Abstract
Lack of data on the hydrogeochemistry of coastal aquifers around the south Caspian Sea warranted the present hydrochemical and quality assessment studies. The study involving classical integrated methods, multivariate statistical analyses, and GIS applications was carried out on the groundwater encountered in coastal Sari–Neka plain, Mazandaran Province, Northern Iran. The present survey indicates that the groundwater encountered at majority (~ 68%) of the sampling locations is of Na+–SO42− category and constitutes deep meteoric percolation type and the same at the remaining ~ 32% of the sampling locations relates to Na+–HCO3− category and belongs to shallow meteoric percolation class. The groundwater situated at ~ 64% and ~ 36% of the sampling locations belongs, respectively, to freshwater and brackish water categories. The groundwater is specified by Na+ > Ca2+ > Mg2+ > K+ and HCO3− > Cl− > SO42− > NO3−. It pertains to Ca2+-Mg2+-HCO3−, mixed, and Na+-Cl− hydrochemical facies. Reverse cation–anion-exchange process was found dominant at majority (~ 76%) of the sampling sites. Dissolved solids were originated chiefly from connate brine water confined to the aquifer sedimentary beds, dissolution of aquifer materials, ion-exchange, and different anthropogenic sources. Groundwater encountered at ~ 71% and ~ 27% of the bore well locations belongs to good and excellent categories of drinking water, while that at ~ 73% of the sampling locations pertains to (C3S1) irrigation water class of Richards (Diagnosis and improvement of saline and alkali soils (USDA. Agriculture handbook, Vol. 60). U.S. Department of Agriculture, Washington DC, 1954). The results of the present study are useful for planning sustainable management and protection of the groundwater in coastal areas, especially around the Caspian Sea, and serve as a database for international researchers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In coastal regions of several countries (e.g., Spain, Greece, Cyprus, Hong Kong, Turkey, Iran, Nigeria, Australia, USA, and India), groundwater encountered in different geological settings exhibits diversified hydrochemical features, and quality-wise constitutes a few graded categories of water meant for irrigation and drinking purposes. Furthermore, the groundwater confined to coastal aquifers/alluvial plains are exposed to diverse levels of salinization, and are attributed to the involvement of one or more terrain—specific contaminants (e.g., seawater, brine/saline water interfaced/underlying fresh groundwater horizon, connate [palaeomarine] water trapped in aquifer rocks, and various pollutants of anthropogenic origin) (Brown and Misut 2010; Cheng and Ouazar 2003; Ergil 2000; Gholami et al. 2015; Gilabert-Alarcón et al. 2018; Giménez and Morell 1997; Jamshidzadeh 2020; Janardhana and Khairy 2019; Khairy and Janardhana 2013, 2014; Kim et al. 2004; Kreitler 1993; Liu et al. 2017; Ozler 2003; Petalas and Diamantis 1999; Vandenbohede and Lebbe 2012).
Insufficient understanding of the hydrogeochemistry of coastal aquifers around the south Caspian Sea makes it necessary to do this survey. The present work deals with the characterization, classification, and quality assessment of the groundwater encountered in coastal alluvial plain from Sari to Neka, in Mazandaran province, Northern Iran. The present investigation was carried out based on the classical methods of interpretation of hydrochemical data, multivariate statistical analyses of the physicochemical parameters of the groundwater, and GIS techniques. The obtained data provide details on the groundwater with respect to the physicochemical characteristics, source-wise and salinity-wise categorization, classifications, degree of evolution, natural mechanisms controlling hydrochemistry, hydrochemical features, ion-exchange processes, sources of dissolved solids, and standard of groundwater for human consumption and agricultural needs.
Materials and methods
Study area
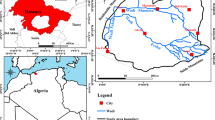
The study region is located in a coastal plain land between Sari and Neka townships in the eastern part of Mazandaran province, Iran (Fig. 1a). The study area, designated as Sari–Neka plain, is limited in the south by the foothills of Alborz Mountain range and in the north by the Caspian Sea coastline. The flow lines of the Siahroud river in the west and Nekaroud river in the east were selected by the present authors to demarcate the adjacent boundaries of the study area. Sari–Neka plain (Fig. 1b) covers an area of ~ 985 km2 and extends from 36°32´ to 36°52´ latitudes and 52°56´ to 53°25´ longitudes. The ground level elevation, near the southern boundary, is about 136 m above mean sea level (m.s.l.) and the same at the northern boundary is at an elevation of about 26 m below m.s.l. (i.e., at the present level of the Caspian Sea). The coastal geomorphic features of the Mazandaran Province include plateaus, alluvial fans, and flood plains (Vahdati Daneshmand and Saidi 1991).
The study area consists of a thick sequence of unconsolidated-to-semi-consolidated marine, deltaic, and fluvial deposits of Tertiary and Quaternary age. Sand and clay are the major fractions of the sediments, with relatively lesser amounts of silt and calcareous material. Exploratory bore wells reveal the extension of the sedimentary beds to a depth of more than 250 m (SazabShargh 2010). Jackson et al. (2002) consider that the sedimentary formation of the study area was possibly accumulated in the post-Oligocene foreland basin setting of Caspian Sea. The study area was developed consequently of the regression of the Caspian Sea, which, at one period, protracted till the foothill region of the Alborz mountains (Dewan and Famouri 1964). This evolutionary feature of the study region indicates that the sedimentary beds may hold a significant amount of palaeomarine water.
The groundwater flows from south to north. Near the shoreline, the water table is encountered at a depth of 2–3 m below the ground level and the same at the southern part of the study region is encountered at a depth of 100–110 m below the ground level. The precipitation in the territory varies from 500 to 800 mm/year and the climate is essentially sub-humid (Source: Iranian Meteorological Organization). The majority of the population makes use of the groundwater for drinking purposes, while about 40% of the agricultural activities are carried out utilizing the groundwater (SazabShargh 2010).
Sampling and hydrochemical analysis
During the month of October 2017, 66 groundwater samples were collected in duplicate at 66 bore wells of the groundwater quality network of the Sari–Neka aquifer (Fig. 1b) by the Mazandaran Regional Water Authority (MRWA) staff. Before collecting the water samples, the water was pumped out from bore wells for about 10 min to remove stagnant groundwater. Prior to sampling, water was filtered through 0.45 Millipore membrane. Portable electrical conductivity and pH meter (WWW COND 340i, Germany) were made use of for measuring Electrical Conductivity (EC) and pH of the groundwater at the sampling sites. Cation analysis was done by acidifying 100 ml of filtered groundwater sample with nitric acid, while un-acidified groundwater samples were utilized for the analyses of anion contents. The laboratory tasks were done during a week after sampling. Chemical analysis of the groundwater samples, following the standard procedure (APHA 1995), was accomplished at MRWA, Sari city, Iran. Charge Balance Error (CBE) in each sample as percent was computed following below mentioned mathematical expression (Freeze and Cherry 1979) and were well within the desirable limits of ± 5%:
where Z is the ionic valance, mc is the molarity of cation species, and ma is the molarity of anion species.
GIS analysis
The hard copy of the topographic map (1:25,000) of the study area, published by the National Cartographic Center of Iran, was digitized using ARCGIS 10.5 version software for preparation of the base map of the study area. The exact places of the sampling spots (i.e., bore well locations) were determined in the field using Garmin GPSMAP 64sx, and accurate placement of latitudes and longitudes was carried out using a GIS platform. Plans showing the areal extent of availability of the groundwater with desirable and permissible limits of the concentration of TDS, Ca2+, Mg2+, Na+, HCO3−, SO42−, Cl−, and NO3− prescribed for potable water by Institute of Standards and Industrial Research of Iran (ISIRI) (2009) and the plan depicting the areal extent of occurrence of three types of potable water (demarcated based on the Water Quality Index) were created utilizing ArcGIS software.
Multivariate statistical analysis
During the present study, SPSS (Version 18) software package was used to carry out multivariate statistical analyses of the analytical data of 12 physicochemical variables (viz., EC, TDS, pH, TH, Ca2+, Mg2+, Na+, K+, HCO3−, SO42−, Cl−, and NO3−) of the groundwater samples (n = 66) of the study area. The multivariate statistical analyses comprise: (1) Computation of Spearman’s correlation coefficient matrices to establish affinity between pairs of physicochemical parameters in the samples for identification of various origins of dissolved solids in the groundwater, (2) Principal Component Analysis (i.e., R-mode factor analysis) of the analytical data to determine and decipher relationships within physicochemical parameters of the groundwater and to extract the agents controlling the chemistry of groundwater (Davis 2002), and (3) Hierarchical Cluster Analysis (HCA) of the hydrochemical variables using Ward's linkage procedure (Ward 1963) to find multivariate resemblances in groundwater chemistry and to group the objects (viz., physicochemical variables of the groundwater) into specific domains, so that the entities within a domain are the same but dissimilar compared to other clusters (Singh et al. 2005).
The normality of distribution of the physicochemical variables in the groundwater was ensured by Kolmogorov–Smirnov and Shapiro–Wilk tests (Nematollahi et al. 2018). The tests revealed that the P values of the analytical data are less than 0.05, thus indicating the regular statistical distribution of the physicochemical variables of the samples.
Estimation of water quality index
Groundwater's suitability for the domestic needs of the study region was appraised from the calculated values of Water Quality Index (WQI). The determination of WQI is a ranking approach that represents the combined effect of discrete physicochemical variables of natural waters on the entire standard of freshwater (Mitra 1998). The WQI of the groundwater samples were computed following the procedure from the published literature (e.g., Saba and Umar 2016; Tarawneh et al. 2019).
The selection of the “Hydrochemical variables” for computation of WQI was made based on their relative significance in defining the water quality for drinking consumption. During the present study, the analytical results of 11 hydrochemical variables of the water samples, namely, pH, TH, TDS, Ca2+, Mg2+, Na+, K+, HCO3−, SO42−, Cl−, and NO3−, were selected. The highest weight number, which is 5, was assigned to 6 hydrochemical variables (viz., TDS, Ca2+, Na+, K+, HCO3−, and NO3−) as their higher concentration can cause significant deterioration of quality of potable water. Weight number 4 was provided to pH as its values away from 7 indicate the deteorated quality of the potable water. Weight number 3 was allocated to Mg2+ and Cl−, as these hydrochemical variables are known to cause less damage to the quality of potable water. Weight number 2 was assigned to TH, as its higher values can indicate limited deterioration of the quality of potable water. The lowest weight number 1 was allotted to SO42− as the latter is known to cause insignificant deterioration of quality of potable water (Sajil Kumar et al. 2013). Weight numbers provided to the selected hydrochemical variables are shown in Table 1.
Calculation of the value of "Relative Weight" (Wi) of the hydrochemical variables was carried out as stated by the formula
where wi is the weight number allotted to the ith hydrochemical variable, Wi is the “Relative weight” of the ith hydrochemical variable, and n is the whole number of selected hydrochemical variables (n = 11). The obtained values of Wi of the hydrochemical variables are provided in Table 1.
Computation of the value of "Quality rating” of the ith hydrochemical variable (qi) was established using the following mathematical expression:
where Ci represents the concentration of ith hydrochemical variable in the considered groundwater sample, and Si is the permissible limit of the concentration of the ith hydrochemical variable as per the guidelines of ISIRI (2009) for potable water.
Calculation of the value of ‶Sub-Index″of the ith hydrochemical variable (SIi) of the considered groundwater samples was carried out according to the following equation:
Calculation of the value of WQI of the considered groundwater sample was established using the following equation:
Evaluation of the groundwater quality for agricultural uses
Assessment of groundwater standard for agricultural use was carried out on the basis of the values/ratings generated from ten parameters and three parameter-based bivariate diagrams devised by prominent investigators. The considered parameters and diagrams can reveal salinity- and alkalinity-related and ion-specific hazards of irrigation waters to soil and vegetation. Table 2 provides the framework for the appraisal of the groundwater quality for agricultural purposes.
Results and discussion
Physicochemical characteristics of groundwater
The pH values of the groundwater samples (n = 66) vary from 6.8 to 9.1 (av. = 7.3) and indicate that majority (~ 85%) of samples are mildly alkaline (Table 3). The values of TDS and EC of the groundwater vary, respectively, from 372 to 2595 mg/l (av. = 1061 mg/l) and from 520 to 3630 µS/cm (av. = 1484 µS/cm). Total hardness (TH) of the groundwater varies from 115 to 1285 mg/l (av. 339 mg/l). Based on the classification proposed by Sawyer and McCarty (1967), the groundwater at the majority (~ 74%) of the sampling locations can be designated as very hard class (TH > 300 mg/l). At the remaining ~ 22% and ~ 4% of the sampling locations, the groundwater belongs to hard water (TH: 150–300 mg/l) and moderately hard water (TH: 75–150 mg/l) classes, respectively. The groundwater, on average and in terms of meq/l, is specified by Na+ > Ca2+ > Mg2+ > K+ and HCO3− > Cl− > SO42− > NO3−. Average concentration of individual cations to total concentration of cations is 45.07% Na+, 34.80% Ca2+, 20.06% Mg2+, and 0.06% K+. On average, the anions are composed of 51.62% HCO3−, 30.14% Cl−, 16.12% SO42−, and 2.11% NO3−. The average concentration of (Na+ + K+) (6.76 meq/l) is lower than that of (Ca2+ + Mg2+) (8.02 meq/l) and the average concentration of (HCO3− + SO42−) (10.10 meq/l) is higher than that of (Cl− + NO3−) (4.72 meq/l). The average concentration of total ions (Tz− + Tz+) equals 29.8 meq/l.
Classification of groundwater
Generally speaking, there are various classifications of groundwater. The principal ones are based on: Cl−, SO42−, and HCO3− contents; quantities of Base Exchange Index (r1); quantities of Meteoric Genesis Index (r2); and concentration of TDS (i.e., salinity).
Categorization of groundwater depending on Cl−, SO4 2−, and HCO3 − contents
Groundwaters, according to their Cl−, SO42−, and HCO3− contents, can be classified as normal chloride (Cl− < 15 meq/l), normal sulfate (SO42− < 6 meq/l), and normal bicarbonate (HCO3− content between 2 and 7 meq/l) types (Soltan 1998). In 66 samples, 38 groundwater samples are affiliated to chloride, sulfate, and bicarbonate water types. Out of the 66 groundwater samples, 65, 62, and 39 samples represent normal chloride, normal sulfate, and normal bicarbonate types. Furthermore, out of 66 samples, 62 samples belong to both chloride and sulfate water types, 38 are related to both sulfate and bicarbonate groups, and 39 belong to both chloride and bicarbonate types. The above data show that the groundwater at ~ 98% of the sampling sites belongs to chloride type, at ~ 94% of the sampling locations are of sulfate type, and at ~ 59% of the bore well locations belongs to bicarbonate group.
Categorization of groundwater based on the base exchange index (r 1)
Base Exchange Index (r1) as the criterion, Matthess (1982) grouped the groundwater into two categories viz, “Na+-SO42−” and “Na+–HCO3−” types. The value of r1 is arrived at with the following expression:
The computed r1 values of the water samples collected at 66 sampling sites are given in Table 4 and the same data are also presented graphically in Fig. 2a. The r1 values of the groundwater samples at 44 bore well locations designate them as of Na+–SO42− type as their r1 values are < 1. At the remaining 22 bore well locations, r1 values of the groundwater samples are > 1 and thus belong to Na+–HCO3− group.
Categorization of groundwater depending on the values of meteoric genesis index (r 2):
Meteoric Genesis Index (r2) of the water samples gathered from the study region was computed using the following equation:
The r2 values of the water samples gathered at 66 sampling sites are provided in (Table 4) and the same data are presented in Fig. 2b. The results show that at 43 bore well locations, the groundwater belongs to deep meteoric percolation type (r2 values < 1) and the groundwater at the remaining 23 sampling sites is of shallow meteoric percolation type (r2 values > 1) (Soltan 1998).
Comparison of r1 and r2 values implies that at 43 sampling sites, the groundwater is allied to Na+–SO42− type and constitutes the deep meteoric percolation type. Out of the remaining 23 sample locations, the groundwater at 22 locations related to “Na+–HCO3−” type and conform to shallow meteoric percolation type, and at BW No. 40 locations, the groundwater is of Na+–SO42− type but constitutes shallow meteoric percolation type. Based on the above information, the groundwater encountered at a large number of the sampling sites belongs to Na+-SO42− type and constitutes deep meteoric percolation type.
Categorization of groundwater depending on TDS concentration
Davis and Wiest (1966) categorized natural waters based on the concentration of Total Dissolved Solids (TDS) into freshwater (TDS < 1000 mg/l), brackish water (TDS between 1000 and 10,000 mg/l), saline water (TDS between 10,000 and 1,00,000 mg/l), and brine water (TDS > 1,00,000 mg/l). The water samples gathered at 66 sampling sites in the study region belong to two types: freshwater and brackish water. Freshwater type of groundwater appeared at 38 places, whereas the brackish water type is found at 28 sites. Table 5 provides the locations of the bore wells yielding fresh and brackish ground waters in the study area.
Degree of evolution
The dissolved load in groundwater increases in proportionate to its length of flow (Freeze and Cherry 1979) and anion chemistry reveals the degree of its evolution in the subsurface environment (Chebotarev 1955). The average analytical data of the groundwater samples show relatively higher levels of HCO3− (7.77 meq/l) followed by Cl− (4.40 meq/l), SO42− (2.48 meq/l), and NO3− (0.32 meq/l). Samples containing bicarbonate as the principal anion, as in the present case, imply the zone of dynamic groundwater movement and least residence time.
Natural mechanisms controlling hydrochemistry
Plots of hydrochemical data on graphs of TDS versus Gibbs’s ratio I [(Na + + K +)/(Na + + K + + Ca2+)] and TDS versus Gibbs’s ratio II [Cl−/(Cl− + HCO32-)] (Gibbs 1970) give a clue on the sources of dissolved solids in the groundwater. These diagrams reveal the importance of three major natural mechanisms controlling water chemistry, viz., mineral weathering, atmospheric precipitation, and evaporation and fractional crystallization. On the graph of TDS versus Gibbs’s ratio I (Table 4; Fig. 3a), the samples plot mainly in the rock dominance and evaporation dominance fields. On the bivariate TDS versus Gibbs’s ratio II diagram (Table 4; Fig. 3b), the samples plot mainly near and above the boundary lines of rock dominance and evaporation dominance fields. The above data indicate that the hydrochemistry of the groundwater is controlled mainly by rock weathering reactions and minimally by evaporation processes (Khairy and Janardhana 2013; Şehnaz et al. 2020). The samples which did not plot in the specified domains may indicate the influence of anthropogenic sources (Adimalla and Venkatayogi 2017).
Hydrochemical facies
The plot of the Hydrochemical data of the groundwater samples (n = 66) on Piper diagram (Piper, 1944) (Fig. 4) show that, cation facies-wise, 16 samples are calcium type, 14 samples are sodium type, 7 samples are magnesium type, and in 29 samples, none of the cations dominates. Anion facies-wise, 28 samples are bicarbonate type, 12 samples are chloride type, 1 sample is sulfate type, and in 25 samples, none of the anion ions dominates. Among the groundwater samples, hydrochemical facies-wise, 27 samples belong to Ca2+–Mg2+–HCO3− facies, 13 samples belong to Na+–Cl− facies, and 26 samples belong to mixed facies (i.e., Ca2+Mg2+–Cl− hydrochemical facies of Back, 1966).
Piper trilinear diagram (Piper 1944) showing the hydrochemical characteristics and hydrochemical facies of the groundwater samples
Ion-exchange reactions
It is observed that groundwater encountered at different locations of a given terrain, barring rare exceptions, participates in ion-exchange reactions with clay minerals (exchanger) of the aquifer matrix (Cardona et al. 2004). The type of ion-exchange reaction (i.e., either direct cation-exchange reaction or reverse cation–anion-exchange reaction) witnessed by the water at a given location may vary seasonally, but invariably depends on its hydrochemical composition.
Ion-exchange reactions witnessed by water at the 66 sampling sites were evaluated based on the values of Chloro-Alkaline Indices (CAI-I and II) of the groundwater samples. The CAI values were computed according to the equations provided by Schoeller (1977) and the obtained data are provided in Table 4. The data reveal that during the sampling season, the water at 50 sampling sites was specified by negative CAI–I and II values implying the water in these locations was witnessing reverse cation–anion reaction. At the remaining 16 sampling sites, the groundwater samples were characterized by positive values of CAI–I and II and the latter designate direct cation-exchange reaction of waters with clay minerals (exchanger) of the aquifer matrix.
It is well known that direct cation-exchange reaction provides a certain amount of alkalies while a similar amount of alkaline earths is being removed from the groundwaters. Reverse cation–anion-exchange reaction involves the addition of a certain amount of alkaline earths and simultaneous removal of similar amount of alkalies from the groundwater. In the study region, the ion-exchange reactions resulted in a significant addition of alkaline earths and removal of similar amounts of alkalies. These data suggests that the ion-exchange process can be considered as one among the few sources of alkaline earths to the groundwater of the study region (Jamshidzadeh 2020; Nematollahi et al. 2018).
Multivariate statistical analyses
Correlation matrix
Correlation analysis has a pivotal role in the hydrogeochemical investigation. The correlation matrix can demonstrate the dependence between different parameters, thus revealing the association between different variables and controlling factors. A correlation coefficient near 1 or at 1 demonstrates a good positive relationship and a correlation coefficient around zero shows no relationship between two variables at a significant level of p < 0.05. Generally, the parameters with r > 0.7 show a strong correlation, and r between 0.5 and 0.7 indicates moderate correlation (Şehnaz et al. 2020). The computed correlation matrices of the analyzed physicochemical variables (n = 12) of the groundwater samples are provided in Table 6. Among the variables, EC shows high positive correlation with TDS (0.99), Na+ (0.81), and Cl− (0.88), and moderate positive correlation with K+ (0.67), HCO3− (0.61), and SO42+ (0.54). Likewise, TDS shows high positive correlation with Na+ (0.79), and Cl− (0.88) and moderate positive correlation with K+ (0.66), HCO3− (0.58), and SO42+ (0.53). Na+ shows high positive correlation with Cl− (0.86), moderate positive correlation with K+(0.54), and weak positive correlation with HCO3− (0.37) and SO42+ (0.41). K+ shows moderate positive correlation with Cl− (0.63) and weak positive correlation with HCO3− (0.47) and SO42− (0.32). Mg2+ shows weak positive correlation with Ca2+(0.25), HCO3− (0.23), and SO42− (0.24) and very weak positive correlation with K+ (0.14). Ca2+ shows very weak positive correlation with K+ (0.15) and HCO3− (0.11). HCO3− shows weak-to-very weak positive correlation with K+ (0.47), Na+ (0.37), Mg2+ (0.23), and Ca2+(0.11). Likewise, SO42− shows weak-to-very weak positive correlation with K+ (0.32), Na+ (0.41), and Mg2+ (0.24). Cl− shows high-to-moderate positive correlation with Na+ (0.86) and K+ (0.63). The observed high positive correlation between EC and TDS, high-to-moderate positive correlation between Na+, K+, and Cl− and their weak positive correlation with Mg2+, HCO3−, and SO42−, and weak-to-very weak positive correlation of Mg2+ with Ca2+, K+, HCO3−, and SO42− may confirm the presence of connate saline water in the Quaternary deposits of the aquifer (Khairy and Janardhana 2013).
In groundwaters, a significant amount of alkaline earths is derived essentially from mineral weathering/dissolution aided by H2CO3 and H2SO4, and in such cases, alkaline earths (Ca2+ ± Mg2+) exhibit very high-to-high positive correlation with HCO3− and SO42−. In the present study, the alkaline earths were found to exhibit weak-to-extremely weak correlation with HCO3− and SO42−. The observed poor positive correlation of alkaline earths with genetically related acids (i.e., HCO3− and SO42−) can be explained if the net contributions of alkaline earths from ion-exchange processes witnessed by the groundwater are considered. As mentioned in the section of ion-exchange reactions, the groundwater was found witnessing reverse cation–anion-exchange reaction with exchanger (clay minerals) of the aquifer matrix at 50 out of 66 groundwater sampling locations. This reverse cation–anion-exchange reaction at majority of the sampling sites provided the significant concentration of alkaline earths to the groundwater. Hence, the observed weak-to-very weak positive correlation of alkaline earths with HCO3− and SO42− has to be attributed to the significant derivation of alkaline earths from the ion-exchange process.
Factor analysis
Factor analysis is a multivariate statistical approach that can be used for studying the correlation between a set of variables (Yu and He 2006). The esential objective of the factor analysis is to discover the role of each variable and also simplify the structure of the pure data. Kaiser–Meyer–Olkin (KMO) Measure of Sampling Adequacy (MSA) is calculated in SPSS and is often used to distinguish if a dataset is “suitable” for factor analysis. In this research, a KMO value of 0.52 was attained, so the hydrochemical data are proper to carry out Principal Component Analysis (PCA). PCA was used as a factor analysis method with considering of varimax rotation technique and eigenvalue > 1(Yang et al. 2016). Factor analysis of the analytical data of the physicochemical variables of the groundwater brought to light 4 factors with Eigenvalues > 1. These factors have been extracted from the principal factor matrix after varimax rotation. These independent factors account for 80.57% of the total variance of the data set (Table 7). Factor-1 accounts for 31.19% of the total variance and has strong positive loadings of TDS (0.936), Na+ (0.927), and Cl− (0.894), moderate positive loadings of EC (0.680) and SO42− (0.601) and weak positive loading of HCO3− (0.429) and very weak positive loadings of K+ (0.22) and Mg2+ (0.12). The strong-to-very weak positive loadings of Na+, Cl−, HCO3−, SO42−, K+, and Mg2+ of the Factor-1 may be related to connate saline water of the sedimentary layers of the aquifer, which now constitutes the admixed component of the groundwater. Factor-2 accounts for 25.2% of the total variance and has strong positive loadings of TH (0.969), Mg2+ (0.809), and Ca2+ (0.742) and moderate positive loading of SO4−2 (0.623). The high-to-moderate positive loadings of Ca2+, Mg2+, and SO4−2 of the Factor-2 may indicate derivation of significant concentration of alkaline earths from weathering of carbonate minerals, including dolomite. Factor-3 accounts for 12.74% of the total variance and has moderate positive loadings of HCO3− (0.742) and K+ (0.550) and weak positive loading of Ca2+ (0.253). The observed positive loadings of HCO3−, K+, and Ca2+ of the Factor-3 may indicate derivation of a certain amount of K+ of the groundwater from weathering of K-bearing silicate minerals aided by carbonic acid. Factor-4 accounts for 11.43% of the total variance, and has strong positive loading of NO3− (0.828) and moderate positive loading of EC (0.567). High positive loading of NO3− of the Factor-4 may indicate the presence of the same in the groundwater as a consequence of pollution caused by anthropogenic inputs (e.g., nitrogenous fertilizers, industrial effluents, human and animal waste, and bio-combustion).
Cluster analysis
Cluster analysis performed on physicochemical variables of the groundwater samples brought to light two major clusters (Fig. 5). Cluster 1 consists of EC, pH, Na+, HCO3−, and NO3−. Cluster 2 includes Ca2+, Mg2+, and SO4−2. Variables of the cluster 1, especially Na+, HCO3−, and NO3−, may indicate their derivation from connate saline (palaeomarine) water of the sedimentary beds of the aquifer, weathering of alkali feldspars aided by carbonic acid, and inputs of anthropogenic sources, which include animal and human waste and fertilizers. Variables of cluster 2 suggest an influx of significant amounts of alkaline earths through dissolution of carbonate minerals, including dolomite, aided by H2SO4. The grouping of three variables of cluster 1 (i.e., Ca2+, Mg2+, and SO4−2) is consistent with the loadings of factor-2 of the factor analysis data provided earlier in the section of Factor analysis.
Sources of dissolved solids in groundwater
In natural system dissolution of carbonate, silicate, and sulfide minerals and consequent generation of HCO3−, SO42− and H2SO4 are represented by the following reactions:
In ground waters of the study area, the carbonic acid (H2CO3) played a predominant part in comparison to sulfuric acid (H2SO4) during dissolution process as could be made out from the average value of > 0.50 of HCO3−/(HCO3− + SO42−) equivalent ratio (C-ratio; Brown et al. 1996). The source of Cl− ion could either be saline water trapped in the basin or anthropogenic inputs or both. The connate saline water, now admixed with the groundwater, in reality, constitutes the Caspian seawater of Quaternary age. The admixed connate water component of the groundwater can provide a significant concentration of Cl− and Na+ and relatively lesser quantities of other major ions to the groundwater. The amount of ionic load received by the groundwater from the admixed component of the connate saline water at any given location of the study area depends on its quantity and hydrochemical composition. The hydrochemical composition of the Caspian seawater of Quaternary age is not known, but it may not differ significantly from the hydrochemical composition of the present-day Caspian seawater. The present-day Caspian seawater, on average, contains in (meq/l) Ca2+ = 44.00; Mg2+ = 19.20; Na+ = 100.80; K+ = 2.60; HCO3− = 3.40; SO42− = 2.70; Cl− = 158.80 (Khairy and Janardhana 2013).
In the study area, anthropogenic inputs (from the agricultural field and domestic wastewater) rendered the groundwater at several localities significantly saline. For example, six samples (BW nos. 2, 37, 38, 41, 59, and 61) contain high concentration of Cl−, ranging from 319.1 to 652.3 mg/l (av. = 456.75 mg/l) (Table 3). At these sites, the water also contains elevated concentration of Na+, varying from 206.9 to 620.7 mg/l (av. = 433.15 mg/l) (Table 3). The observed high concentrations of Cl− and Na+ at several localities were possibly originated from anthropogenic inputs.
High concentrations of Ca2+ and Mg2+ might have been originated from three sources: mineral weathering, ion-exchange process, and palaeomarine water in the basin. During the weathering process, minerals belonging to both carbonate and silicate mineral groups can provide alkaline earths. Hence, it is necessary to identify the mineral group(s) involved during weathering events, based on the hydrochemical data of the groundwater samples.
Groundwater samples of the study area contain significant concentration of sulfate and the latter may be considered to indicate the participation of carbonate minerals during weathering events. During mineral dissolution/weathering process, Ca2+ and Mg2+ derived exclusively from the dissolution of carbonates (calcite and/or dolomite) may be recognized by comparing the theoretically deduced values of Ca2+/HCO3−, Mg2+/HCO3−, Ca2+/SO42−, and Mg2+/SO42− molar ratios related to weathering of carbonates (mixtures of calcite and dolomite in proportions ranging from 0 to 100% of each mineral) which vary, respectively from 0.25 to 0.50, from 0 to 0.25, from 0.5 to 1.00, and from 0 to 0.50 (Khairy and Janardhana 2013).
In this study region, the values of one or two of the above-mentioned molar ratios (viz., Ca2+/HCO3−, Ca2+/SO42−, Mg2+/HCO3−, and Mg2+/SO42−) in 30 groundwater samples are within the limits of the theoretically deduced values (TDV) of molar ratios originated from the exclusive dissolution of carbonates. For example, the values of Ca2+/HCO3− molar ratio in seven samples vary from 0.25 to 0.42 (TDV = 0.25 to 0.50). The values of Mg2+/HCO3− molar ratio in 20 samples range from 0.03 to 0.25 (TDV = 0 to 0.25). The values of Ca2+/SO42− molar ratio in five samples vary from 0.52 to 0.93 (TDV = 0.50 to 1.00). The values of the Mg2+/SO42− molar ratio in nine samples vary from 0.17 to 0.37 (TDV = 0 to 0.50). These values of molar ratios in the identified 30 groundwater samples support the derivation of alkaline earths from the exclusive dissolution of carbonates. Molar ratios of the remaining samples possibly point to the derivation of alkaline earth from dissolution of silicate rocks.
The presence of significant concentrations of HCO3− in the samples validates the participation of silicate minerals during weathering process and they can further be confirmed from the values of certain molar ratios of the hydrochemical data. For example, the values of (Na+ + K+)/Tz+, (Ca2+ + Mg2+)/HCO3−, and (Ca2+ + Mg2+)/(Na+ + K+) molar ratios reveal the dissolution of minerals of carbonate and/or silicate groups (Sarin et al. 1989). In the present case, the values (Table 4) of the above-mentioned molar ratios suggest the chemical weathering of minerals of both silicate and carbonate groups as the source of alkaline earths (Singh et al. 2005). The plots of the values of Mg2+/Na+ and Ca2+/Na+ molar ratios (Fig. 6) on the graph of Gaillardet et al. (1999) indicate the effect of weathering of both carbonate and silicate minerals in the course of the accession of cations (Singh et al. 2012). Also, the groundwater witnessed an influx of alkaline earth during the ion-exchange process.
Bivariate Mg2+/Na+ versus Ca2+/Na+ diagram (Gaillardet et al. 1999) showing the plots of the hydrochemical data of the groundwater samples
The alkali content of the samples might have been originated from four sources: weathering of alkali feldspars, ion-exchange processes, admixed connate saline water, and anthropogenic inputs. The values of Na+/Cl− molar ratio of the samples were considered and it was found that 50 out of 66 samples have values > 1 implying significant participation of alkali feldspars during weathering process (Stallard and Edmond 1983; Meybeck 1987; Morgan and Stumm1996). At the remaining 16 bore well sites, the water might have acquired Na+ ± K+ during the ion-exchange process.
Groundwater samples contain, on average, high concentration of Na+ (av = 152.50 mg/l) and low concentration of K+ (av = 4.1 mg/l) among cations. The observed domination of Na+ over K+ and low concentration K+ may be attributed to the Na+-rich and K+- poor nature of the dissolved solids in the connate saline water component of the groundwater and anthropogenic inputs. Other reasons for the observed difference in the concentrations of Na+ and K+ include: high mobility of Na+ vis-à-vis K+ during mineral weathering (Milliot 1970), ion-exchange process, and participation of a limited amount of K-feldspars during weathering process in comparison with Na-bearing aluminosilicates.
Suitability of the groundwater for drinking purpose
The suitability of the groundwater of the studty area for drinking purpose was evaluated following two different methods. The first method involved comparision of the values/concentrations of 11 physicochemical variables (viz., pH, TDS, TH, Ca2+, Mg2+, Na+, K+, HCO3−, SO42−, Cl−, and NO3−) of the groundwater samples (Table 3) with the permissible limits of the values/concentrations of the same physicochemical variables prescribed by Institute of Standards and Industrial Research of Iran (ISIRI 2009) for potable water (Table 8). The second method of evaluation was based on the computed values of the water quality index (WQI) of the groundwater samples.
The permissible limits of the values/concentrations of physicochemical variables prescribed by ISIRI (2009) for drinking water are: pH (6.5–9), TDS (1500 mg/l), TH (500 mg/l), Ca2+ (300 mg/l), Mg2+ (150 mg/l), Na+ (200 mg/l), K+ (200 mg/l), HCO3− (600 mg/l), SO42− (400 mg/l), Cl− (400 mg/l), and NO3− (50 mg/l). In the present studies, the values/concentrations of pH in 1 sample, TDS in 7 samples, TH in 14 samples, Mg2+ in 1 sample Na+ in 14 samples, HCO3− in 12 samples, SO42− in 2 samples, Cl− in 4 samples, and NO3− in 4 samples exceed the permissible limits prescribed by ISIRI (2009). Concentrations of Ca2+ and K+ in all groundwater are within the prescribed limits of the ISIRI (2009). Table 8 provides the details of the groundwater samples having values/concentrations of physicochemical variables exceeding the prescribed limits suggested by ISIRI (2009).
Figure 7 shows the areal distribution of groundwater with desirable and permissible limits of concentrations of Mg2+ (A), Na+ (B), HCO3− (C), SO42− (D), Cl− (E), NO3− (F), TDS (G), TH (H), and pH (I) recommended by ISIRI (2009).
Plans showing the areal extent of occurrence of the groundwater with desirable and permissible limits of concentrations of Mg2+ (A), Na+ (B), HCO3− (C), SO42− (D), Cl− (E), NO3− (F), TDS (G), TH (H), and pH (I) prescribed by ISIRI (2009) for potable water
The values of the WQI of the groundwater samples, which were calculated based on the approach explained in the section of methodology, are provided in Table 9. In the published literature (e.g., Tarawneh et al. 2019), the values of WQI of waters are grouped into five classes, which correspond to five types of potable water (Table 10). Details of the groundwater samples belonging to the above-mentioned water classes are provided in Table 11. The obtained data designate that, in the study region, groundwater fitting to “excellent”, “good”, and “poor” water classes are encountered, respectively, at 37, 28, and 1 sampling sites (Table 10). Figure 8, which is prepared based on the values of the WQI of the groundwater samples, shows the spatial distribution of potable groundwater belonging to "excellent", "good", and “poor” categories.
Suitability of water for irrigation
Criteria for judging the quality of water for irrigation needs considered here are listed in Tables 9 and 12. The results indicated certain limitations of the usage of the groundwater for irrigation purposes.
Based on the amounts of Exchangeable Sodium Percentage (Tijani 1994), groundwater at ~ 95% of the sampling sites is suitable for irrigation needs (Table 12). Likewise, the ratings provided by the graphs of the data PI versus Tz+ + z- also suggest that the groundwater encountered at the majority (i.e., ~ 86%) of the bore well locations belongs to the Class I category of irrigation water (Table 12). The values/ratings of other parameters and bivariate diagrams brought to light the following specific and valuable information on the possible damage to soil and vegetation due to the excess presence of certain ions/groups of ions in groundwater.
The values/ratings provided by Salinity hazard (Richards 1954) show that the region is devoid of excellent category of irrigation water. Good quality groundwater for irrigation purposes is encountered only at ~ 5% of the bore well locations. At the remaining ~ 85% and ~ 10% of the sites, the water allied, respectively, to fair/medium and poor/bad classes (Table 12). The data reveal high-to-very high salinity encountered at majority (~ 95%) of the sampling sites.
A high content of Na+ in irrigation waters can cause the exchange of Na+ in water with Ca2+ and Mg2+in soil, and this chemical activity can reduce the permeability and internal drainage in soils (Collins and Jenkins 1996; Saleh et al. 1999). Furthermore, the continued application of water with high Na+ and HCO3− can also adversely affect the soil permeability (Doneen 1964; Davis and Wiest 1966; Raghunath 2007). Harmful effects of Na+ in irrigation water are also assessed from the values of % Na+, Kelley’s ratio, Permeability Index as well from the graph of % Na+ versus EC (μS/cm) (Wilcox 1955). The classification of water concerning % Na+ indicates that the groundwater at ~ 27% of the sampling locations is not suitable for irrigation purposes (Table 12). According to the ratings of Kelley’s ratio, the groundwater encountered at ~ 26% of the bore well locations is unsuitable (Table 12). Permeability Index data suggest that the groundwater at ~ 44% of the sampling locations is unsuitable (Table 12). The ratings generated from the plots of the data on the Wilcox diagram indicate that the groundwater encountered at ~ 8%, ~ 10%, and 6% of the borewell locations belongs to permissible to doubtful, doubtful to unsuitable, and unsuitable categories of irrigation water, respectively (Table 12). Thus, the ratings provided by % Na+, Kelley’s ratio and Wilcox diagram together indicate that the water at ~ 27% of the sampling sites is marginally suitable/not suitable, whereas ratings inferred from the Permeability Index suggest that groundwater extracted from half of the sampling sites is unfit for application in the agricultural field.
The quantity of HCO3− and CO32− present in excess of Ca2+ and Mg2+ tend to rise sodium ions in water (Eaton 1950). The values of the related parameter Residual sodium carbonate (RSC, Lloyd and Heathcote 1985) at ~ 7% and ~ 14% of the bore well sites belong, respectively, to doubtful (marginal quality) (Singh et al. 2012) and not suitable categories (Table 12). Bicarbonate in excess in irrigation waters is considered as harmful to soils. The ratings of the Bicarbonate hazard (Mandel and Shiftan 1981) suggest that the groundwater at ~ 71% of the sampling locations may cause an increasing problem, inducing alkalinity to the soil and at ~ 27% of the well locations’ severe problem (Table 12). The high content of chloride in irrigation waters is also considered as detrimental to soils and plant growth. A high concentration of chloride, combined with excess sodium, promotes the formation of acidic soil. According to the values/ratings of the chloride hazard, the groundwater encountered at ~ 20% and ~ 7% of the bore well locations belong, respectively, good-to-hazardous, and hazardous-to-very hazardous categories of irrigation water (Table 12).
Mg2+-rich irrigation water can cause ill effects on clay dispersion and hydraulic conductivity of soils, thus affecting the agricultural produce in the region. The values of Magnesium hazard (expressed as Mg2+ratio—Lloyd and Heathcote 1985; Szabolcs and Darab, 1964) indicate that at ~ 16% of the bore well locations, the groundwater belongs to the unsuitable category of irrigation water (Table 12).
Plots of EC (salinity hazard) and SAR (alkalinity hazard) values of the groundwater samples of the study area on USSL diagram (Richard, 1954) (Fig. 9) reveal that the groundwater at ~ 78% and ~ 5% of the sampling locations belongs, respectively, to C3-S1 and C2-S1 categories of irrigation water (Table 12). Low sodic (S1) nature of the groundwater indicates that it can be safely used in most types of soils with little danger of development of harmful levels of exchangeable sodium. However, water characterized by high (C3) and very high (C4) salinity prohibits its use on fine-textured soils with minimal drainage. The data obtained from the USSL diagram (Fig. 9) suggest that the groundwater encountered at ~ 95% and ~ 5% of the bore well locations can be utilized for lands growing, respectively, salt-tolerant and semi salt-tolerant crops.
U.S Salinity Laboratory diagram (Richards 1954) showing the irrigation water classes of groundwater samples
Figure 10 provides details of spatial distribution of groundwater belonging to seven identified irrigation water classes of Richards (1954). In the study area, groundwater belonging to C3-S1 irrigation water class of Richards (1954) is widely encountered and is located at 48 out of 66 bore well locations (Fig. 10). Groundwater encountered at NW and NE regions of the study area belongs to essentially very high saline irrigation water classes.
Plan showing the spatial distribution of irrigation water classes of Richards (1954) in the study area
Summary and conclusions
The amalgamation of geological knowledge, geochemical data, and statistical treatment of the hydrochemical data was made use of the classification, characterization, and evaluation of the quality of water of coastal aquifer at Sari–Neka region in Iran for drinking and irrigation needs. Source-wise, groundwater of the studied region at about 68% of the bore well locations constitutes Na+-SO42− type and belongs to deep meteoric percolation type. In about 30% of the bore well locations, the groundwater constitutes Na+-HCO3− type and belongs to shallow meteoric percolation type. Salinity-wise, at about 64% and at about 36% of the sampling sites, the groundwater belongs to freshwater and brackish water types, respectively. Molar concentrations of various ions show that cations and anions abundances, respectively, are Na+ > Ca2+ > Mg2+ > K+ and HCO3− > Cl− > SO42− > NO3−. Hydrochemical facies-wise, ~ 42%, ~ 41%, and ~ 17% of the samples belong, respectively, to Ca2+Mg2+HCO3−, mixed (Ca2+Mg2+Cl−; Back, 1996), and Na+-Cl− facies.
The bicarbonate (HCO3−) content of the groundwater appears to be generated owing to the carbonate and silicate weathering process supplemented by carbonic acid and oxidation of sulfide minerals. Sulfate (SO42−) content is probably related to the dissolution of carbonate minerals aided by sulfuric acid (H2SO4) and oxidation of sulfide minerals. The average value of C-ratio (0.78) of the groundwater samples indicates the significant role played by the carbonic acid (H2CO3) during the weathering process. Cl− content appears to have two origins: palaeomarine water of the Quaternary formations of the aquifer, now blended with the freshwater and anthropogenic inputs. Alkaline earths were acquired mainly from 3 sources: carbonate and silicate mineral weathering process, and reverse cation–anion-exchange reaction between groundwater and clay minerals of the aquifer and palaeomarine water. Alkalies were provided by feldspars, direct cation-exchange reaction, connate saline water, and anthropogenic inputs.
Water Quality Index (WQI) used as a tool to assess the quality of the groundwater for drinking purposes indicated excellent, good, and poor classes, respectively at 37, 28, and 1 sampling sites. Graphs of the hydrochemical data of the groundwater samples on USSL diagram of Richards (1954) indicate that about 95% of the samples belongs to highly saline (C3) and very highly saline (C4), and alkalinity-wise, low-to-very high sodic (S1 to S4) irrigation water classes, thus implying that the groundwater is suitable for irrigation of land growing salt-tolerant crops. In the remaining ~ 5% of the sampling sites, groundwater belongs to (C2-S1) irrigation water class and is suitable for the cultivation of semi-salt-tolerant crops.
The present study provides a valuable example for understanding the phenomena of hydrochemical processes and quality assessment of groundwater in parts of the Caspian Sea coastal area that is encountered in five countries. Given that, there are several causes for the deterioration of quality of the groundwater. In terms of salinization, the groundwater samples from the area could potentially represent a variety of water types providing an opportunity to test the performance of many of the available methodologies, including classic integrated hydrogeochemical methods, graphical and statistical techniques and GIS analysis for better understanding of the hydrochemical system, and utilization and sustainable management of the groundwater resources of the study area in a more effective way.
References
Adimalla N, Venkatayogi SJEES (2017) Mechanism of fluoride enrichment in groundwater of hard rock aquifers in Medak, Telangana State, South India. Environ Earth Sci 76(1):45
APHA (1995) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington DC, p 100
Back W (1966) Hydrochemical facies and ground-water flow patterns in the Atlantic Coastal Plain. US Geol. Survey Prof. Paper, p 498
Bouwer H (1978) Groundwater hydrology. McGraw-Hill Inc, New York
Brown CJ, Misut PE (2010) Aquifer geochemistry at potential aquifer storage and recovery sites in coastal plain aquifers in the New York City area, USA. Appl Geochem 25(9):1431–1452. https://doi.org/10.1016/j.apgeochem.2010.07.001
Brown G, Sharp M, Tranter M (1996) Subglacial chemical erosion: seasonal variations in solute provenance, Haut Glacier d’Arolla, Valais, Switzerland. Ann Glaciol 22:25–31
Cardona A, Carrillo-Rivera J, Huizar-Alvarez R, Graniel-Castro E (2004) Salinization in coastal aquifers of arid zones: an example from Santo Domingo, Baja California Sur. Mexico. Environ Geol 45(3):350–366
Chebotarev II (1955) Metamorphism of natural waters in the crust of weathering—1. Geochim Cosmochim Acta 8(1–2):22–48. https://doi.org/10.1016/0016-7037(55)90015-6
Cheng AH, Ouazar D (2003) Coastal aquifer management-monitoring, modeling, and case studies. CRC Press, Berlin
Collins R, Jenkins A (1996) The impact of agricultural land use on stream chemistry in the Middle Hills of the Himalayas, Nepal. J Hydrol 185(1–4):71–86. https://doi.org/10.1016/0022-1694(95)03008-5
Davis J (2002) Statistics and data analysis in geology. Wiley, New York, p 656
Davis S, De Wiest J (1966) Hydrogeology, vol 2. Wiley, New York
Dewan M, Famouri J (1964) The soils of Iran. Food and Agriculture Organization of the United Nations
Doneen LD (1964) Notes on water quality in agriculture. Department of Water Science and Engineering, University of California, Davis
Eaton FM (1950) Significance of carbonates in irrigation waters. Soil Sci 69(2):123–134
Ergil M (2000) The salination problem of the Guzelyurt aquifer, Cyprus. Water Res 34(4):1201–1214. https://doi.org/10.1016/s0043-1354(99)00253-5
Freeze RA, Cherry J (1979) Groundwater. Prentice-Hall, Englewood Cliffs
Gaillardet J, Dupré B, Louvat P, Allègre CJ (1999) Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem Geol 159(1–4):3–30. https://doi.org/10.1016/s0009-2541(99)00031-5
Gholami V, Aghagoli H, Kalteh AM (2015) Modeling sanitary boundaries of drinking water wells on the Caspian Sea southern coasts, Iran. Environ Earth Sci 74(4):2981–2990. https://doi.org/10.1007/s12665-015-4329-3
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170(3962):1088–1090. https://doi.org/10.1126/science.170.3962.1088
Gilabert-Alarcón C, Daesslé LW, Salgado-Méndez SO, Pérez-Flores MA, Knöller K, Kretzschmar TG et al (2018) Effects of reclaimed water discharge in the Maneadero coastal aquifer, Baja California, Mexico. Appl Geochem 92:121–139
Giménez E, Morell I (1997) Hydrogeochemical analysis of salinization processes in the coastal aquifer of Oropesa (Castellón, Spain). Environ Geol 29(1–2):118–131. https://doi.org/10.1007/s002540050110
ISIRI (2009) Chemical specifications of drinking water, ISIRI no. 1053, 5th ed. Institute of Standards and Industrial Research of Iran, Tehran (in Persian)
Jackson J, Priestley K, Allen M, Berberian M (2002) Active tectonics of the south Caspian basin. Geophys J Int 148(2):214–245
Jamshidzadeh ZJEP (2020) An integrated approach of hydrogeochemistry, statistical analysis, and drinking water quality index for groundwater. Assessment 7:781–804
Janardhana MR, Khairy H (2019) Simulation of seawater intrusion in coastal aquifers: a case study on the Amol-Ghaemshahr coastal aquifer system, Northern Iran. Environ Earth Sci 78(24):695. https://doi.org/10.1007/s12665-019-8711-4
Kelley WP (1951) Alkali soils; their formation, properties, and reclamation. Reinold Publication Corporation, New York
Khairy H, Janardhana MR (2013) Hydrogeochemical features of groundwater of semi-confined coastal aquifer in Amol-Ghaemshahr plain, Mazandaran Province, Northern Iran. Environ Monit Assess 185(11):9237–9264. https://doi.org/10.1007/s10661-013-3248-6
Khairy H, Janardhana MR (2014) Hydrogeochemistry and quality of groundwater of coastal unconfined aquifer in Amol-Ghaemshahr plain, Mazandaran Province, Northern Iran. Environ Earth Sci 71(11):4767–4782. https://doi.org/10.1007/s12665-013-2868-z
Kim K, Rajmohan N, Kim HJ, Hwang G-S, Cho MJ (2004) Assessment of groundwater chemistry in a coastal region (Kunsan, Korea) having complex contaminant sources: a stoichiometric approach. Environ Geol 46(6–7):763–774. https://doi.org/10.1007/s00254-004-1109-x
Kreitler CW (1993) Geochemical techniques for identifying sources of ground-water salinization, edited by K. C> Smoley. CRC Press, Boca Raton
Liu Y, Jiao JJ, Liang W, Kuang X (2017) Hydrogeochemical characteristics in coastal groundwater mixing zone. Appl Geochem 85:49–60
Lloyd J, Heathcote J (1985) Natural inorganic hydrochemistry in relation to groundwater: an introduction. Oxford University Press
Mandel S, Shiftan Z (1981) Groundwater resources investigation and development. Water Pollution (A Series of Monographs). Academic Press
Matthess G (1982) The properties of ground-water. Wiley, New York
Meybeck M (1987) Global chemical weathering of surficial rocks estimated from river dissolved loads. Am J Sci 287(5):401–428
Milliot G (1970) Geology of clays. Springer, New York
Mitra BK (1998) Spatial and temporal variation of groundwater quality in sand dune area of Aomori Prefecture in Japan. Paper no. 062023. In: 2006 ASAE Annual Meeting
Morgan JJ, Stumm W (1996) Aquatic chemistry: chemical equilibria and rates in natural waters. Wiley
Nematollahi MJ, Clark MJR, Ebrahimi P, Ebrahimi M (2018) Preliminary assessment of groundwater hydrogeochemistry within Gilan, a northern province of Iran. Environ Monit Assess 190(4):242. https://doi.org/10.1007/s10661-018-6543-4
Ozler MH (2003) Hydrochemistry and salt-water intrusion in the Van aquifer, east Turkey. Environ Geol 43(7):759–775. https://doi.org/10.1007/s00254-002-0690-0
Parkhurst D, Appelo C (2011) User’s guide to PHREEQC (version 2.18. 5570)-a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. USGS Water Resources Investigations Report. USGS Water Resources Investigations Report , pp. 99–4259
Petalas C, Diamantis I (1999) Origin and distribution of saline groundwaters in the upper Miocene aquifer system, coastal Rhodope area, northeastern Greece. Hydrogeol J 7(3):305–316
Piper A (1944) A graphical procedure in the geochemical interpretation of water analyses. Am Geophys Union Trans 25:914–923
Raghunath HM (2007) Hydrology: principles, analysis and design. New Age International
Richards L (1954) Diagnosis and improvement of saline and alkali soils (USDA. Agriculture handbook, Vol. 60). US Department of Agriculture, Washington DC
Saba NU, Umar R (2016) Hydrogeochemical assessment of Moradabad city, an important industrial town of Uttar Pradesh, India. Sustain Water Resour Manag 2(3):217–236. https://doi.org/10.1007/s40899-016-0053-8
Sajil Kumar PJ, Elango L, James EJ (2013) Assessment of hydrochemistry and groundwater quality in the coastal area of South Chennai, India. Arab J Geosci 7(7):2641–2653. https://doi.org/10.1007/s12517-013-0940-3
Saleh A, Al-Ruwaih F, Shehata M (1999) Hydrogeochemical processes operating within the main aquifers of Kuwait. J Arid Environ 42(3):195–209
Sarin M, Krishnaswami S, Dilli K, Somayajulu B, Moore W (1989) Major ion chemistry of the Ganga-Brahmaputra river system: Weathering processes and fluxes to the Bay of Bengal. Geochim Cosmochim Acta 53(5):997–1009
Sawyer CN, McCarty PL (1967) Chemistry for sanitary engineers (2ed.). McGraw-Hill, Berlin
SazabShargh (2010) Integrating studies of water resources. Sazab Shargh Consulting Engineering, Mashhad
Schoeller H (1977) Geochemistry of groundwater. Groundwater studies—an International Guide for Research and Practice, chapter 15. UNESCO, Paris, pp 1–18
Şehnaz Ş, Şener E, Davraz A, Varol S (2020) Hydrogeological and hydrochemical investigation in the Burdur Saline Lake Basin, southwest Turkey. Geochemistry 80(4):125592
Singh AK, Mondal G, Singh PK, Singh S, Singh T, Tewary B (2005) Hydrochemistry of reservoirs of Damodar River basin, India: weathering processes and water quality assessment. Environ Geol 48(8):1014–1028
Singh AK, Mondal GC, Singh TB, Singh S, Tewary BK, Sinha A (2012) Hydrogeochemical processes and quality assessment of groundwater in Dumka and Jamtara districts, Jharkhand, India. Environ Earth Sci 67(8):2175–2191. https://doi.org/10.1007/s12665-012-1658-3
Soltan ME (1998) Characterisation, classification, and evaluation of some ground water samples in upper Egypt. Chemosphere 37(4):735–745. https://doi.org/10.1016/s0045-6535(98)00079-4
Stallard RF, Edmond JM (1983) Geochemistry of the Amazon: 2. The influence of geology and weathering environment on the dissolved load. J Geophys Res Oceans 88(C14):9671–9688. https://doi.org/10.1029/JC088iC14p09671
Tarawneh MSM, Janardhana MR, Ahmed MM (2019) Hydrochemical processes and groundwater quality assessment in North eastern region of Jordan valley, Jordan. HydroResearch 2:129–145. https://doi.org/10.1016/j.hydres.2020.02.001
Tijani MN (1994) Hydrogeochemical assessment of groundwater in Moro area, Kwara state, Nigeria. Environ Geol 24(3):194–202. https://doi.org/10.1007/bf00766889
Vahdati Daneshmand F, Saidi A (1991) Sari; 1:250,000 scale geological quadrangle map of Iran. Geological Survey of Iran, Tehran
Vandenbohede A, Lebbe L (2012) Groundwater chemistry patterns in the phreatic aquifer of the central Belgian coastal plain. Appl Geochem 27(1):22–36. https://doi.org/10.1016/j.apgeochem.2011.08.012
Ward JJH (1963) Hierarchical grouping to optimize an objective function. J Am Stat Assoc 58(301):236–244
WHO (1997) Guidelines for drinking-water quality (Surveillance and control of community supplie, Volume 3). World Health Organization, Geneva
Wilcox L (1955) Classification and use of irrigation waters, vol 969. US Dept. of Agriculture, Washington D.C.
Yang Q, Li Z, Ma H, Wang L, Martín JD (2016) Identification of the hydrogeochemical processes and assessment of groundwater quality using classic integrated geochemical methods in the Southeastern part of Ordos basin, China. Environ Pollut 218:879–888
Yu J, He X (2006) Data statistical analysis and SPSS application. Posts & Telecommunications Press, Beijing
Acknowledgements
The authors are grateful to Prof. B. Krishna Rao, Mysuru, India for his support and to F. Mahbobi, Mazandaran RegionalWater Authority, Sari City, Iran for logistics.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khairy, H., Janardhana, M.R. Classification, hydrochemical characterization, and quality assessment of groundwater of coastal aquifer at Sari–Neka plain, Northern Iran. Environ Earth Sci 81, 3 (2022). https://doi.org/10.1007/s12665-021-10115-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-021-10115-9