Abstract
Scandium addition in Al–Si alloys can reduce grain size and modify the structure from flake-like to fibrous. To use this Sc-modified Al–Si alloy in practical applications, there are many factors that need to be studied, including porosity formation. However, there is limited available information with regard to the effect of Sc on porosity formation. This research used the Tatur test mold to investigate the effect of strontium and scandium on porosity formation in Al–6Si–0.3 Mg alloys. Sc addition promoted the fibrous structure of eutectic silicon and reduced grain size. Sc addition also resulted in smaller and more dispersed porosity across the samples compared to that of unmodified samples. Once the Sc addition reached 0.4 wt%, the τ phase (AlSi2Sc2) was clearly observed. It is believed that the τ phase promoted more oxidation during casting and eventually led to higher numbers of porosity in the casting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
It is important for foundry aluminum alloy to have good castability, which is defined as the ability of an alloy to be cast without the formation of defects such as cracks, segregations, pores or misruns. There are many alloy dependent phenomena that determine castability; for example, fluidity, macrosegregation, hot tearing and porosity formation tendency [1].
One of many methods to evaluate the porosity formation tendency is Tatur test. The Tatur test utilizes a permanent mold of fixed geometry like a right circular cone. It has been used for quantitative measurement of microporosity and macroporosity [2, 3]. The porosity formation tendency is closely related to eutectic solidification, resulting from the modification of eutectic Si. In the case of using a conventional modifier, Arbenz [4] compared unmodified and Na-modified Al–Si casting samples and found that the unmodified samples had large porosities. In contrast, the Na-modified samples had microporosities dispersed throughout the samples. Ware et al. [2] found that Na promoted the eutectic growth from the mold wall. The porosity distribution in the sample from the Tatur tests revealed that Na modification resulted in an increased amount and more complete distribution of porosity compared to the unmodified alloy. Different levels of Sr additions resulted in different porosity distributions. The samples with low Sr addition levels (105 ppm), which were taken from the Tatur test, exhibited some concentration of porosity at the center of casting. These results suggest that a relatively good feeding of eutectic mushy zone still exists and, under these casting conditions, extends from the mold wall for lower Sr levels. However, at higher Sr addition levels (192 ppm), the porosities are completely distributed.
In general, porosities in the unmodified alloy are irregular and interconnected. Dinnis et al. [5] found that Sr addition resulted in the porosity becoming well dispersed and round, but was reduced in the hot spot of the casting. The eutectic grains in the Sr-modified alloy grew to a much larger size and finally trapped liquid pools upon impingement of the eutectic phases. This resulted in a better distributed porosity on the eutectic grain boundaries and was dispersed all over the casting. Tiedje et al. [6] found that, a different modifier strongly effected the nucleation and growth of the eutectic cell. In the unmodified alloy, porosity was usually located towards the center of the casting, but particularly near the surface of its fine eutectic cells. The Na-modified castings also tended to have less porosity, with porosity being found closer towards the center of the casting. In the large cell size of Sr modified alloy, which seemed to solidify in a more mushy manner, porosity was distributed throughout the casting.
Recently, Sc additions in Al–Si alloys have been found to reduce grain size [7] and modify the structure from flake-like to fibrous [8–10]. To use this Sc-modified Al–Si alloy in practical applications, there are many factors that need to be studied, including porosity formation. Limited information on the effect of Sc on porosity formation is available. To study the porosity formation tendency, the Tatur test was successfully applied to evaluate the effects of alloying elements in different aluminum alloys. For example, Gruzleski and Bernard [3] used the Tatur test mold to investigate the effects of Sr and Na to modify aluminum–silicon alloys.
Therefore, the objective of the present work is to investigate the effects of Sc additions on the porosity formation tendency, which is one of the major concerns for aluminum alloy castability.
2 Experimental Procedure
2.1 Tatur Test Mold
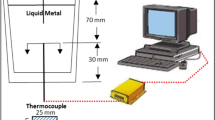
The Tatur mold was made of copper with a right circular cone like shape. Similar to previous work, the inside diameter and height of the cone were 100 and 105 mm, respectively [11]. The right circular cone shape of Tatur mold enabled the solidification order, to be from the surface into the center of the sample in a symmetrical direction. The solidification of narrower top part of right circular cone would be completed before the bottom part. There was no molten aluminum to effectively feed the bottom part of casting in a Tatur mold, thus resulting in microporosity and macroporosity at the hot spot. Before pouring molten aluminum, the mold was preheated in a furnace at 60 °C for approximately 30 min. To control the pouring height, the average pouring rate was maintained at approximately 130 cm3/s. A specially designed mechanism was installed for the pouring cup and a plug was preheated in a separate resistance furnace to 650 °C. The temperature of the melt was measured using a K-type thermocouple to ensure that the pouring temperature was stable at 700 °C for all experiments. The experimental set up is shown in Fig. 1.
2.2 Melt Preparation
The Al–6Si–0.3 Mg aluminum alloy ingots used in this work were made from primary aluminum to minimize any possible contamination effects by trace elements. These were prepared from the same batch to minimize any undesired variations. Master alloys of Al-10 wt% Sr and Al-2 wt% Sc were used to add minor Sr and Sc to obtain a variety of chemical compositions. Sr was used as a reference because it is one of the most conventional treatments to modify eutectic silicon. The chemical compositions for each of the treated alloys were determined by Spark Emission Spectrometer and are shown (along with the alloy designations) in Table 1. The Al–6Si–0.3 Mg alloy charge of 1080 grams was melted in a silicon carbide crucible in a 12-kW induction furnace and all of the charge was later poured into the Tatur mold. Covering and cleaning flux was used at approximately 0.5 wt% of the alloy. Argon purge through a stainless steel tube (6-mm inside diameter) was used to flush out hydrogen. The degassing time was approximately 1 min for each experiment. Flow rate and pressure were set at 4 L/min and 0.2 MPa, respectively. Subsequently, dross was carefully removed before pouring. The melt was heated up to 800 °C before the addition of the master alloys (Al-10 wt% Sr or Al-2 wt% Sc) to obtain the target compositions in each batch. The melt was then poured into the pouring cup as shown in Fig. 1. The melt was held until it reached 700 °C. The plug was then pulled up to let the melt cover the Tatur mold. Each of the experimental conditions was repeated 5 times.
All castings were then cut into half, through the center, and were carefully ground and polished to give a clear surface for visual inspection. The samples were also scanned and their respective microporosities were quantitatively analyzed using an image analyzer software, iSolution DT. To understand the microporosity formation, the samples were also analyzed using an optical microscope, scanning electron microscope, and the EDS technique.
3 Results and Discussions
3.1 Macrostructure Analysis
It have been confirmed that three different eutectic solidification modes can operate in hypoeutectic Al–Si alloys, determined by composition and casting conditions. These are (1) Mode I: nucleation on the dendrites, (2) Mode II: heterogeneous nucleation of eutectic grains in the interdendritic liquid, and (3) Mode III: nucleation and growth adjacent to the mold wall (opposite the thermal gradient) [12–14]. Moreover, the differences in eutectic solidification modes have been shown to influence the formation and distribution of porosity [15].
The macrostructures of the samples from the various conditions are shown in Fig. 2. This figure clearly shows that the microporosities of the unmodified Al–6Si–0.3 Mg alloys are distributed across the cross section of the samples. The piping is found in the center of the casting for every sample because the hot spot is at the center of casting of the mold. This leads to a large number of porosities concentrated at the center of the samples. It has been reported that the solidification mode of unmodified hypoeutectic Al–Si alloys is Mode I (Fig. 3a) with the eutectic Si nucleation sites and growth next to the tips of the aluminum dendrite [15]. Normally, porosities are likely to form on the eutectic grain boundaries. The numerous eutectic Si nucleation events and the eutectic evolves with a very irregular interface in unmodified alloys which can lead to unfed liquid pockets on the irregular eutectic grain boundaries. Thus the shapes of porosities are very irregular with a high degree of interconnection. This analysis is supported by the observation that the hot spot segment of the Tatur mold castings made from the unmodified Al–6Si–0.3 Mg alloy shows a large amount of irregular and interconnected porosity in the hot spot area in center of the sample (Fig. 2a). This solidification mode results in much more porosities at larger sizes as shown in the microstructures.
Sr addition results in finer and more widely distributed porosities across the cross section of the specimens compared to the unmodified Al–6Si–0.3 Mg alloys as shown in Fig. 2a, b. These results are consistent with those of previous works [2, 3, 5, 15, 16]. There is much less concentrated porosity in the center of the casting than in the Al–6Si–0.3 Mg alloys that is unmodified at the hot spot around the piping. The Sr addition promotes mode II eutectic solidification, which features eutectic grains nucleation and grows in the spaces between dendrites (Fig. 3b). The eutectic cells are likely to be present in the interdendritic areas and block the feeding channel during the solidification at lower solidification fractions [15]. However, in case of Sr modified alloy casting, the eutectic is characterized by a low nucleation frequency, thus helping Sr-modified eutectic grains to grow to a large size [17]. Moreover, the eutectic grains form in the melt between dendrites and there is no eutectic front growing from the surface towards the center of the casting [17]; therefore, the eutectic does not form a solid shell at the mold surface. Thus, the final liquid phase becomes isolated in many parts of the sample resulting in the porosities being more dispersed [6].
Previous work has demonstrated that Sc is simultaneously a grain refiner and a modifier [8, 10]. In this work, Sc addition in Al–6Si–0.3 Mg alloy promotes fibrous eutectic silicon and reduces its grain size. The trend for distributed porosity across the cross section of the specimens has been found to be similar to those of Sr-modified samples. It has been found that the distribution of porosity strongly depends on the amount of Sc added, i.e., more Sc addition results in more porosity in more dispersed forms. Pandee et al. [18] found that Sc addition in Al–7Si–0.3 Mg has altered the macroscopic eutectic growth mode; the propagation of a defined eutectic front from the mold walls (Mode III in Fig. 3c) is in the opposite direction of the heat flux. This is similar to previously published work with the addition of Na, Ca, and Y. Na modifications increases microporosity compared to Sr addition [2]. The Na modification promotes eutectic growth from the mold wall, also known as solidification mode III. That eutectic solidification involves nucleation at or next to the wall and the growth front is opposite to the thermal gradient. The growth front of the eutectic is expected to be relatively flat and gradually moves toward the center of the sample. The feeding channels in the solid network remains open until the last stage of solidification. Porosity has been expected to be less dispersed and this is shown in Fig. 3d [2, 15]. Ware [2] found that Na modifications increases the amount of porosities in a more dispersed form in the Tatur sections compared to the unmodified alloy. This result is similar to the effect of Sc that has been found in this research.
Campbell and Tiryakioğlu [19] found that Sr additions reduces the surface tension of the Al–Si melt, thus making it easier to nucleate porosity. In addition, Sr promotes the rate of oxidation and increases the tendency for microporosity formation resulting in more porosities [20]. Kim et al. [21] found that Sc addition, just like Na, decreases the surface tension. As a result, Na addition significantly decreases the porosity size and causes it to be more dispersed compared to that in the unmodified alloy [22]. This result agrees well with the effect of Sc modification found in this research.
3.2 Quantitative Results of Porosity
From the cross section of the center of the unmodified, Sr-, and Sc-modified samples, microporosities were determined using an image analyzer software. The results are shown in Table 2. It has been found that the porosity areas at the cross section are reduced from 3.34 to 3.08 and 2.38 % with 0.01 %Sr and 0.2 %Sc additions, respectively. However, the porosity area of 0.4 %Sc modified samples increases to 3.67 %.
There are many porosities found in the hot spot, which is the last solidified part. In the unmodified samples, the percentage of porosities is 14.35 %. However, the percentages of porosities are reduced to 8.92, 6.85 and 5.01 % after modification with 0.01 %Sr, 0.2 %Sc and 0.4 %Sc respectively. These results confirm that the modification of Al–6Si–0.3 Mg alloys using either Sr or Sc can reduce number of porosities at the hot spot.
3.3 Microstructure Analysis
Micrographs shows that the microporosities in the unmodified Al–6Si–0.3 Mg alloys are irregular and interconnected. Specifically, there are areas with large porosities around the center of the hot spot as shown in Fig. 4. In the Sr modified samples, the microporosity is smaller and more dispersed as compared to the unmodified Al–6Si–0.3 Mg alloy. This result is in agreement with the previous works [5, 16]. This result suggests that the feeding of the eutectic mushy zone is reduced, possibly due to the increased nucleation of eutectic colonies that blocked the feeding channels [2].
Recently, Pandee et al. [18] suggested that Sc addition in Al–Si alloy changes the eutectic growth to mode III, which is similar to Na addition. Thus, Sc redistributes the porosities and a few small porosities are scattered around the cross section of the samples. This result agrees with previous published work [16]. However, when the addition of Sc reaches 0.4 wt%, more porosities are observed and they are more scattered than the case with 0.2 wt% Sc addition as shown in Fig. 2c, d. It has been proposed that 0.4 wt% Sc addition results in AlSi2Sc2 intermetallic phase formations (τ phase), which is not found in the samples with 0.2 wt% Sc addition [18]. SEM micrographs and EDS analysis results of 0.4 wt% Sc modified samples are shown in Fig. 5. These results confirm that the white phase is the τ phase. There are microporosities adjacent to the τ phases between eutectic silicon. It is believed that the τ phase promotes more oxide formation during casting.
Campbell and Tiryakioğlu [19] and Miresmaeili et al. [20] explained that more oxide increases the number of porosities found in the casting. It is also possible that the τ phases obstruct the liquid metal feed. Similarly, Moustafa [23] proved that the precipitated long branched β-Al5FeSi in Al–Si eutectic alloys result in the formation of large shrinkage cavities due to the limitation of liquid metal to feed the inter-cavity space during solidification.
4 Conclusion
-
1.
The unmodified Al–6Si–0.3 Mg alloy had the largest number of microporosities at the hot spot around the piping. The percentage of porosity area at the hot spot was 14.35 %. Micrographs showed that the microporosities were irregular and interconnected.
-
2.
The microporosity of Sr-modified Al–6Si–0.3 Mg alloy was relatively small and well dispersed across the samples compared to that of the unmodified Al–6Si–0.3 Mg alloy. The percentage of porosity area at the hot spot reduced to 8.92 %.
-
3.
By adding Sc into Al–Si alloys, it was clear that the solidification mode was Mode III as already published in our previous work [18]. Sc addition promoted fibrous structure of eutectic silicon and reduced grain size. Sc addition also resulted in smaller and more dispersed porosity across the samples compared to that of unmodified samples. Sc addition in Al–Si alloy changed the eutectic growth to mode III, which was similar to Na addition. Thus, Sc redistributed the porosities, with a few small porosities that were scattered around the cross section of the samples. In addition, the percentage of porosity area at the hot spot was reduced to 6.85 and 5.01 % for 0.2 and 0.4 %Sc addition, respectively.
-
4.
Once the Sc addition reached 0.4 wt%, the τ phase (AlSi2Sc2) was clearly found. It is believed that τ phase promotes oxidation during casting which eventually leads to higher porosity count in the casting.
References
Sabatino M. D. and Arnberg L., Trans Ind Inst Met, 62(4–5) (2009) 321.
Ware T. N., Dahle A. K., Charles S., Couper M. J., Effect of Sr, Na, Ca & P on the castability of foundry alloy A356.2, ASM Materials Solutions 2002 Conference and Exposition, 2nd International Aluminum Casting Technology Symposium, Columbus, Ohio, USA, (2002) p. 159.
Gruzleski J. E., Bernard C. M., The Treatment of Liquid Aluminium-Silicon Alloys, American Foundrymen’s Society, Inc., USA (1990) p. 63.
Arbenz H., Lunkerneigung von Aluminium-Guslegierungen, Giesserei (1962) p. 105.
Dinnis C. M., Otte M. O., Dahle A. K., Taylor J. A., Metall. Mater. Trans. A, 35A (2004) 3531.
Tiedje N. S., Taylor J. A., Easton M. A., Metall. Mater. Trans. A, 43 (2012) 4846.
Toropova L. S., Eskin D. G., Kharakterova M. L., Dobatkina T.V., Advanced Aluminum Alloys Containing Scandium: Structure and Properties, Baikov Institute of Metallurgy, Moscow, Russia (1998) p. 133.
Prukkanon W., Srisukhumbovornchai N., Limmaneevichitr C., J. Alloys. Compd., 477 (2009) 454.
Prukkanon W., Srisukhumbowornchai N., Limmaneevichitr C., J. Alloys. Compd., 487 (2009) 453.
Patakham U., Kajornchaiyakul J., Limmaneevichitr C., J. Alloys. Compd., 542 (2012) 177.
Argo D. and Gruzleski J. E., AFS Trans., 96 (1988) 65.
Nogita K., McDonald S. D., Zindel J. W., Dahle A. K., Mater. Trans., 42 (2001) 1981.
Nogita K., Knuutinen A., McDonald S. D., Dahle A. K., J. Light. Met., 1 (2001) 219.
Dahle A., Nogita K., McDonald S., Zindel J., Hogan L., Metall. Mater. Trans. A, 32 (2001) 949.
Lu L., Nogita K., Mcdonald S. D., Dahle A. K., Eutectic solidification and its role in casting porosity formation, JOM, (2004) p. 52.
Lu L., Nogita K., Dahle A. K., Mater. Sci. Eng. A, 399 (2005) 244.
McDonald S. D., Nogita K., Dahle A. K., J. Alloys. Compd., 422 (2006) 184.
Pandee P., Gourlay C. M., Belyakov S. A., Ozaki R., Yasuda H., Limmaneevichitr C., Metall. Mater. Trans. A, 45A (2014) 4549.
Campbell J., Tiryakioğlu M., Mater. Sci. Tech., 26 (2010) 262.
Miresmaeili S. M., Campbell J., Shabestari S. G., Boutorabi S. M. A., Metall. Mater. Trans. A, 36A (2005) 2341.
Kim M., Hong Y., Cho H., Metals. Mater. Int. (2004) 10(6):513.
McDonald S. D., Dahle A. K., Taylor J. A., StJohn D. H., Metall. Mater. Trans. B, 35B (2004) 1097.
Moustafa M. A., J. Mater. Proc. Tech., 209 (2009) 605.
Acknowledgments
This work was financially supported by the National Research University Project of Thailand’s Office of the Higher Education Commission. We graciously acknowledge the Royal Thai Government Scholarship (Ministry of Science and Technology) for Mr. Kongkiat Puparattanapong for his Ph.D. study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Puparattanapong, K., Limmaneevichitr, C. Effect of Scandium on Porosity formation in Al–6Si–0.3 Mg Alloys. Trans Indian Inst Met 69, 1587–1594 (2016). https://doi.org/10.1007/s12666-015-0732-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-015-0732-4