statistics-archive/yearbook-archive.html.
Morris J P, Riott J P and Illig E G, JOM 18 (1966) 803.
Ellis A F and Glover J, J Iron Steel Inst 209 (1971) 593.
Guezennec A G, Huber J C, Patisson F, Sessiecq P H, Birat J P and Ablitzer D, ISIJ Int 44 (2014) 1328.
Lis T, Nowacki K, Z_elichowska M and Kania H, Metalurgija 54 (2015) 283.
Pickles C A, Can Metall Quart 46 (2007) 125.
Lin X, Peng Z, Yan J, Li Z, Hwang J Y, Zhang Y, Li G and Jiang T, J Clean Prod 149 (2017) 1079.
Ma N Y, On in-process separation of zinc from EAF dust, Proceedings of EPD Congress (2011) p 947.
Ma N Y, J Clean Prod 112 (2016) 4497.
Nyirenda R L, Miner Eng 4 (1991) 1003.
Stewart C, Recovery of Zinc from EAF dust in the Steel Industry, Intergalva conference, Liverpool, England (2015).
Pickles C A, J Hazard Mater 150 (2008) 265.
International Zinc Association, 2013. Zinc recycling; https://www.zinc.org/basics/zinc recycling.
Environmental Protection Agency, 1991; https://www.epa.gov/rcra/resource-conservation-and-recovery-act-rcra-regulations#haz.
Gorez J P, Gros B, Birat J P, Grisvard J, Huber C and Le Coq X, Rev Met Paris 100 (2003) 17.
Nakayama T and Taniishi H, Eng Tech Rev 2 (2011) 25.
Buzin P J W K, Heck N C and Vilela A C F, J Mater Res Tech 6 (2017) 194.
Kukurugya F, Vindt T and Havlik T, Hydrometallurgy 154 (2015) 20.
Mager K, Meurer U and Wirling J, JOM 55 (2003) 20.
Masson N and Briol P, A Brief Summary of Zinc Oxide Processing Methods Available for the Bongara Deposit, Belgium (2017).
Harald R, Klaus D H, Rieger J and Reiter W, J Sustain Metall 5 (2019) 310.
Morcali M H, Yucel O, Aydin A and Derin B, J Min Metall Sect B Metall 48 (2012) 173.
Kanari N, Mishra D, Arteche A and Gaballah I, Waste Manag 2 (2002) 221.
Antrekowitsch J, Rosler G and Steinacker S, Chem Ing Tech 87 (2015) 1498.
Wu Y, Jiang Z, Zhang X, Wang P and She X, Int J Min Metall Mater 20 (2013) 636.
Kuwauchi Y and Barati M, ISIJ Int 53 (2013) 1097.
Suetens T, Klaasen B, Van Acker K and Blanpain B, J Clean Prod 65 (2014) 152.
Suetens T, Van Acker K, Blanpain B, Mishra B and Apelian D, JOM 66 (2014) 1119.
Frieden R, Hansmann T, Roth J L, Solvi M and Engel R, Acta Metall Slovaka 7 (2001) 33.
Kurunov I F, Metallurgist 55 (2012) 634
Bratina J E and Lenti K M, Iron Steel Technol 5 (2008) 118.
Verscheure K, Van Camp M C, Blanpain B, Wollants P, Hayes C and Jak E, Investigation of zinc fuming processes for the treatment of zinc containing residues, (ed) Nilmani M, Rankin W J, Melbourne, Australia (2005), p 237.
Verscheure K, Van Camp M C, Blanpain B, Wollants P, Hayes C and Jak E, Metall Mater Trans B 38 (2007) 21.
Verscheure K, Van Camp M C, Blanpain B, Wollants P, Hayes C and Jak E, Metall Mater Trans B 38 (2007) 13.
Holtzer M, Kmita A and Roczniak A, Arch Foundry Eng 15 (2015) 126.
Itaya H, Katayama H, Hamada T, Sato M, Ushijima T and Momokawa H, Kawasaki Steel Tech Rep 22 (1990) 3.
Hara Y, Ishiwata N, Itaya H and Matsumoto T, ISIJ Int 40 (2000) 231.
Hughes S, Reuter M A and Baxter R, lead and zinc 2008 (2008) 147
Zhou T, China Nonferr Metall 31 (2002) 49.
Nakayama M, SEAISI Q 41 (2012)
Peng Z and Hwang J Y, Int Mater Rev 60 (2015) 30.
Sun X, Hwang J Y and Huang X, JOM 60 (2008) 35.
Zhou Y, Wu L, Wang J, Wang H and Dong Y, High Temp Mater Proc 34 (2015) 177.
Sebastian M T, Ubic R and Jantunen H, Int Mater Rev 60 (2015) 392.
Muller J I R and Mayer W A, AIP conference proceedings 1653, 020074 (2015).
Wang J, Zhang Y, Cui K, Gao J, Hussain S and Algarni T S, J Clean Prod 298 (2021) 126788.
Piret N L, World Metall Erzmetall 65 (2012) 306.
Nakayama T and Taniishi H, Nippon Steel Eng Tech Rev 2 (2011) 25
Roth J L, Frieden R, Hansmann T, Monai J and Solvi M, Rev Met Paris 98 (2001) 987.
Hoang J, Reuter M A, Matusewicz R, Hughes S and Piret N, Min Eng 22 (2009) 742.
Acknowledgements
The work is a part of the UAY project sponsored by MHRD-MoS-ASP, SAIL, Durgapur.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
KiranKumar, T., Roy, G.G. A Review on Processing of Electric Arc Furnace Dust (EAFD) by Pyro-Metallurgical Processes. Trans Indian Inst Met 75, 1101–1112 (2022). https://doi.org/10.1007/s12666-021-02465-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-021-02465-6