Abstract
High velocity oxide fuel sprayed NiCrAlY coatings on the Superni 76 superalloy have been oxidized cyclically at 900 °C up to 200 cycles. Weight change measurements were taken after each cycle to obtain kinetics of the oxidation process. The surface and cross-section of oxidized samples have been examined by XRD, FESEM/EDS to characterize the corrosion products. The formation of Al2O3 at the surface as well as at the coating/scale interface as a sub-layer was observed. Other oxides such as Ni (Al, Cr)2O4 and Cr2O3 were also present above this Al2O3 sub-layer. An oxidation mechanism has been proposed for the oxidation of the coating for 200 cycles by correlating the results after different cycles of oxidation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Life time prediction of the coatings for any component is required for its successful application. Different life time prediction models have been developed and used to estimate the life time of the coatings [1, 2]. To assess the life time one has to understand the mechanism of degradation in a given environment. The overall mechanism of degradation can be understood by stepwise study where the sample is subjected to oxidising environment for different length of exposure time. By detailed examination of corrosion products after different stages of exposure one can determine the overall mechanism of degradation and can also estimate the lifetime of the component in a given set of environment. The NiCrAlY coatings have been used commercially for protecting the gas turbine engine components from the oxidizing environment. Various researchers have studied the oxidation behavior of these coatings both under cyclic and isothermal conditions [3–8].Formation of slow growing Al2O3 in the scale is found to provide the desired protection against high temperature oxidation. However, formations of other oxides in the scale along with the stable alumina have also been reported. Some researchers have detected the θ alumina in early stages of the oxidation, the formation of which depends on temperature and time of the oxidation [5, 9]. This metastable state transforms itself to stable alumina upon successive heating. The content of Al in the MCrAlY coating is also crucial for the formation of a pure α-Al2O3 scale as a protective layer on the surface to suppress the oxidation [10, 11]. However from the machinability point of view, MCrAlY coatings are usually selected with a low Al (less than 12 wt%) and high Cr content (15–30 wt%) [12]. MCrAlY coatings have been developed by various thermal spray processes. High velocity oxide fuel (HVOF) is widely used technique to develop the bond coat as it is reported to be superior to other thermal spray techniques [13, 14]. Further it is reported that the dispersion of oxide particles formed by the free interaction of powder particles with air during the time of flight leads to improved oxidation resistance of the coatings [15].
It is important to study the degradation of NiCrAlY coatings under severe conditions. The mechanism of isothermal oxidation for NiCrAlY coatings have been also studied with different times of exposure by some researchers [5, 9]. However, there are no stepwise oxidation studies reported on the Ni–21Cr–10Al–1Y coating, under cyclic conditions. Therefore, the present work has been focused to understand the oxidation mechanisms of the NiCrAlY coatings developed by HVOF technique, at 900 °C, as a function of different exposure periods, up to 200 cycles. The oxidized coatings were characterized by XRD and FESEM/EDS to elucidate the oxidation mechanisms under different exposure periods of the coatings.
2 Experimental
2.1 Coating Formation
The Superni76 was procured from M/s Mishra Dhatu Nigam Limited, Hyderabad, India, in the form of rolled sheets from which specimens of desired dimensions were cut. These specimens were polished and grit blasted with alumina prior to coating. NiCrAlY powder with nominal composition (Ni-bal, Cr-21 %, Al-10 %, Y-1 %) obtained from Praxair Inc., US was sprayed by using HVOF gun (JP5000) at Industrial Processors & Metallizers (P) Ltd, New Delhi, India. The parameters used to deposit the NiCrAlY coatings have been given in Table 1.
2.2 Oxidation Studies
The coated samples were weighed with alumina boat and put in the constant temperature region of the furnace which was maintained at 900 °C. After 1 h boat with the samples were taken out from the furnace and cooled in air for 20 min by which time it came to the ambient temperature and samples with alumina boat was again weighed. Two samples of the coated alloy were subjected to cyclic oxidation for 200 cycles with weight change being noted after each cycle. A curve between specific weight change and no. of cycles was then obtained from the weight change measurements from the two samples at a difference of each cycle.
To understand the mechanism of the oxidation at different stages, six coated samples were kept in the furnace and were subjected to cyclic oxidation test at 900 °C. One sample each was withdrawn after 5, 15, 30, 50, 75, 100 and 200 cycles respectively to understand the stepwise formation of the scale.
2.3 Characterisation Techniques
The surface and cross-section of the samples exposed to 200 cycles of oxidation were examined for morphological and compositional details by means of FESEM with attachment of EDS (FEI Company, Quanta 200F) and X-ray mapping technique. For the cross-section study the samples were cut by means of Low Speed Diamond cutter (MS-10, Ducom Instruments Pvt. Ltd.). The phase analysis was done by using the X-ray Diffraction (Bruker AXS D-8Advance Diffractometer with CuKα radiation). Samples withdrawn after exposure of 5, 15,30,50,75 and 100 cycles were also subjected to similar analysis.
3 Results
3.1 As Sprayed Coating
The surface morphology and composition of the as sprayed coatings is shown in Fig. 1a. Some melted and unmelted particles of the powder can be seen on the as sprayed surface. The EDS results show the presence of some oxygen in the coating. The cross-sectional image shows the presence of some oxide particles and some amount of porosity as shown in Fig 1b. The coating thickness was ~220 μm.
3.2 Oxidation Kinetics
The curve between specific weight change and number of cycles for each cycle is shown in Fig. 2. There is an accelerated oxidation in a narrow region up to ~15 cycles after that the rate gradually tapers off and a power law is followed with power equal to 0.2708. The best fit of the curve has been drawn and it is found that the curve does not follow the parabolic rate law but it is governed by following equation:
where, Y is the specific weight change (g/cm−2), X is the time (h)
Majority of the plot follows the above equation but there are deviations which can be seen in the curve. This indicates that there are steps at around 15th, 50th, 80th and 130th cycles. These steps may be related to the change in compositional and microstructural features of the oxides as described in next sections. The scale remained intact, adherent and crack free and no spallation was seen even after 200 cycles of oxidation as shown in Fig. 3.
3.3 X-ray Diffraction Study
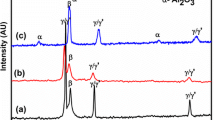
The XRD pattern of as received coated samples and oxidized samples after different no. of cycles is shown in Fig. 4. As sprayed coating exhibit the Ni3Al and NiAl phase. The different oxide phases which are observed after different periods of oxidation cycles are: α-Al2O3, Ni (Al, Cr)2O4 and Cr2O3. The smaller intensity of the oxide phases as compared to the Ni3Al and NiAl phases indicates the excellent oxidation resistance of the coating. After 15 cycles, the major oxide was a spinel Ni (Al, Cr)2O4 with some peaks of Alumina. Al2O3 peaks continued to grow and it became a major phase after 50 cycles. Some of Cr2O3 peaks were also observed after 50 cycles.
3.4 SEM/EDS Analysis of Oxides
The surface morphology of the oxides grown on the coating is shown in Fig. 5. After 15 cycles of oxidation, some needles started appearing which can be seen only at higher magnification and after 50 cycles, this morphology became very distinct. After 100 cycles, oxide nodules without needles were observed. The surface composition of the major coating elements at various times has been summarised in Table 2. The Al concentration on the surface increases up to 50 cycles and after that it becomes almost constant up to 200 cycles. The cross-sectional SEM images and mapping of the scale are shown in Figs. 6 and 7. After five cycles, an oxide layer which is rich in Al and Cr has formed as shown in Fig. 7a. There are some areas in which Al2O3 stringers have started forming below the coating/scale interface (Fig. 6a). The Al2O3 stringers continue to form inside the coating, below the outer oxide scale in the subsequent cycles. After 30 cycles, the discontinuous layer of Al2O3 has formed (Fig. 6b) which becomes almost continuous after 75 cycles (Fig. 6c). After 100 cycles, the distinct alumina sub-layer can be observed, which has grown below the outer oxide layer (Figs. 6d, 7b). The outer surface of the scale consisted of some oxidized and unoxidized areas. The X-ray maps of the cross-section after 100 cycles clearly indicate that the coating is getting oxidized internally as O is migrating inward through the coating and oxidizing Al at the coating/scale interface. Some Cr-rich regions are observed in the upper most layers adjacent to the Al2O3 sub-layer. Further with the increasing exposure time up to 200 cycles, the Al is getting oxidized internally which is evident from the Fig. 6e and a thicker Al2O3 layer is developed.
4 Discussion
The needle like morphology of the oxides observed up to 50 cycles corresponds to the fast and outward growth of the Al2O3. This type of morphology has also been reported by Toma et al. [9] where they have suggested that formation of these needles supports the mechanism of outward growth of the oxide. These needles appeared only up to 50 cycles and only oxide nodules were observed after 100 cycles. This change in morphology from needles to nodules could be the indication of the change from outward diffusion of Al to purely inward diffusion of oxygen.
The cross-sectional analysis of the scale after five cycles exposure reveal that initially Al and Cr rich scale is formed which grows outward by the diffusion of these elements. The scale consists of mainly Ni (Al, Cr)2O4 and some Al2O3 as revealed by XRD results. There are some regions in which the Al2O3 is formed inside the coating, just below the coating/scale interface as shown in Fig. 6a. This internal oxide has grown by diffusion of oxygen through discontinuous Cr and Al rich scale. This type of behaviour has also been explained by Kear et al. [16] in their study on NiCrAl alloy where they suggested that initially spinels i.e. Ni (Al, Cr)2O4 and NiO grow on the coating surface, then Cr start diffusing and forms a healing layer at alloy/oxide interface below the spinels and NiO layer. This healing layer acts as getter for the formation of stable and slow growing Al2O3 below the interface by the inward diffusion of oxygen. However, in the present case, instead of distinct Cr2O3 layer, a mixed layer of Al2O3 and Ni (Al, Cr)2O4 is formed after five cycles. The inward diffusion of oxygen to form Al2O3 precipitate could also be a consequence of the presence of Y in the coating. Y in the coating is found to retard the outward diffusion of Al and facilitate the inward diffusion of oxygen by modifying electronic structure of Al2O3 by condensation of Al-vacancies at the alloy/scale interface and thus retards the cationic diffusion [17–19]. The excellent spallation resistance to the cyclic oxidation which was observed in present case is also attributed to the presence of Y. It has also been reported by Delaunay et al. [20] that the stresses in the oxide are greatly reduced by adding Y in MCrAl and NiAl coating.
Further, with increasing oxidation time, the Al2O3 continue to form below the external layer of oxides by the inward diffusion of the oxygen shown in Fig. 6b. After 75 cycles, the complete and almost continuous Al2O3 sub-layer has formed Fig. 6c. The X-ray maps after 5 cycles and 100 cycles of oxidation clearly indicate the start and continuous growth of the Al2O3. This Al2O3 sub-layer may be blocking the diffusion of the Al to the surface and the migration of the O into the coating as indicated by the almost negligible weight gain of the coating between 100 and 200 cycles. Some Cr-rich regions just above the Al2O3 sub-layer could be Cr2O3 as detected by the XRD.
After 100 cycles, there was negligible oxygen uptake; however thickness of Al2O3 sub-layer increased up to 200 cycles as shown in Fig. 6d. It means that the Al is getting oxidized internally below the initially grown sub-layer of Al2O3 due to availability of larger Al concentration. This observation is also supported by the similar finding of the fact which was observed by Giggins and Pettit [21] that the initially grown oxides (spinels and Cr2O3) are dissolving to release oxygen which combine with Al to form stable and slow growing Al2O3. In present case, the Cr-rich oxides which were observed just above the Al2O3 sub-layer had got dissolved in alumina layer which is further confirmed by disappearance of Cr2O3 peaks in XRD pattern after 100 cycles. From the above discussion, the oxidation mechanism up to 200 cycles can be divided into four steps as illustrated in Fig. 8.
The first step exists up to five cycles and indicates accelerated weight change due to the formation of spinels and some amount of Al2O3. This corresponds to very fast diffusion of Al and its reaction with O. The second step up to 50 cycles indicates decreased outward diffusion of Al and increased inward diffusion of O resulting in formation of slow growing Al2O3. In the third step these Al2O3 stringers grow into a continuous sub-layer up to 100 cycles. The last step refers to growth of Al2O3 layer due to internally available O formed due to dissolution of the Cr2O3 in the Al2O3 sub-layer.
5 Conclusions
Following conclusions can be drawn from the above study:
-
1.
Ni–21Cr–10Al–1Y coating on Superni76 exhibits excellent oxidation resistance to cyclic oxidation up to 200 cycles at 900 °C in air.
-
2.
The mechanism of oxidation of the HVOF sprayed NiCrAlY coatings at 900 °C in air may be divided into four stages:
-
i)
Initially up to ~5 cycles, spinels and alumina start forming and growing on the surface by the outward diffusion of the elements. This step is the fastest step of the oxidation.
-
ii)
Further increase in exposure time up to 50 cycles, Al2O3 starts forming discontinuous sub-layer below coating/scale interface by inward diffusion of oxygen.
-
iii)
Between 50 and 100 cycles, the discontinuous Al2O3 becomes continuous sub layer.
-
iv)
Last step from 100 to 200 cycles is the slowest step, which corresponds to the dissolution of Cr2O3 to form Al2O3.
-
i)
References
Smialek J L, Acta Mater 51 (2003) 469.
Smialek J L, and Auping J V, Oxid Met 57 (2002) 559.
Puetz P, Huang X, Yang Q, and Tang Z, J Therm Spray Technol 20 (2011) 621.
Huang X, Puetz P, Yang Q, Tang Z, Surf Eng 27 (2011) 368.
Li M H, Zhang Z Y, Sun X F, Li J G, Yin F S, Hu W Y, Guan H R, and Hu Z Q, Surf Coat Technol 165 (2003) 241.
Mahesh R A, Jayaganthan R, and Prakash S, Surf Eng 27 (2011) 332.
Higuero V, Belzunce, F J and Riba J, Surf Eng 25 (2009) 319.
Brandl W, Toma D, Krüger J, Grabke H J, Matthäus G, Surf Coat Technol 94–95 (1997) 21.
Toma D, Brandl W, Koster U, Oxid Met 53 (2000) 125.
Czech N, Schmitz F, Stamm W, Surf Coat Technol 76–77 (1995) 28.
Hutchinson J W, Evans A G, Surf Coat Technol 149 (2002) 179.
Warnes B M, Surf Coat Technol 163–164 (2003) 106.
Taheri M, Valefi Z and Zangeneh-Madar K, Surf Eng 28 (2012) 266.
Ferdinando M D, Fossati A, Lavacchi A, Bardi U, Borgioli F, Borri C, Giolli C and Scrivani A, Surf Coat Technol 204 (2010) 2499.
Toma D, Brandl W and Ko¨ster U, Surf Coat Technol 120–121 (1999) 8.
Kear B H, Pettit F S, Fornwalt D E, and Lemaire L P, Oxid Met 3 (1971) 557.
Ramanarayanan T A, Raghavan M, and Petkovic-Luton R, Oxid Met 22 (1984) 83.
Young E W A and Wit J H W, Oxid Met 26 (1986) 351.
Heuer H, Hovis D B, Smialek J L, and Gleeson B, J Am Ceram Soc 94 (2011) 146.
Delaunay D, Huntz A M, and Lacombe P, Corros Sci 20 (1980) 1109.
Giggins C S, and Pettit F S, J. Electrochem Soc 118 (1971) 1782.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rana, N., Jayaganthan, R. & Prakash, S. Stepwise Oxidation Mechanism of HVOF Sprayed NiCrAlY Coatings in Air. Trans Indian Inst Met 67, 393–400 (2014). https://doi.org/10.1007/s12666-013-0362-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-013-0362-7