Abstract
A study of trace elements using Instrumental neutron activation analysis was carried out. The purpose of study was to determine the concentration of trace elements, naturally occurring radionuclides and pollution Indices. The study Shawa-01 Oil Well is located in Kohat Basin, Khyber-Pakhtunkhwa, Pakistan. The 21 samples of Shawa-01 Well were provided by the Oil and Gas Development Company Limited (OGDCL). 20 trace elements were determined by NAA. Standard reference materials IAEA-SL1 (Lake Sediment) and IAEA-S7 (Soil) were used to assure the quality of results. Shawa-01 Well rock extracts contain trace elements like As, Ce, Co, Cr, Cs, Eu, Hf, K, La, Mn, Rb, Sb, Sc, Sm, Ta, Th and V. Al was found to be higher as compared to other world regions due to clay contents. Other elements were found to be in comparable range. Annual Dose rates were found to be in the safe limit which implies a safe working environment. Pollution Indices, like Enrichment factor (EF), geo-accumulation index (Igeo), Pollution Index (PI) and Integrated Pollution Index (IPI) were also calculated. Elements like Sb, As and Cr show pollution levels above safe limits which may be due to contamination of soil from different sources with the passage of time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Trace element content of oil field rock extracts has been investigated in different countries. It has been an active area of research for a long time. Elemental composition information is helpful in many areas such as exploration, environment and refining (Oluwole et al. 1993). Trace element concentration values and relationship of these concentrations with one another can be used for exploration of oil fields. Such data can also be used for soil-source rock and oil-source rock correlations and can provide useful information on the origin of oil (Cariale 1984; Hitchon et al. 1975). Frankenberger (Frankenberger 1994) analyzed the trace element concentrations of the Taranaki Basin of New Zealand. INAA was chosen as the analytical method because analysis could be done nondestructively on samples without pretreatment and with rapid turnaround times. Man power requirements are small and could be reduced even further by automation of the counting and reporting operations (Kinsley et al. 1996). A large range of elements can also be quantified by neutron activation analysis with high sensitivity. Therefore, this technique was employed in this work. The study was designed to determine trace element contents and its distribution in well cuttings; particularly in source formation, as depth of well changes. This is the first study of trace element concentrations carried out in the Kohat Sub-basin.
In northern Pakistan, Kohat Basin consists of a complex tectonic area or plateau, with moderate to steeper dips and disproportionate structures formed by a large number of faults and thrusts. It is thought to have been formed by transgressional tectonic, based on salt affected or cellar involved thrust or reverse faulting (Paracha 2001). Due to the complexity of this region, the Kohat Basin of Khyber-Pakhtunkhwa (KPK), Pakistan was selected for hydrocarbon and oil exploration study using Neutron Activation Analysis (NAA). NAA has vast applications in research including hydrocarbon and crude oil exploration. In the current study, Oil and Gas Development Corporation Limited (OGDCL) provided the samples. OGDCL is the largest petroleum exploration and production company in Pakistan.
Humans are exposed to natural radioactivity which is considered as the major source of radiation and it represents more than 75% of all ionizing radiation (UNSCEAR 2000). Naturally occurring radionuclides commonly known as NORM compose the majority of the dose received by a person each year at approximately 80% of the total amount. It is approximately 150 years since oil was discovered in the continental United States and the mention of radioactivity in mineral oils and natural gases occurred in 1904, just 8 years after the discovery of radioactivity by Henri Becquerel in 1896. The problems arising from naturally occurring radioactive material wastes from oil and gas industry needs to be scrutinized. Uncontrolled release of activities associated with enhanced levels of NORM can contaminate the environment and pose a risk to human health. These risks can be alleviated by the adoption of controls to identify the source of NORM, and by control of NORM contaminated equipment and waste while protecting workers. Radioactive materials such as Uranium and Thorium occur in trace concentrations in rock formations. Decay of these unstable radioactive elements produces other radionuclides that, under certain conditions (dependent on pressure, temperature, acidity, etc.) in the subsurface environment, are mobile and can be transported from the reservoir to the surface with the oil and gas products being recovered. The level of NORM accumulation can vary substantially from one facility to another, depending on geological formation, operational and other factors (Landsberger et al. 2014). Therefore, naturally occurring radionuclides were also measured in this study using Gamma Spectrometry. Industrial waste water discharged from the industries such as textile dyeing, petroleum, metal finishing, automobile, electro-plating, and leather tanning cause heavy metals entering into the lifecycle. Therefore, this becomes one of the most important environmental problems in the world (Magdi et al. 2020). Heavy metals can also be investigated for Shawa-01 well rock samples, because ultimately, it will affect the environment during handling.
Geology of Kohat region
The sedimentary basin in Pakistan is divided in to two main basins; (1) Indus Basin and (2) Baluchistan Basin. These basins have developed through different sediment logical and geotectonic events. The Indus Basin is further subdivided into Upper and Lower Indus Basin by Sargodha High due to geological and tectonic processes. Kohat and Potwar Sub-basins are further sub-divisions of the Upper Indus Basin. Kohat Basin is separated from the Potwar Basin by the Indus River (Malik et al. 2014).
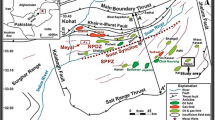
Location of Kohat Basin is shown on the map in Fig. 2. It is not possible to investigate all of the Kohat area in this study because of the large number of wells present there. Different wells were selected from four directions; i.e. one well from the East, one from the West, one from the North and one from the South. In this paper, the data obtained for the Shawa-01 Well is presented and discussed in detail. Shawa-01 Well was selected keeping in view the geology of the Kohat Basin and the well’s geological oil source formation. This well is situated at Shawa Village in Lower Dir District, KPK, Pakistan and is located at 33o14′ 02.68″ N; 71o03′ 53.72″ E in the Kohat Sub-basin. Shawa-01 Well was drilled by the OGDCL, Pakistan. This well is an exploratory well of OGDCL and is still under drilling. The Shawa-01 Well has been drilled up to a depth of 4437 m till now. Its target depth is 5550 m (Pakistan Petroleum Information Services 2016).
Methodology
Methodology used is shown in Fig. 1.
Sample collection
At the time of sample collection, the OGDCL had collected and processed samples up to a depth of 3600 m. Other samples are under process of transportation, washing and drying. Samples were washed with kerosene oil to make the samples fluid free. Samples were collected in dry form. Each sample was collected in a clean polythene bag. Weight of each sample taken was 100 ± 10 g. Samples were collected from different depths according to geological formations as shown in Table 1. Each sample was taken in a band of 5 m depth to cover relevant formations. Samples consist of soil and non-uniform pieces of ditch cuttings. Samples were ground and sieved with a 305 size mesh to homogenize grain size. Grain size for 305 mesh is < 40 µm. This grinding was necessary to obtain homogenized samples for NAA.
Neutron activation analysis (NAA) procedure
Sample preparation for NAA
Homogenized solid samples were obtained after crushing, grinding and sieving. The samples listed in Table 1, in duplicate, along with 2–3 standard reference materials (SRMs), were used for analysis. Samples were encapsulated in labeled clean capsules made of polyethylene. These capsules were further packed in polyethylene rabbits. These rabbits were thermally sealed and labeled prior to irradiation.
Irradiation schemes at PARR-II
The prepared rabbits were irradiated for 30 s, 1 h and 5 h for the sequential/short, intermediate and long irradiation protocols, respectively, at the Pakistan Research Reactor II (PARR-II) irradiation facility. These irradiation protocols, as given in Table 2, have been devised by consideration of the nuclear properties of the elements being studied. PARR-II is a miniature neutron source reactor (MNSR) and was operated at a power of 30 kW and has a neutron flux of 1 × 1012 n/sec.cm2 (Fig. 2).
An element is detected by NAA, if the selected isotope has a significant neutron activation cross-section and a high abundance. The gamma ray which is used to obtain quantitative results should be selected so that its probability of emission is highest and there are no interfering peaks. These two parameters were achieved by consideration of the nuclear data.
A suitable decay time is provided after irradiation for safe handling of the samples or to allow shorter lived radionuclides to decay. The measurements were done using a Canberra model (AL-30) high purity Germanium detector (HPGe). The detector was attached to a PC-based multi-channel analyzer (InterTechnique model pro-286e) through a sensitive spectroscopy amplifier (Ortec model 2010). Intergamma, version 5.03 software was used for data acquisition. The system resolution for the 1332.5 keV peak of 60Co is 1.9 keV. Different computer programs were used to acquire spectra (Journal of Radioanalytical and Nuclear Chemistry 1993). The counting system is calibrated daily using calibrated γ-ray sources.
Spectra were analyzed using Gamma Cal software (Wasim 2010). This software is based on visual basics and is used for obtaining quantitative results. It can be used for sequential/short, intermediate and long irradiation schemes as well as for radionuclide measurements. Decay factors and background correction are incorporated in the calculations and are obtained from nuclear data libraries in this software.
Standards used
To perform quantitative analysis standard reference materials (RMs) were used. These were selected so that the matrix material matched the sample matrix. Furthermore, it was also desirable that the RMs contained a large number of elements with certified values. Details of the standards used are given in the table below (Table 3);
Radionuclide measurement procedure
Samples of Shawa-01 Well as given in Table 1 were also investigated for their naturally occurring radionuclides content. The Standard Reference Materials used were soil samples; SO0103 and SO0303, which were obtained during the Environmental Measurement Laboratory (EML) of U.S. DOE’s Quality Assessment Programme (QAP) conducted from March 1998 (QAP 48) to May 2004 (QAP 60).
The area surrounding the detector and its shielding were cleaned before starting the measurements of Shawa-01 Well samples. All the covers of work surfaces were replaced and background and blank spectra obtained. Each sample was sealed in clean, pre-weighed and labeled polythene container. Identical containers were used for samples, standards and blank and efforts were made to keep the depth of every sample and standard in the container the same. Samples/standards were sealed and stored for around a month to obtain secular equilibrium. The gamma spectrometry system used to obtain the spectra for each sample/standard and blank was the same as that used for elemental analysis using NAA. All spectra were obtained under identical conditions. The counting time was 16 h and 3 replicate spectra were obtained for each sample.
Results and discussion
Quality assurance data
SRMs from IAEA, namely IAEA-SL1 (Lake Sediment) and IAEA-S7 (Soil), were used to assure the quality of results. Comparison of results obtained with the IAEA certified data is shown in Table 4 for both standards.
In the 21 samples of Shawa-01 Well {Sw-1, Sw-2, Sw-3…sw-pnb-6} analyzed, 23 elements were determined. In Table 5, only those elements are presented which were measured in all samples due to space constraints. For the same reason, the measurement uncertainties have not been shown but are similar to those given for the QA materials. Samples are grouped according to depth of the exploratory Shawa-01 Well. Table 5 shows the presence of trace elements but no clear cut relationship like that observed in New Zealand rock extracts (Frankenberger 1994) was found. Shawa-01 Well rock extracts contain trace elements such as As, Ce, Co, Cr, Cs, Eu, Hf, K, La, Mn, Rb, Sb, Sc, Sm, Ta, Th and V.
Yb was measured in some samples but not in all samples, so this element is excluded from data. Al, Ba, Fe, K and Mg were measured in higher amounts which are to be expected in soil samples. Na may be present due to salt content. The elements of interest are S, As, Sb, Ni, from refining and environmental considerations (Oluwole et al. 1993). However, sulphur and Ni cannot be measured using conventional NAA, while As and Sb were measured in all 21 samples.
Trace element concentrations vary as depth of Shawa-01 Well changes. Variation of trace elements in the original source rock actually represents the variations in trace elements concentrations of oil samples as shown in the study of New Zealand oil fields (Frankenberger 1994). It means that it is not possible to develop any direct relationship with source rock trace element concentrations to trace element concentrations of oils for New Zealand oil fields. China oil samples and source rocks are rich in Cl and Br. Br and Cl may be present due to salt contents but Yemen oil fields are not rich in these elements (Frankenberger 1994).
Finally, we compared the trace element concentrations of Shawa-01 Well with source rock extracts of other regions of the world. Panoba formation is the source formation and source formations provide information about origin of oil. Therefore, comparison has been made between samples of Panoba formation and samples from Tranaki Basin and Yemen rock extracts as shown in Table 6. Waipawa Shale, Maui-4 coal, Kapuni # 2 coal sample, Toko-1 sample C and North Cape coal samples were taken from different areas of New Zealand’s Taranaki Basin (Frankenberger 1994). Comparison of these samples showed that:
-
1.
Al is higher in Kohat Basin rock extracts as compared to other regions of the world like Yemen rock extracts and New Zealand coal extracts of Taranaki Basin. This is due to high clay contents in Kohat Basin.
-
2.
Arsenic concentration in Panoba formation ranges from 7.01 to 35.41 ppm which is almost comparable to New Zealand rock extracts and Yemen rocks.
-
3.
Some trace elements such as Ce, Hf, K, La, Ta and Th are present in Shawa-01 Well rock extracts but these elements are not reported in Yemen rock and coal extracts of New Zealand Taranaki Basin. This difference may be due to geological reason or these elements may not have been measured in these studies. Geological aspects of one area of world differ from other areas of the world.
-
4.
Coal extracts of Taranaki Basin of New Zealand shows the presence of Br, Cl and Zn. Yemen rock extracts also contain Bromine and chlorine in the same concentration as New Zealand extract. The presence of these trace elements is probably due to salt contents of rock samples.
-
5.
Eu shows little variation with a concentration range of 0.2 to 1.98 ppm in Shawa-01 samples. Table 6 shows that New Zealand coal extracts and Athabasca oil sand also have little variation in Eu concentrations. Other elements, in Table 5, show little variation include Ce, Cr, Cs, Hf, Sc, Sm, Ta, Th and La.
-
6.
Vanadium concentrations were higher in rock extracts of Shawa-01 Well of Kohat-Sub Basin than Yemen rock extracts, Waipawa Shale, Maui-4 coal, Kapuni # 2 coal sample, Toko-1 sample C and North Cape coal. This indicates that deposition environment is high in vanadium in Kohat Sub Basin and favorable conditions are present for the complexing of Vanadium in clay mineral and in organic compounds in Kohat Sub-Basin.
Athabasca oil sand which consists of mixture of solids and bitumen was also investigated by INAA. Eleven elements (Al, K, Na, Ti, Mn, Cl, Dy, Sm, Eu, Ba and V) were determined with reasonable precision in the 23, 0.5 g oil sand test portions using INAA (Kinsley et al. 1996). Al, Eu, Mn, Na, Sm and V have comparable values with Athabasca oil sand.
Radionuclide results
Activity concentration
The radiation exposure to human beings due to the activity concentrations of 226Ra, 232Th and 40 K were assessed considering the time of stay in the radiation area. The exposure is of two types; outdoor and indoor. The outdoor exposure is assessed for \(\upgamma \)-rays emitted by terrestrial materials and for the time of stay of a person in the outdoor. For outdoor exposure, the radiation indices namely radium equivalent activity (Raeq), outdoor external dose (Dout) and annual outdoor effective dose (Eout) were calculated. For the measurement of indoor hazard of radioactivity, indoor external dose (Din) and annual indoor effective dose (Ein) were calculated. Procedures adopted during present study for the calculation of outdoor and indoor radiation indices and annual effective doses are given below.
Radiological assessments
Indoor hazard indices
Radium Equivalent (Raeq) was calculated using the following equation (Beretka et al.1985; Ibrahim 1999; Mollah et.al 1986; El-Taher 2010; UCS 30).
Here, ARa,, ATh and AK are the activity concentrations of 226Ra, 232Th and 40 K, respectively.
Outdoor External Dose (Dout) in nGyh−1 was estimated using the following equation (UNSCEAR 2000):
Annual Outdoor Effective Dose (Eout) was estimated using the following equation (UNSCEAR 2000):
Indoor External Dose (Din) was estimated using the following equation by (EC 1999):
Here, 0.5 is taken as half the time the population in that area would spend at home.
Annual Indoor Effective Dose (Ein) was estimated as per (UNSCEAR 2000).
Excessive life time cancer risk (ELCR)
The excessive life time cancer risk was calculated using the following relation:
Here, ELCRout and ELCRin are the excessive life time cancer risks for outdoor and indoor exposures, respectively. LE is the life time expectancy and is taken as 66 years. RF is the fatal risk factor per Sievert and is 0.05 as per (ICRP 60) (Tables 7, 8).
The value of Ra-226 ranges from 5.4 ± 1.3 to 1125.4 ± 510.1 Bq. kg−1, Th-232 was below detection limit, because it was found only in one sample Sw-6 and K-40 in the samples ranged from 26.1 ± 6.7 to 1206.8 ± 153.5 Bq. kg−1. The average values of Ra-226, Th-232 and K-40 for Murree formation (Sw-1–Sw-15) were 49.4 ± 41.3, 17.4 ± 0.0 and 394.0 ± 109.3, respectively. These values are lower as compared to the world average apart from the value for K-40 which is higher due to large clay contents. The average values of Ra-226 and K-40 for Panoba formation (sw_pnb_1 to sw_pnb_6) are 845.6 ± 460.3 and 703.4 ± 89.5, respectively. Th-232 was below detection limit. These values are comparable to radionuclide values of source formations of oil well of Az Zubair oil field, Basra, Iraq given in Table 9 (Sahar 2016). Panoba formation is a source formation, higher concentrations of radionuclides like Ra-226 are expected as oil and gas reserves are known to accumulate radionuclides in higher concentrations (Smith et al. 1999; Arogunjo et al. 2004; Avwiri et al. 2007).
Radionuclides detected in the well cuttings are naturally occurring radionuclides. These were not anthropogenic radionuclides like Cs-137 (Jibiri et al. 2011). Therefore, it was safe for the working staff and for handling oil in the refinery.
The average values of absorbed dose rates Dout and Din were 140.14 and 277.58 nGy h−1, respectively. The effective dose rate Eout and Ein average values were 0.171 and 1.362 mSv y−1. The total effective dose rate was 1.53 mSv y−1. The recommended annual dose limit for the public (UNSCEAR 2000) is1 mSv y−1. The outdoor effective dose rate was less than 1 mSv y−1 which was 0.171 mSv y−1. Consequently, these samples are safe to handle.
It is clear from Table 8 that the activities of radionuclides are independent of the well depth. Every formation rocks may have its unique properties which may affect the concentration of radionuclides (Alharbi et al. 2013). It depends on lithological variations as evident from the study of activity concentration of the radionuclides for drilling mud samples of ZB-269 oil well in Az Zubair oil field using gamma ray spectrometry. The average values of activity concentration for this oil well were greater than the recommended values (Sahar 2016).
The cumulative average lifetime cancer risks due to the radionuclides are lower than 1: 10,000 levels for contribution from a single source activity concentration (Sahar 2016). These values are low and do not affect the employees’ health and environment like refinery operation, transportation and use of these soils in construction. However, if well cuttings from the oil fields exceed the limit, proper disposal methods should be adopted according to the regulatory requirements of Pakistan Nuclear Regulatory Authority (PNRA).
Pollution studies through calculations of pollution indices
The Enrichment Factor (EF) and Geo-accumulation Index (Igeo) Eqs. (8) and (9) are indicators used to assess the presence and intensity of anthropogenic contaminant deposition on surface soil. These indices of potential contamination are calculated by the normalization of one metal concentration in the top soil with respect to the concentration of a reference element (Maurizio B 2016). A reference element is an element particularly stable in the soil, which is characterized by the absence of vertical mobility and/or degradation phenomena. The constituent chosen should also be associated with finer particles (related to grain size), and it should not be possible to change its concentration by anthropogenic means. Typical elements used in many studies are Al, Fe, Mn, Rb and Sc, while total organic carbon and grain size are also used. Aluminum is a conservative element and a major constituent of clay minerals, and it has been used successfully by several scientists (Ackerman 1980).
Here, RE is the concentration of metal, adopted as Reference Element. The numerical results are indicative of different pollution level. Values of 0.5 ≤ EF ≤ 1.5 suggest that the trace metal concentration may come entirely from natural weathering processes (Zhang et al. 2002). However, an EF > 1.5 indicates that a significant portion of the trace metals was delivered from non-crustal materials so, these trace metals were delivered by other sources, like point and non-point pollution (Zhang et al. 2002; Yongming et al. 2006; Klerks et al. 1989; Sutherland et al. 2000). With EF index, quality of well cuttings can be assessed as suggested in Table 10 (Yongming et al. 2006).
Enrichment Factors were calculated for Shawa-01 well with respect to Aluminum and results are given in Table 12. Values between 2 and 5 are in bold. The values which are double underlined show EF between 5 and 20. Values above 20 and below 40 are in bold and single underlined. Most values of enrichment factor are in the range of 5 and 20 for all Shawa-01 well samples of Kohat Basin. It shows that samples are significantly enriched in trace element at this site. Few elements are above 40 such as, Sb and Cr which shows extremely high enrichment in all samples. Enrichment Factor for some elements like Ta, Th and V only have values in Panoba Formation samples. This simple analysis shows this site to be significantly polluted by As, Sb and Cr.
The Geo-accumulation Index (Igeo) is defined as shown in Eq. (9) (Muller 1979).
Here, Cn is the measured concentration of the element in soil dust, Bn is the geochemical background value and the constant 1.5 allows us to analyze natural fluctuations in the content of a given substance in the environment and to detect very small anthropogenic influence. Geo-accumulation Index values are classified in seven classes as shown in the Table 11 (Muller 1981).
Geo-accumulation Index (Igeo) values were calculated for all the samples of Shawa-01 well and results are shown in Table 12. Most of the values in the Table 13 are less than zero or near to zero. Igeo values between 1 and 2 are in bold while the Igeo values above 2 are underlined. Igeo values of Sb in most of the samples are above 2 and Cr has Igeo value above 2 in 2 samples; Sw-11 and Sw-15. The well cuttings of Shawa-01 well are moderate to heavily contaminate in Sb. Igeo values of Sb for Panoba formation samples (sw-pnb-3 to sw-pnb-6) are near to 4. It means Panoba formation is heavily contaminated in Sb due to the presence of oil and gas reservoirs near source formation. Igeo values between 1 and 2 are observed in some samples which shows that they are moderately contaminated due to As, Cr and Sb.
Pollution Index was calculated using Eq. 10:
Here, Cn in measured concentration and Bn is the background concentration. Pollution Index (PI) classification is given in Table 14 (Tokalioglu et al. 2006).
Shawa-01 well samples were further evaluated for pollution. Pollution Indices were calculated for the Shawa-01 Well and the results are given in Table 15. In Table 15, PI values above 3 are double underlined and the values between 1 and 3 are in bold and single underlined. Most of the PI values of Shawa-01 well samples are below 1. Some elements such as, Cr and Sb show PI values greater than 3. Sb and Ta are measured near their detection limits. Therefore, these elements have higher measurement uncertainties (Faiz et al. 2013). PI values of As, Cr, Cs, Sm, and Th in some samples are between 1 and 3 which shows middle level pollution. From Table 14, it is clear that As and Cr are the main elements of pollution concerned. This may be due to migration of oil and gas from different sites which may have higher pollution activity. Integrated Pollution Index (IPI) is defined as the mean value of pollution Index and is classified as given in Table 16 (Chen et al. 2005).
The IPI values were calculated and are also given in Table 15. The IPI values above 2 are in bold and double underlined, while the values between 1 and 2 are single underlined. The IPI value of Sb is highest among all other elements. Elements such as As and Cr also have IPI values greater than 2. It means that these elements show high level of pollution. Elements such as Mn and Sm have IPI values between 1 and 2 which shows middle level pollution. The IPI values of all other element are less than 1.
Conclusions
In this study of source, rock samples collected from Shawa-01 well were analyzed. INAA has been successfully employed to measure the trace elements such as Al, As, Ce, Co, Cr, Cs, Eu, Fe, Hf, K, La, Mn, Na, Rb, Sb, Sc, Sm, Ta, Th and V and comparison was made with world other regions. It is concluded that Shawa-01 well shows heterogeneous behavior with variation in its elements composition. This study shows that the developed INAA technique can be used to determine the elemental composition of rock samples of any oil well where minor differences can show major changes in the environment. It provides information about the elements present in the source rocks of oil well which will be helpful during drilling, processing and handling. Measurement of natural radioactivity level can also provide useful information for environmental studies. Calculation of Pollution indices from trace element concentrations shows that this area is moderately to highly pollute by As, Cr and Sb.
Future recommendations
Shawa-01 Well is an exploratory well, therefore, at the time of collection of samples trace elements study of crude oils could not be conducted. Further NAA may be applied to the elemental analysis of rock extracts from other oil fields in Pakistan. A relationship can be established to find origin of the crude oil by comparing measured trace elements contents of crude oils and rock extracts. Trace elements determination using neutron activation analysis can be used to find the age and source of oil fields in Pakistan by comparing the elemental concentration of same source rocks of different oil fields of different areas and same areas. But this is not included here, because this area of study requires large data collection of various oil fields and coal extracts and their analysis which is again a separate project (Tables 12, 13, 14, 15, 16).
References
Ackerman F (1980) A procedure for correcting the grain size effect in heavy metal analyses of estuarine and coastal sediments. Environ Techno Lett 1:518–527. https://doi.org/10.1080/09593338009384008
Alharbi WR, Abbady AGE (2013) Measurement of radon concentrations in soil and the extent of their impact on the environment from Al-Qassim. Saudi Arabia Nat Sci 5:93–98. https://doi.org/10.4236/ns.2013.51015
Amin SA (2016) Assessment of naturally occurring radioactive material (NORM) in the oil drilling mud of Az Zubair oil field, Basra Iraq. Environ Earth Sci 75:769. https://doi.org/10.1007/s12665-016-5564-y
Arogunjo AM, Farai IP, Fuwape IA (2004) Impact of oil and gas industry to the natural radioactivity in the delta region of Nigeria. J Phys 16:131–136
Avwiri GO, Enyinna PI, Agbalagba EO (2007) Terrestrial radiation around oil and gas facilities in Ughelli Nigeria. J Appl Sci 7:1543–1546. https://doi.org/10.3923/jas.2007.1543.1546
Beretka J, Mathew PJ (1985) Natural radioactivity of Australian building materials, industrial wastes and by- products. Health Phys 48:87–95. https://doi.org/10.1097/00004032-198501000-00007
Cariale JA (1984) Oil/Rock correlation study on North slope of alaska. In: Claypool M (Ed) AAPG studies in geology series No. 20 Tulsa: 203
Chen TB, Zheng YM, Lei M, Huang ZC, Wu HT, Chen H, Fan KK, Yu K, Wu X, Tian QZ (2005) Assessment of heavy metal pollution in surface soils of urban parks in Beijing, China. Chemosphere 60:542–551. https://doi.org/10.1016/j.chemosphere.2004.12.072
Dybczynski R, Suschny O (1979) Final reports on the intercomparison run SL-1 for the determination of trace elements in a Lake Sediment Sample. IAEA/RL/64
EC (1999) Radiological protection principles concerning the natural radioactivity of building materials. Radiat. Protect. Directorate General Environment. Nuclear Safety and Civil Protection, European Commission, Luxembourg 112
El-Taher A (2010) Gamma spectroscopic analysis and associated radiation hazards of building materials used in Egypt. Radiat Protect Dosim 138(2):166–173. https://doi.org/10.1093/rpd/ncp205
Faiz Y, Siddique N, Tufail M (2013) Pollution level assessment of road dust from Islamabad Expressway. The Nucleus 50(3):241–251. https://doi.org/10.1080/10934529.2012.664994
Frankenberger A (1994) Trace elements in New Zealand oils and their significance for analytical chemistry, geochemistry and oil classification.
Hitchon B, Filby RH, Shah KR (1975) The role of trace metals in petroleum. In: Yen TF (Ed.) Ann Arbor Science Publ 11
Ibrahim N (1999) Natural activities of 238U, 232Th and 40K in building materials. J of Environ Radioact 43:255–258. https://doi.org/10.1016/S0265-931X(98)00033-2
ICRP (1991) Publication 60: ann. ICRP 21:1–3
Jibiri NN, Amakom CM (2011) Radiological assessment of radionuclide contents in soil waste streams from an oil production well of a petroleum development company in Warri, Niger delta Nigeria. Indoor Built Environ 20(2):246–252. https://doi.org/10.1177/1420326X10378806
J Radioanal Nuclear Chem (1993) 168(1):145–152
Kinsley KD, Byron K, John MMD (1996) Estimation of the fines content of Athabasca oil sands using instrumental neutron activation analysis. Can J Chem 74:583–590. https://doi.org/10.1139/v96-062
Klerks PL, Levinton JS (1989) Rapid evolution of metal resistance in a benthic oligochaete inhabiting a metal-polluted site. Biol Bull 176:135–141. https://doi.org/10.2307/1541580
Landsberger S, Landsberger SG, Graham G (2014) Radioactive waste in oil exploration. NORM Environ Radioact 1:16–30
Magdi B, Saziye B, Şeniz ÖY (2020) Heavy metal removal investigation in conventional activated sludge systems. Civil Eng J 6(3):470–477. https://doi.org/10.28991/cej-2020-03091484
Malik AM, Ahmed N (2014) Paleocene carbonate of Kohat-Potwar Sub-Basin of Upper Indus Basin Pakistan. Sci Int (lahore) 26(5):2337–2350
Maurizio B (2016) The importance of Enrichment Factor (EF) and Geo-accumulation Index (Igeo) to evaluate the soil contamination. J Geol Geophys. https://doi.org/10.4172/2381-8719.1000237
Mollah AS, Ahmed GU, Husain SR, Rahman MM (1986) The natural radioactivity of some building materials used in Bangladesh. Health Phys 50(6):849–851
Muller G (1979) Schwermetalle in den sedimenten des Rheinse Veranderungen seitt. Umschau 79:778–783
Muller G (1981) Die Schwermetallbelastung der sedimente des Neckars und seiner Nebenflusseeine Bestandsaufnahme. Chem Ztg 105:157–164. https://www.statistik-bw.de/LABI/LABI.asp?ID=3177612
Oluwole AF, Asubiojo OI, Nwachukwu JI, Ojo JO, Ogunsola AJA, Filby RH, Fitzgerald S, Grimm C (1993) Neutron activation analysis of Nigerian crude oils. J Radioanal Nucl Chem 168(1):145–152. https://doi.org/10.1007/bf02040887
Paracha (2001) 2D Seismic interpretation study of near Top Kohat. Pakistan oil fields Limited: 1–32
Pszonicki L, Hanna AN, Suschny O (1984) Report on intercomparison run IAEA/Soil-7 for the determination of trace elements in soil. IAEA/RL/112. http://www.SpectrumTechniques.com/
Smith KP, Blunt DL, Williams GP, Arnish JJ, Pfingston M, Herbert J, Haffenden RA (1999) An assessment of the disposal of petroleum industry NORM in non-hazardous landfills. National petroleum technology office, US Department of Energy Report No - DOE/BC/W-31-109-ENG-38-8. https://doi.org/10.2172/13061
Sutherland RA, Tolosa CA, Tack FMG, Verloo MG (2000) Characterization of selected element concentrations and enrichment ratios in background and anthropogenically impacted roadside areas. Arch Environ Contam Toxicol 38:428–438. https://doi.org/10.1007/s002449910057
Tokalioglu S, Kartal S (2006) Multivariate analysis of the data and speciation of heavy metals in street dust samples from the organized industrial district in Kayseri (Turkey) Atmos. Environ 40:2797–2805. https://doi.org/10.1016/j.atmosenv.2006.01.019
UCS 30 USB Computer Spectrometer, Spectrum Techniques, Tennessee, USA.
UNSCEAR (2000) Sources and effects of ionizing radiation. In: report of the United Nations Scientific Committee on the Effects of Atomic Radiation report to the General Assembly, with scientific annexes
Pakistan Petroleum Information Services (2016). Upstream petroleum activities
Wasim M (2010) gamma Cal – a useful computer program for neutron activation analysis and radioactivity calculations. The Nucleus 47(2):149–152
Yongming H, Peixuan D, Junji C, Posmentier ES (2006) Multivariate analysis of heavy metal contamination in urban dusts of Xi’an, Cent China. Sci Total Environ 355:176–186. https://doi.org/10.1016/j.scitotenv.2005.02.026
Zhang J, Liu CL (2002) Riverine composition and estuarine geochemistry of particulate metals in China-weathering features, anthropogenic impact and chemical fluxes. Estuar Coast Shelf Sci 54:1051–1070. https://doi.org/10.1006/ecss.2001.0879
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nusrat, M., Siddique, N., Wazir, Z. et al. Neutron activation analysis and gamma spectrometry of Shawa oil well of Kohat basin, Pakistan. Environ Earth Sci 81, 11 (2022). https://doi.org/10.1007/s12665-021-10126-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-021-10126-6