Abstract
Porous titanium (Ti) and its alloys are promising materials for orthopedic applications due to their low elastic modulus, high strength, excellent corrosion resistance, and biocompatibility. In this study, the porous Ti–xNb–5Ag (x = 25, 30 and 35 wt%) alloys were synthesized using the powder metallurgy approach. The effects of Nb content on the porosity, mechanical properties, and electrochemical corrosion behavior of the alloys were investigated. XRD analysis revealed that the porous alloys mainly consist of α-Ti, β-Ti, intermetallic compound (Ti4Nb), and oxides of TiO2 and NbO phases. Porous alloys possess the porosity ranging from 57 to 65%, due to the addition of NH4HCO3 (45 wt%). Increase in Nb content lead to a reduction in the elastic modulus and compression strengths of the sintered porous Ti–xNb–5Ag alloys. All three developed porous Ti–xNb–5Ag alloys show the optimum combination of elastic modulus and compression strength, which is suitable for orthopedic applications. These porous alloys exhibit excellent electrochemical corrosion resistance in the simulated body fluids, and the samples having low porosity exhibit higher corrosion resistance than high-porosity samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Titanium (Ti) and its alloys are the most extensively studied metallic biomaterial to replace the hard tissues such as dental, total hip replacement, knee, spinal, and joints [1,2,3]. As known, β-Ti alloys are widely used for clinical applications due to their low elastic modulus and excellent biocompatibility, which shows biological acceptance in the human body environment [4, 5]. A few researchers have attempted to fabricate the Ti-based alloy with addition of strong β stabilizing elements such as Nb, Mo, Zr, Sn, and Ta to understand the influence on the microstructure and mechanical properties of the implant materials [6,7,8]. Further, Ag and Cu when alloyed with the Ti matrix, exhibit excellent antibacterial and high corrosion resistance properties [9, 10]. Nb element stands for the upper most alloying element with Ti, which promotes a β-phase in the developed alloys. It helps to reduce the elastic modulus of the implant materials [11,12,13,14]. Nazari et al. [12] prepared metastable β Ti–(5–40) Nb–3Mo alloys via powder metallurgy (PM) route and reported the remarkable reduction in the compression strength and elastic modulus from about 1370–1180 MPa to 95–37 GPa, respectively. Liu et al. [13] fabricated Ti–Nb–Zr alloys using the arc melting process with the addition of various Nb contents from 5 to 15 wt%. It is found that with increasing Nb content, both the compression strength (850–795 MPa) and elastic modulus (62–38 GPa) reduce significantly. Xu et al. [15] prepared β-type Ti–Mo–Nb alloys through the arc melting process with the addition of 3–10 wt% of Nb contents. The developed alloys possess compression strength of about 1918–1717 MPa and elastic modulus ranging from 28 to 24 GPa. These types of developed β-Ti alloys exhibit a very small reduction in elastic modulus with increasing Nb content. However, the obtained mechanical properties of these alloys does not match with the required bone properties (elastic modulus of 0.1–30 GPa and compression strength of about 2–200 MPa) [16, 17]. Still, researchers have made an effort to mimic the bone properties by the introduction of porous structures in Ti-based alloys to minimize the stress-shielding effect (occurred due to mismatch of elastic modulus between implants and bone). In recent years, a few studies have reported on microstructures and mechanical properties of the Ti–Nb alloys with introduction of the pore structure into bulk alloys [18,19,20]. Various processing techniques have been used to prepare the porous Ti alloys, including gas entrapped technique, additive manufacturing process, and powder metallurgy (PM) method [21,22,23]. Several studies have reported that PM is the best choice for fabricating the porous Ti alloys, due to low operating cost and controlled processing atmosphere to avoid oxidation using high purity argon or vacuum. Also, the required porosity level, pore size, shape and distributions can be achieved by using the various space holder materials [17, 23]. Oliveira et al. [18] fabricated the porous Ti–35Nb alloy with the addition of ammonium bicarbonate (two different sizes of 355 and 425 μm). They found the porosity of about 59 and 63%, and the elastic modulus of about 2.6 and 25 GPa, respectively, after sintering at 1200 °C. Further, XRD results exhibited α-Ti, β-Ti, intermetallic compound Ti4Nb and few oxides formation (NbO, TiO, Ti3O) at 1200 °C of sintering. However, these oxides disappear with further sintering of the porous alloy at 1300 °C. These oxide formations are quite common during the sintering operation due to the presence of impurities as an interstitial element (H, O, N and C) along with the presence of β-stabilizer elements. Rao et al. [20] synthesized two different porous alloys with the addition of Nb content Ti–(20 and 35) Nb–15Zr alloys by adding NH4HCO3 content (50%). With increasing Nb content from 20 to 35 wt%, a significant reduction in the compression strength from 102 to 73.4 MPa, and the elastic modulus from 2.5 to 1.2 GPa, respectively, are observed, showing that the addition of Nb significantly reduces the elastic modulus of the alloys, which is similar to the cancellous bone (0.01–3.0 GPa) properties. Yilmaz et al. [19] developed porous Ti–16Nb alloys with the porosity of 4–60% due to the addition of space holder material (0–70 vol%) which was sintered at 1200 °C for 3 h. The elastic modulus and the compression strength of developed alloys decrease from 96 to 15 GPa and from 1450 to 100 MPa, respectively. Further, the examination of electrochemical corrosion properties of porous alloys in SBF shows a high corrosion rate (278.9 × 10−3 mpy) for the high-porosity sample as compared to the low-porosity sample (166.2 × 10−3 mpy). Similarly, results were discussed by Xu et al. [24] for the porous Ti–(5–20)Mo alloy and by Li et al. [25] for porous Ti–Cu alloys. The reported literature confirms that porosity in Ti alloys has an essential role in the compression strength and elastic modulus of implant materials. The biocompatibility of the implant materials strongly depends on corrosion behavior in the human body as leaching of metal due to the electrochemical corrosion reactions with body fluids affects the life of metallic implants. The present study focused on developing Ag-added porous Ti–xNb–5Ag alloys (x = 25, 30 and 35 wt%) and to examine the role of Nb content on the porosity, microstructure, mechanical and electrochemical corrosion properties.
2 Experimental details
2.1 Preparation of porous Ti–xNb–5Ag alloy
In the present work, three different porous Ti–xNb–5Ag (x = 25, 30, and 35 wt%) alloys were fabricated (namely T25, T30, and T35) using a powder metallurgy method. Commercially available powders of Ti (< 45 µm), Nb (< 45 µm), and Ag (<5 µm) were ball-milled for 20 h using a high-energy planetary ball milling, with the ball-to-powder ratio of 20:1 and rotation speed of 250 rpm. The ball-milled powders were mixed with the 45 wt% of ammonium bicarbonate (NH4HCO3). The mixed powders were cold-compacted under the uniaxial load of 500 MPa to prepare green compacts using a steel die. Sintering operation was carried out in two stages: firstly, at 200 °C for 2 h to eliminate space holder materials; secondly, at 1200 °C for 3 h in a high-level vacuum (10−5 mbar), with the heating and cooling rates maintained at 10 °C/min. Phase identification and surface morphology of the developed alloy powders and sintered porous alloys were carried out using X-ray diffraction (XRD, JEOL DX GE-2P, Japan) and scanning electron microscopy (SEM) with energy-dispersive X-ray spectroscopy (EDX) (SEM-JEOL-Japan).
2.2 Porosity and mechanical properties
Porosity and density of the porous T25, T30, and T35 alloy were calculated using Archimedes’ principle. Uniaxial compression tests were conducted on the porous alloys at a strain rate of 0.001 mm/s to determine the mechanical properties (Shimadzu, Autograph AG-X plus, 100KN, Japan). Porous samples were prepared with the dimensions of ∅ 10 mm × 15 mm, according to the ASTM standard E9–09. The elastic modulus of the porous alloy was determined from the linear region of the typical compression stress–strain curves.
2.3 Electrochemical corrosion behavior
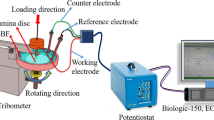
Electrochemical corrosion behavior of the developed porous alloys measured using the three-electrode system in simulated body fluid (SBF) at 37 °C according to Ref. [26]. The porous alloys (with the exposed surface area of ~ 1.0 cm2), platinum electrode, and saturated calomel electrode (SCE) served as a working electrode, a counter electrode, and a reference electrode, respectively. The open circuit potential (OCP) measurement was recorded for 1800 s in SBF for all the porous samples. The potentiodynamic polarization curve was recorded in the range from − 0.7 to 2 V versus SCE at a scan rate of 0.5 mV/s. For electrochemical impedance spectroscopy (EIS), tests were performed in the frequency range from 100 kHz to 10 mHz with an amplitude of 10 mV. All the experiments were conducted using the electrochemical work station (Biologic series SP-150, France), and the obtained data were analyzed using EC Lab software.
3 Results and discussion
3.1 Microstructure and XRD analysis of ball-milled powders
SEM images and XRD analysis of T25, T30, and T35 alloy powders after 20 h of ball milling are shown in Fig. 1. Figure 1a–c shows that all the developed alloy powders are irregular in shape and are slightly agglomerated particles due to the intense plastic deformation during the high-energy ball milling [8, 27]. The XRD patterns for T25, T30, and T35 powders after 20 h of ball milling are shown in Fig. 1d. Figure 1d of the manuscript is modified and discussed on the basis of 2θ values and planes as follows: It can be seen that the ball-milled powders showed the α-Ti at 2θ values of 38.8°, 40.1° and 76.1° and the corresponding planes of (002), (101) and (112) respectively. The β-Ti is obtained at 2θ values 38.7°, 55.9°, 70.1° and 83.1°, and the corresponding planes of (110), (200), (211) and (220) respectively, while the planes of (111) and (151) are identified for Ti4Nb and TiO2 at 2θ values of 39° and 61.1° respectively. It is also noticed that the peak broadening and peak shift are observed due to the reduction in the crystalline size and the increase in lattice strain, which are commonly observed after ball milling [28].
3.2 Porosity and density
Figure 2 shows the porosity and density of the sintered porous T25, T30, and T35 alloy with the addition of 45 wt% of NH4HCO3. The porous alloys possess a porosity ranging from 57 to 65%. Slightly higher porosity is observed in the sintered porous alloy (more than the addition of NH4HCO3 amount); this is because of the formation of Kirkendall-type pore during the sintering process [24]. The density of sintered porous alloys drastically reduces from 2.5 to 1.5 g/cm3, due to the addition of various Nb content, and the obtained values are found close to the density of human bone (1.8–2.1 g cm−3) [29]. The obtained porosity and density of the developed porous alloys are quite suitable for the use of bone applications.
3.3 Pore morphology and phase analysis
Figure 3 shows the SEM micrographs and XRD analysis of sintered porous T25, T30, and T35 alloys. All three porous alloys exhibit the homogeneous distribution of pores. The developed porous alloys are comprised of the micro sized pores, macro size pores, isolated pores, and interconnected porous network structures. The pore formation in the sintered porous alloys is due to the evaporation of NH4HCO3 [17]. The average pore size of about 130 μm, 136 μm and 142 μm for porous T25, T30, and T35 alloys, respectively, is observed. In our study, more than 100 pores have been measured to determine the average pore size of the porous samples using Image-J software [20]. The average pore sizes of all the porous alloys exhibit more than 100 μm, which is necessarily required to enhance the osseointegration implant’s materials [4, 17]. XRD patterns of the developed porous samples after sintered at 1200 °C are present in Fig. 3d. It is interesting to notice the formation of an intermetallic compound of Ti4Nb and oxides such as NbO and TiO2 peaks in sintered porous alloys along with the α-Ti and β-Ti phases. The obtained compounds such as α-Ti and β-Ti, Ti4Nb, TiO2, and NbO are identified by using the JCPDS codes as α-Ti (89-2762), β-Ti (89-4913), Ti4Nb (65–7479), NbO (42–1125), and TiO2 (49–1433). The peaks of α-Ti are observed at 2θ values of 40.1° and 76.1°, with the corresponding planes of (101) and (112) respectively. The phase of β-Ti is obtained at 2θ values 38.7°, 55.9°, 70.1° and 83.1°, with the corresponding planes of (110), (200), (211) and (220), respectively. The formation of intermetallic compound Ti4Nb diffraction peaks at 2θ values of 38.6° and 70.2° with the corresponding planes of (111) and (113), respectively, confirms the formation of the intermetallic compound in the sintered alloy. The formation of oxides peaks at 2θ values of 61.1°, which corresponds to TiO2 [the corresponding plane of (151)] and at 2θ values of 36.5° 42.5° and 73.9° represented as NbO compounds with corresponding planes of (111) (200) and (311) respectively are also observed. XRD data are in good agreement with the previous research’ [18]. However, α-Ti is detected with the lower intensity as compared to the β-Ti phase; it may be due to the diffusion of Nb into Ti during the sintering process. Nb element is not detected in the sintered porous alloys due to the overlapping of Nb with the β-Ti. Similarly, the formation of intermetallic compounds and oxides are observed in the sintered porous alloy as characterized by C.S.S. Oliveira et al. [18].
3.4 Mechanical properties
Similar, compressive stress–strain curves are obtained for all the sintered porous T25, T30, and T35 alloys, as shown in Fig. 4. The obtained curves exhibit three distinct regions, such as linear elastic region, plateau region, and densification region. However, in the plateau region, the fluctuation in stress value and a constant even increase in strain are observed (average stress in this region is known as plateau stress) and followed by densification. In the densification region, the stress is slightly reduced with the increase in the strain, where all the pore cell walls collapse due to the continuous applied stress. Figure 5 reveals the elastic modulus and compression strengths of porous alloys, which are measured using stress–strain curves (Fig. 4). The reduction in the elastic modulus to about 4, 2.2 and 1.0 GPa and the compression strength to 64, 43, and 22 MPa are observed due to the addition 25, 30 and 35 wt% of Nb content, respectively [20].
3.5 Electrochemical corrosion behavior
Figure 6 shows all the OCP plots for the porous T25, T30, and T35 alloys after immersion in the SBF solution at 37 °C for time 1800 s. It is observed that the OCP values decrease sharply toward the active potential side with increase in the Nb content, which indicates instability of spontaneous passive film formation and its breakdown due to the porosity variations [30]. Further, it is noticed that the OCP shifts toward the noble side for initial few minutes for all these alloys, indicating a rapid passive film formation at available bulks surface area, and a further reduction in OCP toward the active direction is mainly due to the characteristics of the pores [19]. Figure 7 reveals the potentiodynamic polarization curves of the sintered porous alloys in SBF at 37 °C. It is found that similar features of polarization curves for all the sintered porous alloys have a different porosity level. The curves exhibit the active-passivation region and also shows the distinct self-passivation behavior. However, it does not show an exponential growth of corrosion current density on the increasing potential in the presence of porosity like ferrous alloy. It is also noticed that there is a small fluctuation of corrosion current density due to the metastable passivation behavior of the sintered porous Ti alloy. The corrosion parameters such as corrosion potential (Ecorr) corrosion current density (icorr), anodic slope (βa), and cathodic slope (βc) are determined from the Tafel analysis using both anodic and cathodic branches (Fig. 7) as given in Table 1. The Ecorr values of − 0.13 V, − 0.21 V and − 0.37 V (vs. SCE) and icorr values of 0.20 × 10−6 A/cm2, 0.63 × 10−6 A/cm2, and 1.1 × 10−6 A/cm2 have been estimated from the obtained curves, for the porous T25, T30 and T35 alloys, respectively,. Yilmaz et al. [19] and Xie et al. [30] have developed porous Ti–Nb and porous Ti–(4–8)Mo, respectively, and investigated the electrochemical properties of these alloys in SBF. It is found that both the addition of alloying elements and porosity level are playing an important role in the corrosion properties of materials. The results suggest that porous alloys have a high porosity and exhibit a low corrosion resistance as compared to the low-porosity alloys; it is due to the exposure of more surface area to the electrolyte which easily penetrates among the interconnected porosities.
To investigate the passive film charecteristics of porous alloys containing different porosity levels are shown in Fig. 8 (EIS Polts). Nyquist plots (Fig. 8a) exhibit incomplete and large depressed semicircles, indicating a capacitive response of passive film formation on the sample surface. It clearly shows that the semicircle diameter increases in the low-porosity sample indicating a better impedance behavior of porous alloys with the low porosity as similar results are observed using the potentiodynamic polarization test [30, 31]. Bode magnitude and phase angle plot are shown in Fig. 8b, c, respectively, which shows similar curves for all three different porous samples. Porous T25 alloy exhibits a higher modulus of impedance (Z) than other porous alloys, indicating a stable passivity response. Further, it is noticed that initially, the modulus of impedance shows a flat region due to the electrolyte resistance. In the middle and lower frequency ranges, the curves exhibit a linear relationship between modulus and frequency (slope close to − 1) and indicate a characteristic response of a capacitive behavior of passive film [32]. However, the determined impedance values increase with the reduction in porosity. The obtained result shows that the porous alloy with the low porosity exhibits high corrosion resistance than highly porous samples. The obtained Bode phase angle plot shows three distinct characteristics, such as a high-frequency region, a middle-frequency region, and a low-frequency region. In the high-frequency region, it shows phase angle drops toward the 0°, indicating the response of electrolyte resistance. In the middle-frequency region, the phase angle is near to the − 75°, − 65°, and − 60° for porous T25, T30, and T35 alloys, respectively, which exhibit wide-range frequencies indicating the formation of the compact passive film on the alloy surface and near the capacitive response of the passive film. In the low-frequency region, it is observed that the values of the phase angle drastically decreases to a lower value due to the influence of resistance of the passive film. To obtain the typical information for the passive films, the equivalent circuit model (Fig. 8d) is used to analyze the EIS data and results are summarized in Table 2. The model assumes the formation of the oxide film, which is composed of the inner barrier layer and a porous outer layer. All the ‘n’ values are close to 1, indicating that a near capacitive characteristic of passive film is formed on the porous alloys. The Rs is electrolyte resistance (SBF), and a constant phase element representing a shift from the ideal capacitor is used instead of capacitance. Rp and Rb are the resistances of the porous and barrier layers, which is associated with the charge transfer resistance through the porous layer. Qp and Qb corresponds to the capacitances of the porous layer and barrier layer, which is related to the formation of the passive film layer. All the electrical parameters obtained by the equivalent circuit model are summarized (Table 2). The barrier resistance (Rb) is strongly dependent on the formation of the passive film, and it shows higher Rb value for porous T25 (175 kΩ cm2), which indicates high corrosion resistance as compared to porous T30 (32 kΩ cm2) and porous T35 (12 kΩ cm2) alloys. Moreover, the Rb value of all the porous alloys is much higher than Rp values (porous layer), which exhibits that the porous alloy surface is significantly protected by the barrier film. The obtained result shows that the capacitance values Qp and Qb decrease, and the resistances Rp and Rb increase while there is a reduction in porosities. It shows the formation of the total polarization resistance (Rt) of passive layers (Rt = Rp + Rb) on the porous alloys having more resistance with the decreasing porosity to the electrolytes. The lower capacitances of the porous alloys are associated with higher resistance and its characteristics, indicating high corrosion resistance of the developed porous alloys.
4 Conclusions
In the present study, the powder metallurgy method was used to develop porous Ti–xNb–5Ag alloys (x = 25, 30, and 35 wt%) and the effect of Nb content on porosity, microstructures, mechanical properties, and electrochemical corrosion behavior was investigated. The conclusions are summarized as follows:
The porous T25, T30, and T35 alloys were successfully fabricated from the 20-h-ball-milled powders with the addition of 45 wt% of NH4HCO3.
The sintered porous T25, T30, and T35 alloys exhibited the porosities in the range from 57 to 65% due to the addition 25–35 wt% Nb content and 45 wt% of NH4HCO3.
XRD analysis revealed that the sintered porous alloys predominantly consisted of intermetallic compound Ti4Nb and β-Ti phases.
The significant reduction in the compression strength (64–22 MPa) and elastic modulus (4.2–1 GPa) were observed due to the addition of various Nb content (25–35 wt%) in the sintered porous alloy.
The electrochemical corrosion behavior of the porous alloys exhibited excellent corrosion resistance in SBF. It was observed that corrosion potential decreased away from the noble direction, while corrosion current density increased with the increase in Nb content, where porosity also had an essential role in the corrosion behavior of sintered porous alloys.
References
Geetha M, Singh A K, Asokamani R, and Gogia A K, Prog Mater Sci54 (2009) 397.
Spoerke E D, Murray N G, Li H, Brinson L C, Dunand D C, and Stupp S I, Acta Biomater.1 (2005) 523.
Gurappa I, Mater Charact49 (2002) 73.
Gao Z, Li Q, He F, Huang Y, and Wan Y, Mater Des42 (2012) 13.
Majumdar P, Singh S B, Dhara S, and Chakraborty M, Mater Sci Eng C46 (2015) 62.
Yang D, Guo Z, Shao H, Liu X, and Ji Y, Procedia Eng.36 (2012) 160.
Wang X, Li S, Jia M, Hao Y, Yang R, and Guo Z, Chin J Mater Res24 (2010) 378.
Aguilar C, Guerra C, Lascano S, Guzman D, Rojas P A, Thirumurugan M, Bejar L, and Medina A, Mater Sci Eng C58 (2016) 420.
Chen M, Zhang E, and Zhang L, Mater Sci Eng C. 62 (2016) 350.
Takahashi M, Kikuchi M, and Takada Y, Dent Mater J21 (2002) 270.
Lee C M, Ju C P, and Lin J H C, J Oral Reha29 (2002) 314.
Nazari K A, Nouri A, and Hilditch T, J Mech Beh Bio75 (2017) 33.
Liu Q, Meng Q, Guo S, and Zhao X, Prog Nat Sci Mater Inter23 (2013) 562.
Wang Y B, Zheng Y F, Mater Lett62 (2008) 269.
Xu L J, Chen Y Y, Liu Z G, and Kong F T, J Alloys Compd453 (2008) 320.
Hsu H-C, Wu S-C, Hsu S-K, Tsai M-S, Chang T-Y, and Ho W-F, Mater Des47 (2013) 21.
Yue-qin W, Jie T A O, Jin-long Z, and Tao W, Trans Nonferrous Met Soc China.21 (2010) 1074.
Oliveira C S S, Griza S, de Oliveira M V, Ribeiro A A, and Leite M B, Powder Technol.281 (2015) 91.
Yılmaz E, Gökçe A, Findik F, Gulsoy H O, and İyibilgin O, J Mech Behav Biomed Mater87 (2018) 59.
Rao X, Chu C L, and Zheng Y Y, J Mech Behav Biomed Mater34 (2014) 27.
Murray N G D, and Dunand D C, Act Mater52 (2004) 2279.
Murr L E, Gaytan S M, Medina F, Martinez E, Martinez J L, Hernandez D H, Machado B I, Ramirez D A, and Wicker R B, Mater Sci Eng A. 527 (2010) 1861.
Wen C E, Mabuchi M, Yamada Y, Shimojima K, Chino Y, and Asahina T, Scr Mater.45 (2001) 1147.
Xu J L, Tao S C, Bao L Z, Luo J M, and Zheng Y F, Mater Sci Eng C.97 (2019) 156.
Li Y H, Chen N, Cui H T, and Wang F, J Alloys Compd723 (2017) 967.
Kokubo T, and Takadama H, Biomaterials27 (2006) 2907.
Dercz G, and Matuła I, Mater. Tehnol.51 (2017) 795.
Wen M, Wen C, Hodgson P, and Li Y, Mater Des.56 (2014) 629.
Staiger M P, Pietak A M, Huadmai J, and Dias G, Biomaterials.27 (2006) 1728.
Xie F, He X, Cao S, Mei M, and Qu X, Electrochim Acta.105 (2013) 121.
Bai Y, Li S J, Prima F, Hao Y L, and Yang R, Appl. Surf. Sci.258 (2012) 4035.
Lu J, Zhang Y, Huo W, Zhang W, Zhao Y, and Zhang Y, Appl Surf Sci.434 (2018) 63.
Acknowledgment
One of the authors Shivaram M.J wishes to acknowledge MHRD, Govt. of India, for providing research fellowship. The author expresses gratitude to Prof. Rajendra Udupa, Department of Metallurgical and Materials engineering, NITK Surathkal, for his technical inputs and suggestions. Authors also would like to thank Dr. R.B. Mane (MSME, IITH) for helping partially in developing alloys.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shivaram, M.J., Arya, S.B., Nayak, J. et al. Electrochemical Corrosion and Impedance Studies of Porous Ti–xNb–Ag Alloy in Physiological Solution. Trans Indian Inst Met 73, 921–928 (2020). https://doi.org/10.1007/s12666-020-01904-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-020-01904-0