Abstract
The objective of our study was to evaluate if there is heavy metal contamination in the water of rivers and irrigation canals of the Cotopaxi and Tungurahua provinces in Ecuador and assesing the health risk of this contamination in the neighboring population. To date no study has been done on contaminant health risk in Ecuador. For this purpose, 21 water samples collected along the Cutuchi, Pumacunchi and Ambato Rivers, the Latacunga-Salcedo-Ambato irrigation canal, tap water and water from a tannery were analyzed by atomic absorption spectrophotometry. The metals analyzed were Cd, Cr, Pb, Ni, Hg and As. At all points tested at least one of these metals surpassed the permissible limits under the Ecuadorian law or the Environmental Protection Agency. Cr had the highest level of toxicity, with critical values (8.3E + 03; 2.2E + 04; 1.8E + 06; 99.6, for non-carcinogenic risk; and 1.17; 0.63; 217.06, for carcinogenic risk) followed by Cd, Pb, As and Hg. Regarding health risk analyses, there was high risk, both carcinogenic and non-carcinogenic, for all metals analyzed; the most harmful was Cr followed by As. The most damaging exposure route was inhalation, followed by the dermal and ingestion routes. Children were the most vulnerable population for non-carcinogenic risk, while for carcinogenic risk; the population most likely to suffer some type of cancer was the adult one. So, it is clear that the populations living close to these rivers have a high probability of having different diseases and cancer, due to the high heavy metal contamination that exists in these waters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Agricultural activity, oil extraction and mining are the main economic resources of Ecuador and, precisely, these activities are the ones generating the most contamination in this country’s water and soil (González-Carrasco et al. 2011; San Sebastián et al. 2001; Sovacool and Scarpaci 2016) and in the food grown here. One of the main contaminations is caused by heavy metals; largely derived from anthropogenic activity, due to the use of pesticides and fungicides in agricultural activities, (Guo et al. 2014), to the emission of fumes and waste dumping in rivers and canals from industrial processes (Cruz et al. 2015), mining, burning of fossil fuels and incineration of solid waste. Although it can also stem from nature, mainly from volcanic activity (Valderas et al. 2013) and the weathering of rocks or metals present in the soil.
Although some heavy metals in very small amounts are beneficial and necessary for several vital processes in the body (Londoño-Franco et al. 2016); others, however, can be very noxious if they exceed permissible limits (Molina et al. 2012). The damages caused by the exposure to heavy metals depends on the duration of the exposure, the dose, the route of exposure, in addition to the chemical compound to which they are bound and their solubility (Tchounwou et al. 2012). The effects they have on each person will depend on sex, age, diet, lifestyle, personal characteristics and health condition (ATSDR 2012a, b, c, d, 2016a, b; WHO 2007, 2010).
The main health problems caused by overexposure to heavy metals, if they are inhaled, are respiratory problems (asthma, cough, shortness of breath and irregular breathing); while if ingested, they can cause digestive problems (nausea, vomiting and diarrhea), kidney, liver, bladder, lung, and stomach damage and breast cancers (Prieto Méndez et al. 2009; WHO 2007, 2010; ATSDR 2012a, b, c, d, 2016a, b). If the exposure is by dermal contact, allergies, ulcers, redness and swelling of the skin can occur, as in the cases of Ni and hexavalent Cr. Exposure to Cr, Ni and Pb in pregnant women can cause abortions, premature births or low birth weight (Tavakoly et al. 2011), in addition to affecting child development, diminishing mental and learning capacity (WHO 2007, 2010; ATSDR 2012a, c, d), and even leading to infertility (Hertz-Picciotto 2000). On the other hand, intoxication by metals such as Hg and Pb predominantly affect the nervous system, causing disorders at neurological level, such as decreased sense of touch, hearing and vision, emotional instability, tremors, memory loss, cognitive dysfunction, neuromuscular disorders, lack of coordination and delayed mental development (WHO 2007, 2010; Valderas et al. 2013; ATSDR 2012c, 2016b). These effects were detected in children of the Andean regions of Ecuador exposed to Pb contamination (Counter et al. 2015). There, children exposed to this metal had chronic neurocognitive adverse effects, showing decrease in spatial visual acuity and auditory memory over time. Other studies carried out on experimental animals have shown that exposure to Cd during pregnancy causes harmful effects on the offspring (Ohrvik et al. 2006), triggering behavioral alterations, weight reduction, and disorder in prepubertal development. (Alonso-González et al. 2007).
These effects of heavy metals on health can be evaluated using formulas that allow estimating the health risk undergone by people exposed to these contaminants. The Environmental Protection Agency (EPA 2014) has proposed several methods and conducted several studies on this topic, classifying exposure risk to different contaminants as carcinogenic and non-carcinogenic. For this, it is necessary to take into account several factors, such as the dose of the contaminant, the route and the duration of exposure. This way it is possible to assess the probability that the population, exposed to these pollutants, has of suffering the diseases described above or even cancer.
Although Ecuador has legislation (TULSMA 2013, Unified Text of Secondary Environmental Legislation), that regulates the permissible limits of heavy metals and other contaminants in water and soil, several studies have shown evidence that there is contamination by Cd and Pb in soils of the Guayas and Oro provinces (Pozo et al. 2011; Chavez et al. 2015) and by As in the water, soil and rice grown there (Otero et al. 2016). Pb contamination has also been found in soil of the city of Cuenca and its surroundings near the Quinuas River (Hewitt and Candy 1990). Regarding river water, contamination by Pb, Cr and Ni was found in the water of the Santiago River in the province of Esmeraldas (Cruz et al. 2015) and by Cr and Ni in its soil. In this same province, high levels of Hg were found in the Puyano River, as well as Pb and Cd (Tarras-Wahlberg et al. 2001). Not only were high levels of these metals obtained in the water of rivers, but Pb was also found in drinking water of the city of Portovelo (Betancourt et al. 2005).
Although some studies demonstrate metal contamination in soils and rivers of Ecuador, no study has been carried out on the state of the water in the rivers and irrigation canals of the Cotopaxi and Tungurahua provinces and much less on the health risk. Considering all this and taking into account that Ecuador is a developing country, in which one of its main economic resources is still agriculture and that this activity uses the water from rivers and canals to irrigate the crops, a study on the quality of water and the potential health risk entailed by exposure to the contaminants in these waters is needed and mainly in the Cotopaxi and Tungurahua provinces, where the foremost activity is agriculture. Contamination passes from the water to the soil and from there to the food cultivated on it (Liang et al. 2017), which will be ingested later; its concentration increases as it goes through the food chain (Molina et al. 2012) with the resulting increase in health risk. Besides, in these two provinces many inhabitants of the populations close to the rivers and irrigation canals do not have drinking water and they use water from these rivers for domestic use. Therefore, the purpose of our study was: (1) analyzing the concentration of As, Cd, Cr, Pb, Hg and Ni in the water of the rivers in the Cotopaxi and Tungurahua provinces and the Latacunga-Salcedo-Ambato irrigation canal, (2) evaluating exposure to these metals of the nearby population and (3) estimating the health risk of this population.
Methodology
Study area
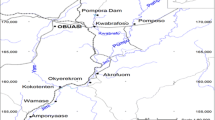
The study area is located in the central region of Ecuador (Fig. 1a) in the Andes mountain range (cordillera), the provinces studied were Cotopaxi and Tungurahua (Fig. 1b) between coordinates 328º NW and 50º NE. The waters analyzed were from the Cutuchi and Pumacunchi rivers in the Cotopaxi province, which later flow into the Latacunga-Salcedo-Ambato irrigation canal, also analyzed, and from the Ambato River in the Tungurahua province, as well as water from a tannery located in the industrial park of Ambato (Fig. 1c, d).
Map of the provinces where the study was conducted. The red marks indicate the points where water sampling was carried out (d) along the Cutuchi, Pumacunchi and Ambato rivers, the Latacunga-Salcedo-Ambato irrigation canal (c) and the tannery in the provinces of Cotopaxi and Tungurahua (b), Ecuador (a)
In these provinces, the rain fall level is in the range of 500–750 mm per year and evapotranspiration is in the range of 650–700 mm/year. Water flow of the Latacunga-Salcedo-Ambato irrigation canal is 4500 L/s, originating from the waters of the Cutuchi and Pumacunchi rivers, with an allocation of 0.7 L/s/ha. This water is used to irrigate the crops in this area that covers 6215.42 ha (MAGAP-SRD 2016).
Sampling
In Fig. 1, the 21 samples analyzed are shown, beginning in the Cutuchi and Pumacunchi rivers and along the irrigation canal, into which they flow, to reach the Ambato River. Samples of tap water and water from one of the many tanneries in the city of Ambato that dump their waste into the irrigation canal were also collected. The water samples were collected following the chain of supervision to ensure there was no manipulation or contamination until they reached the laboratory where they were analyzed. Samples were gathered during the months of October and November, corresponding to the dry season in this Andean region.
The water samples were collected in sterile and sealed PVC containers until the time of testing. The containers used for water collection were rinsed several times with the same water to ensure the same conditions.
For each sample, 1 L of water was collected at about 25 and 50 cm deep in the rivers and in the canal, always in the direction of the current and not at the banks. At the time of sampling, the following parameters were analyzed: pH and temperature. The coordinates of the point, where each sample was collected, were recorded. Once the water was collected, 5% HNO3 (pH 2) was added; then, the container was closed, identified and kept cold until it reached the laboratory, where the samples were kept at 4 ºC until analysis.
Sample analysis
Prior to the sample analysis, the water samples were pretreated, for this, the samples were brought to room temperature. Once the sample was acclimatized, a volume of 100 mL of sample was taken and 15 mL of concentrated HNO3 was added, then the mixture was heated to 250 °C until almost completely dried. Then 2 mL of concentrated HNO3 was added through the walls of the vessel and allowed to dry almost completely, then 10 mL of concentrated HCl was added and heated for 10 min, the mixture was allowed to cool to room temperature and then oxidized with 3 mL of peroxide of hydrogen 30% v/v, then heated for 5 min and allowed to cool. The obtained mixture was filtered and brought to 50 mL with distilled water.
The samples treated were analyzed in ISO 17025:2017 accredited laboratories using atomic absorption spectrophotometry techniques. A flame atomic absorption spectrometer (FAAS), Varian 220 Spectra AA model, serial number EL98023240, was used for element determinations of Cd, Cr, Pb, Ni, Hg and As metals.
Once the equipment was heated and stabilized, the flow rate was selected and the lamp to be used for each metal, the air extractor and the flame were ignited. Subsequently, the capillary was placed in the solution with the specific concentration for each metal to adjust to the maximum absorption specified for each metal. Next, the 5% HNO3 solution that was blank was passed and then the curve parameters were read. Them, after cleaning the capillary well, it is introduced into each of the solutions that were used to elaborate the standard curve, always cleaning the capillary between one reading and another. Once the curve was finished, the capillary was cleaned, passed through the target after checking the selected linearity curve or a correlation coefficient ≥ 0.995, and finally continued reading each water sample.
When samples are aspirated through the capillary they pass into a flame and are atomized using a composite source lamp for each metal. A beam of light is directed through the flame, towards a monochromator and towards a detector that measures the amount of light absorbed by the atomized element in the flame. The amount of energy in the characteristic wavelength absorbed in the flame is proportional to the concentration of the element in the sample in a limited concentration range.
Quality assurance and quality control were assessed using duplicates, reagent blanks, and certified reference materials, with each batch of samples. Matrix interference (Blank) was < 2% for all elements. Triplicates of samples analysis yielded relative percent differences of < 5%. Each calibration curve was evaluated by analyses of quality control standards before, during, and after the analyses of a set of samples. When the recovery rate become out of the recommended range (90–110%), samples were reanalyzed with a new calibration curve. Prior to each analysis, the instrument was calibrated according to the manufacturer’s recommendations and EPA 3010A, Rev. 01,1992 and Standard Methods Ed. 22, 2012, 3111 B. Background correction measurements were made using a deuterium lamp (Hamamatsu Photonic SK.K hollow-cathode lamp), operated at various currents, for the elements of interest. After each analytical run, measuring progress was displayed on the screen, and a visual check was made for linearity and replication. All the standard solutions (1000 mg/L) for Ni, Cr, Pb, Cd, As, and Hg were Merck (Darmstadt, Germany) spectroscopic grade. These solutions were carefully diluted with UHQ water (chemical resistivity: 18.2 MΩ/cm) to the required concentrations according to the EPA 3010A, Rev. 01, 1992 and Standard Methods Ed. 22, 2012, 3111 B.
The pH of the solutions was adjusted by adding HCl and NaOH solutions, and controlled with a Mettler Toledo Seven Compact S220 pH meter. An electrical heater (Binder-ED400) was used throughout the experiments.
Total heavy metal concentrations were analyzed in the water samples collected.
Health risk study
The probability of suffering any health problem, including cancer, due to chronic exposure to the heavy metals studied, which depended on the exposure route: ingestion, inhalation or dermal contact, was estimated for the inhabitants of the study areas.
Hazard identification
In this case the Cutuchi and Pumacunchi rivers, the Latacunga-Salcedo-Ambato irrigation canal and the Ambato River, were chosen since their water is utilized for crop irrigation in the area and for domestic use by the inhabitants of nearby populations that do not have sewage service. Close to these rivers and canal there are several industries that discharge their waste into these waters, in addition to a great agricultural activity in which a large amount of fertilizers and pesticides is used in an uncontrolled manner. To check if the water used in the process of dyeing leather is contaminated, water from these tanneries was also analyzed, since this water is dumped into the irrigation channel.
The metals analyzed were As, Cd, Cr, Pb, Hg, and Ni and the chosen exposure routes were ingestion, inhalation and dermal contact for river, canal and tap water and only dermal contact and inhalation for tannery water. Of these metals, As, Cd, Cr, and Ni are considered carcinogenic by the International Agency for Cancer Research (IARC 2018) and the EPA (USEPA 1989, 1991a, b) and Pb and Hg, as possible carcinogens.
Exposure evaluation
EPA indications were followed (USEPA 1989, 1991a, b, 2004) to calculate the doses received through the different exposure routes (ingestion, inhalation and dermal). In our study, both the adult and the child population were analyzed.
For exposure by ingestion, the equation applied was:
where CDIing is the average dose of daily exposure by ingested water, expressed in mg/kg/day. Cw (mg/L) is the concentration of metal in the water samples analyzed, since concentrations present great variation along the points analyzed, the average value for each one of the metals was considered its concentration, distinguishing between the water from the rivers and canal and the water samples collected inside the tannery; IR is the daily water ingestion rate expressed in L/day. In this study, 1 L was assumed for children and 2 L, for adults. EF is the exposure frequency (days/year); for our study we consider 350 days/year as chronic exposure. ED is the duration of the exposure expressed in years; in this study: 6 for children and 30 for adults; BW is the body weight in kg, for this study, 15 kg for children and 70 kg for adults. AT is the average time of exposure (in days); in our study we consider it as a life expectancy of approximately 70 years. These data were selected following the EPA recommendations (USEPA 1989, 1991a, b).
The dose of dermal exposure was calculated by the following equation:
where CDIderm is the average dose of daily dermal water exposure by ingestion, expressed in mg/kg/day. CW is the concentration of the metal in water. SA is the exposed skin area (cm2); for this study: 6660 cm2 for children and 18,000 cm2 for adults. Kp is the coefficient of skin permeability in water (cm/h); in this study: 0.001 cm/h for As and Cd, and Hg; 0.0001 cm/h for Pb; 0.002 cm/h for Cr and 0.0002 for Ni. ET is the duration of exposure (h/day); in this study: 0.6 h/day. The exposure variables were based on the standards proposed by the EPA (USEPA 1989, 1991a, b, 2004). In the case of the tannery, CDIderm was only calculated for adults since they are the ones, who work inside these industries and are in contact with the water there, it was not calculated for children.
The dose of exposure by inhalation was calculated using the following equation:
where CDIInh is the average dose of daily water exposure by inhalation expressed in mg/kg/day. CW is the concentration of the metal in water. K is the volatilization factor, in this case: 0.0005 × 10,000 L/m3. IRinh (cm) is the daily inhalation rate of the metal; in our study: 15 for adults and 8.7 for children. EF is the exposure frequency (days/year); chronic exposure: 350 days/year for river and canal water and 250 for tannery water. ED in this study was 6 for children and 30 for adults in river and irrigation canal water, and in the case of tannery water, 25 was used for adults. In the case of the tannery, CDIinh was only calculated for adults.
Risk characterization
For the risk characterization, the non-carcinogenic and carcinogenic risks were calculated.
Non-carcinogenic risk
The non-carcinogenic risk after exposure to a toxic substance, in this case heavy metals, is the probability that a person will suffer disease or harmful effect, excluding cancer. This risk (HQ) was calculated using the equations:
where CDI are the exposure doses for each metal analyzed and each exposure route and RfD is the corresponding reference dose for each of the analyzed metals depending on the route of exposure. RfD values were obtained from IRIS EPA (2011), USEPA (1989, 1991a; b), RAIS (1998) and Liang et al. (2017). A non-carcinogenic health risk is considered if HQ is > 1. This risk was not calculated for tannery water in children. The risk index (HI) was calculated to evaluate the sum of the non-carcinogenic risks of all the metals analyzed and all exposure routes. If HI is greater than 1, it indicates a potential adverse effect on human health (USEPA 1989, 1991a, b).
Carcinogenic risk
Carcinogenic risk is defined as the probability that an individual will eventually develop some type of cancer during his life, due to exposure to a potentially carcinogenic contaminant. The risk is proportional to the cumulative dose for life. This risk was evaluated for each of the exposure routes using the formulas:
where CR is the cancer risk expressed without units; CDI are exposure doses for each of the exposure routes and CSF is the cancer slope factor for each of the metals analyzed depending on the route of exposure. CSF values were obtained from IRIS EPA (2011), USEPA (1989, 1991a, b), RAIS (2011) and Liang et al. (2017). The acceptable range for carcinogenic risk is 10–6 to 10–4 (USEPA 1989, 1991a, b). This risk was also not calculated in children for tannery water.
To calculate total cancer risk, all the risks for all metals and all exposure routes were added, using the following equation:
Statistical analysis
The data were analyzed by a non-parametric test using the Graph Pad Prism program for a multiple comparison Kruskal–Wallis one-way ANOVA test, considering p < 0.05 as significant. The Pearson normality test was used to check if the values for each of the metals followed a normal distribution along all the points.
Results
Heavy metal levels in water of the rivers in the Cotopaxi and Tungurahua provinces
Among the parameters analyzed (Table 1), pH values in all samples analyzed were found to range around a neutral pH except in S15 with acid pH (4.0) and S20, alkaline pH (8.5). In these samples, there was a change in temperature with respect to other samples, surpassing the average values of around 16‒17 °C.
Regarding heavy metal concentrations (Table 1), in all the samples examined there was contamination by at least one of the metals analyzed. All metals except Ni exceeded the permissible limits established by the TULSMA, or EPA in at least one of the samples analyzed. The highest levels of contamination were found for Cr with values of 22.2 and 30.2 mg/L for S15 and S16, respectively. In the case of Cd, the highest level was found in S9 (0.23 mg/L); this value was 23 times higher than the permissible limits, according to the Ecuadorian legislation. The main source of contamination in the Cutuchi and Pumacunchi rivers was As, with the highest values found in S2 (0.062 mg/L) and S4 (0.067 mg/L) corresponding to the Pumacunchi River. Pb was found above the permissible limits in the irrigation canal, the tannery water and the Ambato River, with maximum values of 0.2 mg/L (S15), 0.15 mg/L (S16) and 0.18 mg/L (S17 and S18) in tannery water. In S21 from tap water, Pb is exactly within the permissible limit (0.05 mg/L). The highest level of Hg was found in S18 with a value of 0.0084 mg/ml; this metal also exceeded the permissible limits in the Pumacunchi River, the irrigation canal and the Ambato River.
Study of the health risk
The mean value for each metal was used to calculate the average dose of daily exposure in the nearby population for each of the metals analyzed. This dose was calculated for the different routes of exposure: ingestion, inhalation and dermal. The data obtained are shown in Table 2. They were higher for non-carcinogenic risk in children and for carcinogenic risk, in adults. Cr was the metal that presented the highest values for all exposure routes: 14.82 mg/kg/day and Hg, the lowest: 2.96E−08 mg/kg/day. Regarding the route of exposure, the highest values were obtained for inhalation and the values were higher for tannery water than river and irrigation canal water. Cr values of 14.82 mg/kg/day were calculated for adults in the tannery, and 0.18 mg/kg/day for non-carcinogenic risk in children in the rivers, and 5.29 mg/kg/day for carcinogenic risk in adults; the lowest CDI values were obtained for dermal contact.
Regarding non-carcinogenic risk calculation (Table 3), there was health risk (HQ > 1) for all metals analyzed and for all exposure routes, with the highest risk for inhalation. In the case of ingestion, non-carcinogenic risk was found for As: 2.02 in adults and for As, Cd and Cr in children, with values of 4.72; 1.55 and 1.38, respectively. Non-carcinogenic risk was observed for all metals by inhalation, with the highest risk for Cr, followed by Cd, As, Pb, Hg and Ni. This risk was greater in children than in adults for water from rivers and canals. In the case of Cr, the values (8.3E + 03; 2.2E + 04; 1.8E + 06) were well above what is admissible by the EPA, reaching alarming high risk values. By dermal contact there was only non-carcinogenic risk in the case of Cr for adults exposed to tannery water with values exceeding 99 times those established as admissible by the EPA.
The values obtained for carcinogenic risk are presented in Table 4, showing that cancer risk exists for both adults and children, due to the ingestion and inhalation of water; this risk, was higher for the adult population. There was no risk from dermal contact. In the case of CRing, risk is caused by As with values of 0.00039 in the adult population and 0.00018 in children; similar results were obtained for Cr. In the case of inhalation, all metals caused risk, except that Hg was not calculated, because CSF for this metal was not found. The metals that presented the highest carcinogenic risk were Cr > As > Cd > Ni > Pb, with values well above the range allowed by the EPA (10–4‒10–6), especially in the case of Cr for which 217 people who are exposed to this metal by inhalation are at risk of cancer. In the case of dermal contact, CSF was found only for As, so the CR could only be calculated for this metal and it was within normal parameters.
When HI and total CR were calculated (Table 5), it could be observed that there is as much non-carcinogenic risk 1.87E + 06; 3.7E + 04 as carcinogenic 218.5; 0.73, respectively for the adult and children populations studied. In both cases, the risk was higher for the adult population. These values were well above the values established as admissible by the EPA.
Discussion
The results obtained in this study evidence the high contamination by heavy metals present in the water of the rivers and irrigation canal of the Cotopaxi and Tungurahua provinces and the alarming level of health risk to which the inhabitants of this area are exposed.
Contamination of water in the Cotopaxi and Tungurahua provinces
The water of the Cutuchi, Pumacunchi and Ambato rivers and the Latacunga-Salcedo-Ambato irrigation canal presents heavy metal contamination along their entire length. Although not all samples showed contamination by the same metals; at all points analyzed there was contamination by at least one of them, depending on various factors such as pH, redox potential, water ion composition, ion exchange capacity (Prieto Méndez et al. 2009), presence of carbonates and organic matter, and mainly on the anthropogenic activities carried out in the vicinity of the analyzed rivers and canal.
In the case of the Cutuchi and Pumacunchi rivers, the main contamination was caused by As, surpassing the permissible limits established by the Ecuadorian TULSMA along their entire length, as well as at the first points sampled of the Latacunga-Salcedo-Ambato irrigation canal into which these two rivers flow. The high As content can stem from volcanic processes (WHO 2010; Tchounwou et al. 2012), considering that the region where high levels of As appeared is near the Cotopaxi volcano. This volcano was erupting at the time of the study, releasing large amounts of volcanic ash and toxic gases. These high As levels can originate from the ashes fallen into the water due to the eruptive process. Besides, higher levels of As were present in samples of the Cutuchi and Pumacunchi rivers, closer to the Cotopaxi volcano, decreasing as we move away from it. Normal levels were detected in the last points sampled of the canal and the Ambato river, which are the points farthest from the volcano. This is because As is diluted by the flowing water as it moves down the canal and the river, or it is absorbed by organic matter and sediments in its path (Cumbal et al. 2010). These data agree with the results obtained by Cumbal et al. (2009, 2010) in the thermal waters of Papallacta and the hot springs of the Andean region of Ecuador (Carchi, Imbabura, Pichincha, Cotopaxi and Tungurahua), in which high levels of As were observed for all these geothermal sources analyzed. This is in agreement with the fact that all these areas were close to volcanic areas, with equally high levels of As found in the Cotopaxi and Tungurahua provinces. In addition, levels of As exceeding the permissible limits were also found in the rivers and streams near the hot springs, so it is evident that As contamination is caused by the ashes emitted by the volcano.
In these two rivers and the irrigation canal, there was Pb and Cr contamination in the Cutuchi River and the irrigation canal; by Hg in the Pumacunchi River and the entire irrigation canal and by Cd at the entry of both rivers into the irrigation canal. Mainly, this contamination is due to dumping by the many nearby industries (lumber, paper, metallurgy and dairy), rubbish and debris dumped into the river by the residents of the area, burning of urban garbage, gases emitted by the industries and burning of fossil fuels (Mite et al. 2010), apart from the uncontrolled use of pesticides and fertilizers (Jiao et al. 2012; Ruiz et al. 2017) in agricultural activities carried out locally (Breilh et al. 2012), especially floriculture and broccoli, with high content of metals, mostly Cd (Chen et al. 2007; McDowell et al. 2013). In the case of Pb, contamination could also come from lubricator plants in the area that dump their waste into the rivers. The same high Pb content results were obtained in the water of the Guayas River basin by Pozo et al. (2011), due to the release of Pb materials from the city of Guayas. In addition, the management techniques used in agriculture can cause changes in the soil that lead to an increase in runoff, which, in turn, can increase contaminant levels in the water (Ruiz et al. 2017). Similar results were found by Mancilla-Villa et al. (2012) in river and irrigation water in Puebla and Veracruz in Mexico. They found that contamination by As, Cd, Pb and Hg exceeded the permissible limits established by the EPA for irrigation water. In this case, the contamination stemmed from the agricultural activity, and the release of residues from the sugar industry.
In the final part of the irrigation canal and in the Ambato River, high contamination by Cr, Pb and Hg was found. Cr was the most abundant, well above the permissible limits established by Ecuadorian legislation and the EPA. In fact, these data can be considered alarming because of the high concentrations detected and the health problems that may arise. These high levels of Cr, Pb and Hg are mainly generated by industrial waste dumping into the canal and river, since the Ambato industrial park is located in this area. Tanning is one of the main industries in the area. Tannery water was also analyzed and high levels of these same metals were found, mainly Cr with values exceeding by almost 50% those established by the TULSMA and the EPA, because the chemical compounds used in leather tanning and dyeing basically contain Cr salts, in addition to other chemical compounds. The high levels of Cr, Pb and Hg decrease as samples were collected farther away from the industrial park. Therefore, making it clear that the contamination of the canal and the Ambato River comes from the waste that these tanneries and industries release into the rivers. The same contamination by Pb and Cr, as well as Ni, was found in the Santiago River in the province of Esmeraldas with values above the permissible limits (Cruz et al. 2015). This contamination largely originates in the mining and agricultural activities carried out in that area. Mining activity also caused contamination by Pb and Hg in the Puyano River in the Oro province (Betancourt et al. 2005) and by Hg, As, Cr, Pb and Ni in the rivers near the mines in southern Ecuador (Carling et al. 2013).
Other studies in different parts of the country also reveal contamination by heavy metals in river and canal waters, as in the case of the Azuay and El Oro irrigation water where Cd contamination was found (Mite et al. 2010), and As contamination in the Guayas and El Oro rivers (Otero et al. 2016). In addition to contamination in rivers, heavy metal contamination has also been found in soils and crops in several areas of the country (Hewitt and Candy 1990; Mite et al. 2010; Chavez et al. 2015). The problem of heavy metal contamination in water and soil not only exists in Ecuador; it mainly affects developing countries that do not have good drinking water or sewage systems, especially in rural areas. Several studies have shown this problem (Chambi et al. 2012; Hrubá et al. 2012; Mancilla-Villa et al. 2012; Molina et al. 2012; Villamarín et al. 2013; Lu et al. 2015). Studies also show that this contamination exists in developed countries (Turer and Maynard 2003; Sainz et al. 2004; Tóth et al. 2016; Bahloul et al. 2018); so this is a worldwide problem that should be thoroughly studied to find a solution.
Health risk due to heavy metal exposure
After evaluating non-carcinogenic and carcinogenic risk for inhabitants living near the rivers and canals analyzed, it was found that for both children and adults there are non-carcinogenic and carcinogenic risks, because they are exposed to high metal concentrations, exceeding the permissible limits.
Among the routes of exposure, inhalation proved to be the most dangerous route, followed by ingestion and lastly, the dermal route. This is due to the exposure to high metal concentrations and the low value of the reference dose.
With respect to non-carcinogenic risk, children are the most likely to suffer diseases; because they are more vulnerable to metal exposure, since their toxin tolerance is lower. These data agree with those of Zeng et al. (2015), who found children had the highest values for non-carcinogenic risk, in this case by ingestion. Among the different exposure routes, Cr was the most dangerous metal, with alarming HQ values, since they were well above what is considered admissible by the EPA. These values were especially critical in the tannery water analyzed, a disturbing fact, considering that the workers are exposed to this metal by inhalation or by dermal contact and can suffer damages such as nasal ulcers, asthma, respiratory problems, allergy, liver and kidney problems, high levels of proteinuria, and DNA damage (WHO 2007, 2010; Tchounwou et al. 2012). These high Cr values were mostly due to the high concentration found for this metal in the water inside the tannery and in the last points of the canal and the Ambato River. In addition to Cr, there was also non-carcinogenic risk for Cd, As, Pb, Hg and Ni, mainly by inhalation, with disturbing values for Cd and As and by ingestion for As and Cd. Similar results were found in a tailings pond in China that showed non-carcinogenic risk for Cr, As, Cd and Pb (Liang et al. 2017). Other studies corroborate these results (Cabrera et al. 2009). The non-carcinogenic risks caused by As and Cd are vascular problems such as hypertension and diabetes; gastrointestinal disorders, such as vomiting and diarrhea; neurological disorders, when ingested, and headache and lung conditions, when inhaled (Wu et al. 2009; Tchounwou et al. 2012). In the case of Pb and Hg, they predominantly cause neurological and developmental disorders, difficulties in speech and hearing, low IQ and short height, as well as gastrointestinal, cardiovascular, renal and immune system deficiencies (ATSDR 2012c, 2016b; García-García et al. 2015). When HI was calculated for all the exposure routes, alarming values were demonstrated that are considered inadmissible by the EPA. In this case, risk was greater for adults than for children, because HQ was not calculated for children in tannery water samples by inhalation or dermal contact. These data indicate that the populations near these rivers and the irrigation canal have a very high probability of having health problems and chronic diseases, especially children, who are the most vulnerable.
The data obtained for carcinogenic risk show there is high probability for the neighboring population to develop cancer both by ingestion and inhalation of these metals. The latter route poses higher risk, primarily due to the high levels found in the tannery water. Since inhalation and dermal contact risks in tannery water were not calculated for children, they were higher in the adult population. As in the case of non-carcinogenic risk, the highest carcinogenic risk values were determined for Cr. The data were perturbing and inadmissible when compared to those established by the EPA (USEPA 1989, 1991a, b). In the tannery, 217 people are at risk for some type of cancer such as lung cancer by inhalation, and liver, kidney, prostate, and gastric cancer by ingestion (Cantor 1997). In addition to this metal, carcinogenic risk can also be caused by As, Cd, Pb and Ni, data that agree with studies carried out in China (Liang et al. 2017), where carcinogenic risk was found for these metals.
Although several studies have evaluated the health risk by heavy metal exposure in water and other sources (Kurt-Karakus 2012; Peña-Fernández et al. 2014; Duodu et al. 2016; Maigari et al. 2016; Li et al. 2017; Augustsson et al. 2018; Huang et al. 2018), no study has been carried out so far in Ecuador on the health risk from exposure to heavy metals. Therefore, the data provided in this article are novel, relevant and crucial to raise the awareness of the population and the government bodies on the heavy metal contamination problem that exists in this country and most importantly the health consequences this contamination causes to the people exposed.
Conclusions
In our investigation, we found that the Cutuchi, Pumacunchi and Ambato rivers, and the Latacunga-Salcedo-Ambato irrigation canal have heavy metals contamination that is well above the values allowed by the Ecuadorian legislation (TULSMA), being Cr the metal with the highest values. In addition, the data obtained for non-carcinogenic and carcinogenic risk are alarming, since there is a very high probability that the populations near these areas may suffer diseases that include cancer. According to these results, one of the populations most exposed to these risks are the farmers of the area, who use these waters for the daily irrigation of their crops, in addition to the tannery workers, where the highest levels of Cr were obtained.
One of the problems that derives from this contamination is that when the waters of these rivers and channels are used for crop irrigation, these contaminants enter the soil from the water and from there pass on to the food, which will then be ingested by inhabitants of the nearby towns. This increases the bioaccumulation of these metals along the food chain, raising the health risk. The severity of these effects will depend on the duration of exposure and the dose. In addition, it must be kept in mind that fruits and vegetables grown in these areas are not only consumed at a regional level; Ecuador exports them to other countries, especially to Europe. Thus, this problem does not only affect Ecuador, but also the rest of the countries to which these foods are exported and consumed.
Due to the high values obtained for both non-carcinogenic and carcinogenic risks, a thorough epidemiological study is necessary to establish what type of pathologies and diseases are suffered by the inhabitants of these provinces and the populations near these rivers and canals, since some of them can be related to the consumption of these waters and foods grown in those areas.
Taking into account this problem, which not only occurs in the provinces studied but also in the rest of the country, according to the results obtained by other authors, government agencies must establish environmental management measures to ensure the quality of water of rivers and channels and water of domestic use. To do so, government agencies must control the management of waste and its discharge into rivers and channels by industries, and the uncontrolled use of pesticides and fertilizers, which would contribute to reducing these health risks.
On the other hand, procedures for cleaning and decontaminating the water must be developed to eliminate the high concentrations of heavy metals that already exist. One of these procedures could be Phytoremediation through the use of certain plants that have the capacity to absorb these metals and eliminate their high concentrations, which would lead to an adequate maintenance of the ecosystem, avoiding the health problems caused by these heavy metals.
References
Alonso-González C, González A, Mazarrasa O, Güezmes A, Sánchez-Mateos S, Martínez-Campa C, Cos S, Sánchez-Barceló EJ, Mediavilla MD (2007) Melatonin prevents the estrogenic effects of sub-chronic administration of cadmium on mice mammary glands and uterus. J Pineal Res 42:403–410. https://doi.org/10.1111/j.1600-079X.2007.00434.x
ATSDR (2012a) Agency for Toxic Substances and Disease Registry. Toxicological profile for Chromium. U.S. Department of Health and Human Services, Public Health Service, Atlanta, pp 7–435. https://www.atsdr.cdc.gov/toxprofiles/tp7.pdf
ATSDR (2012b) Agency for Toxic Substances and Disease Registry. Toxicological profile for Cadmium. U.S. Department of Health and Human Services, Public Health Service, Atlanta, pp 8–350. https://www.atsdr.cdc.gov/toxprofiles/tp5.pdf
ATSDR (2012c) Agency for Toxic Substances and Disease Registry. Toxicological profile for Lead. U.S. Department of Health and Human Services, Public Health Service, Atlanta, pp 1–413. https://www.atsdr.cdc.gov/toxprofiles/tp13.pdf
ATSDR (2012d) Agency for Toxic Substances and Disease Registry. Toxicological profile for Nickel. U.S. Department of Health and Human Services, Public Health Service, Atlanta, pp 1–283. https://www.atsdr.cdc.gov/toxprofiles/tp15.pdf
ATSDR (2016a) Agency for Toxic Substances and Disease Registry. Addendum to the toxicological profile for Arsenic. U.S. Department of Health and Human Services, Public Health Service, Atlanta, pp 1–166. https://www.atsdr.cdc.gov/toxprofiles/Arsenic_addendum.pdf
ATSDR (2016b) Agency for Toxic Substances and Disease Registry. Addendum to the toxicological profile for Mercury. U.S. Department of Health and Human Services, Public Health Service, Atlanta, pp 4–142. https://www.atsdr.cdc.gov/toxprofiles/mercury_organic_addendum.pdf
Augustsson A, Uddh-Söderberg T, Filipsson M, Helmfrid I, Berglund M, Karlsson H, Hogmalm J, Karlsson A, Alriksson S (2018) Challenges in assessing the health risks of consuming vegetables in metal-contaminated environments. Environ Int 113:269–280. https://doi.org/10.1016/j.envint.2017.10.002
Bahloul M, Baati H, Amdouni R, Azri C (2018) Assessment of heavy metals contamination and their potential toxicity in the surface sediments of Sfax Solar Saltern, Tunisia. Environ Earth Sci 77(1):27. https://doi.org/10.1007/s12665-018-7227-7
Betancourt O, Narváez A, Roulet M (2005) Small-scale gold mining in the Puyango River Basin, Southern Ecuador: a study of environmental impacts and human exposures. Eco Health 2(4):323–332. https://doi.org/10.1007/s10393-005-8462-4
Breilh J, Pagliccia N, Yassi A (2012) Chronic pesticide poisoning from persistent low-dose exposures in Ecuadorean floriculture workers: toward validating a low-cost test battery. Int J Occup Environ Health 18(1):7–21. https://doi.org/10.1179/1077352512Z.0000000002
Cabrera PP, Blanco AV, Toujague R, Leal RM, Acosta F, Jaimez E (2009) Evaluación del riesgo para la salud humana por exposición a arsénico y plomo en la mina Delita y sus alrededores. Revista Ciencias de la Tierra y el Espacio 10:50–62
Carling GT, Diaz X, Ponce M, Perez L, Nasimba L, Pazmino E, Rudd A, Merugu S, Fernandez DP, Gale BK (2013) Particulate and dissolved trace element concentrations in three southern Ecuador rivers impacted by artisanal gold mining. Water Air Soil Pollut 224:1–16. https://doi.org/10.1007/s11270-012-1415-y
Cantor KP (1997) Drinking water and cancer. Cancer Causes Control 8(3):292–308. https://doi.org/10.1023/A:1018444902486
Chambi LJ, Orsag V, Niura A (2012) Evaluación de la Presencia de metales pesados y arsénico en suelos agrícolas y cultivos en tres micro-cuencas del Municipio de Poopó. Rev Bol Quim 29(1):111–119
Chavez E, He ZL, Stoffella PJ, Mylavarapu RS, Li YC, Moyano B, Baligar VC (2015) Concentration of cadmium in cacao beans and its relationship with soil cadmium in southern Ecuador. Sci Total Environ 533:205–214. https://doi.org/10.1016/j.scitotenv.2015.06.106
Chen W, Chang AC, Wu L (2007) Assessing long-term environmental risks of trace elements in phosphate fertilizers. Ecotoxicol Environ Saf 67(1):48–58. https://doi.org/10.1016/j.ecoenv.2006.12.013
Counter SA, Buchanan LH, Ortega F, Laurell G (2015) Neurocognitive status of Andean Children with chronic environmental lead exposure. J Environ Occup Sci 4(4):179–184. https://doi.org/10.5455/jeos.20151029110613
Cruz MC, Ortega MB, Mosalve ER, Mihi DR, Rodríguez ES (2015) Análisis del contenido de metales en aguas, sedimentos y peces en la cuenca del río Santiago, provincia de Esmeraldas. Ecuador Investigación y Saberes 4(2):32–42
Cumbal L, Bundschuh J, Aguirre V, Murgueitio E, Tipán I, Chavez C (2009) The origin of arsenic in waters and sediments from Papallacta Lake in Ecuador. In: Bundschuh J, Armienta MA, Birkle P, Bhattacharya P, Matschullat J, Mukherjee AB (eds) Natural arsenic in ground waters of Latin America occurrence health impact and remediation. Taylor & Francis Group, London, pp 81–90
Cumbal L, Vallejo P, Rodriguez B, Lopez D (2010) Arsenic in geothermal sources at the north-central Andean region of Ecuador: concentrations and mechanisms of mobility. Environ Earth Sci 61(2):299–310. https://doi.org/10.1007/s12665-009-0343-7
Duodu GO, Goonetilleke A, Ayoko GA (2016) Comparison of pollution indices for the assessment of heavy metal in Brisbane River sediment. Environ pollut 219:1077–1091. https://doi.org/10.1016/j.envpol.2016.09.008
Echeverry G, Zapata AM, Paéz MI, Méndez F, Peña M (2015) Valoración del riesgo en salud en un grupo de población de Cali, Colombia, por exposición a plomo, cadmio, mercurio, ácido 2, 4-diclorofenoxiacético y diuron, asociada al consumo de agua potable y alimentos. Biomédica. https://doi.org/10.7705/biomedica.v35i0.2464
EPA (2014) United States Environmental Protection Agency. Conducting a Human Health Risk Assessment. https://www.epa.gov/risk/conducting-human-health-risk-assessment
García-García MSN, Pedraza-Garciga CJ, Martínez M, Leyva CJ (2015) Evaluación preliminar de riesgos para la salud humana por metales pesados en las bahías de Buenavista y San Juan de los Remedios, Villa Clara. Cuba Rev Cub Quim 24(2):126–135
González-Carrasco V, Velasquez-Lopez PC, Olivero-Verbel J, Pájaro-Castro N (2011) Air mercury contamination in the gold mining town of Portovelo, Ecuador. Bull Environ Contam Toxicol 87(3):250–253. https://doi.org/10.1007/s00128-011-0345-5
Guo W, Fu Y, Ruan B, Ge H, Zhao N (2014) Agricultural non-point source pollution in the Yongding River Basin. Ecol Indic 36:254–261. https://doi.org/10.1016/j.ecolind.2013.07.012
Hertz-Picciotto I (2000) The evidence that lead increases the risk for spontaneous abortion. Am J Ind Med 38:300–309. https://doi.org/10.1080/03630242.2010.532760
Hewitt CN, Candy GBB (1990) Soil and street dust heavy metal concentrations in and around Cuenca, Ecuador. Environ Pollut 63(2):129–136. https://doi.org/10.1016/0269-7491(90)90063-I
Hrubá F, Strömberg U, Černá M, Chen C, Harari F, Harari R, Krsnik M (2012) Blood cadmium, mercury, and lead in children: An international comparison of cities in six European countries, and China, Ecuador, and Morocco. Environ Int 41:29–34. https://doi.org/10.1016/j.envint.2011.12.001
Huang Y, Chen Q, Deng M, Japenga J, Li T, Yang X, He Z (2018) Heavy metal pollution and health risk assessment of agricultural soils in a typical peri-urban area in Southeast China. J Environ Manag 207:159–168. https://doi.org/10.1016/j.jenvman.2017.10.072
IARC (2018) International Agency for Research on Cancer. IARC Monographs on the evaluation of carcinogenic risks to humans—Complete list of agents evaluated and their classification volumes 1–122. https://monographs.iarc.fr/list-of-classifications-volumes/
IRIS EPA (2011) Integrated Risk Information System (IRIS) U.S. Environmental Protection Agency. Chemical Assessment Summary National Center for Environmental Assessment. IRIS Assessments. Browse A to Z List of Chemicals. https://cfpub.epa.gov/ncea/iris2/atoz.cfm
Jiao W, Chen W, Chang AC, Page AL (2012) Environmental risks of trace elements associated with long-term phosphate fertilizers applications: a review. Environ Pollut 168:44–53. https://doi.org/10.1016/j.envpol.2012.03.052
Kurt-Karakus PB (2012) Determination of heavy metals in indoor dust from Istanbul, Turkey: estimation of the health risk. Environ Int 50:47–55. https://doi.org/10.1016/j.envint.2012.09.011
Li F, Qiu Z, Zhang J, Liu W, Liu C, Zeng G (2017) Investigation, pollution mapping and simulative leakage health risk assessment for heavy metals and metalloids in groundwater from a typical brownfield, middle China. Int J Environ Res Public Health 14(7):768. https://doi.org/10.3390/ijerph14070768
Liang Y, Yi X, Dang Z, Wang Q, Luo H, Tang J (2017) Heavy metal contamination and health risk assessment in the vicinity of a tailing pond in Guangdong, China. Int J Environ Res Public Health 14(12):1557. https://doi.org/10.3390/ijerph14121557
Londoño-Franco LF, Londoño-Muñoz PT, Muñoz-Garcia FG (2016) Los riesgos de los metales pesados en la salud humana y animal. Biotecnología en el Sector Agropecuario y Agroindustrial 14(2):145–153. https://doi.org/10.18684/BSAA(14)145-153
Lu Y, Song S, Wang R, Liu Z, Meng J, Sweetman AJ, Jenkins A, Ferrier RC, Li H, Luo W, Wang T (2015) Impacts of soil and water pollution on food safety and health risks in China. Environ Int 77:5–15. https://doi.org/10.1016/j.envint.2014.12.010
MAGAP-SRD (2016) Estudio viabilidad de presurización en sistemas de riego públicos del Ecuador, pp 108–126
Maigari AU, Ekanem EO, Garba IH, Harami A, Akan JC (2016) Health risk assessment for exposure to some selected heavy metals via drinking water from Dadinkowa Dam and River Gombe Abba in Gombe State, Northeast Nigeria. World J Anal Chem 4(1):1–5. https://doi.org/10.12691/wjac-4-1-1
Mancilla-Villa ÓR, Ortega-Escobar HM, Ramírez-Ayala C, Uscanga-Mortera E, Ramos-Bello R, Reyes-Ortigoza AL (2012) Metales pesados totales y arsénico en el agua para riego de Puebla y Veracruz, México. Rev Int Contam Ambient 28(1):39–48
McDowell R, Taylor M, Stevenson B (2013) Natural background and anthropogenic contributions of cadmium to New Zealand soils. Agric Ecosyst Environ 165:80–87. https://doi.org/10.1016/j.agee.2012.12.011
Mite F, carrillo M, Durango Wuellins (2010) Avances del monitoreo de presencia de cadmio en almendras de cacao, suelos y agua en Ecuador. In: Conference Paper. XII congreso ecuatoriano de la ciencia del suelo
Molina CI, Ibañez C, Gibon FM (2012) Proceso de biomagnificación de metales pesados en un lago hiperhalino (Poopó, Oruro, Bolivia): Posible riesgo en la salud de consumidores. Ecología en Bolivia 47(2):99–118
Ohrvik H, Yoshioka M, Oskarsson A et al (2006) Cadmium-induced disturbance in lactating mammary glands of mice. Toxicol Lett 164:207–213. https://doi.org/10.1016/j.toxlet.2005.12.008
Otero XL, Tierra W, Atiaga O, Guanoluisa D, Nunes LM, Ferreira TO, Ruales J (2016) Arsenic in rice agrosystems (water, soil and rice plants) in Guayas and Los Ríos provinces, Ecuador. Sci Total Environ 573:778–787. https://doi.org/10.1016/j.scitotenv.2016.08.162
Peña-Fernández A, González-Muñoz MJ, Lobo-Bedmar MC (2014) Establishing the importance of human health risk assessment for metals and metalloids in urban environments. Environ Int 72:176–185. https://doi.org/10.1016/j.envint.2014.04.007
Prieto Méndez J, González Ramírez CA, Román Gutiérrez AD, Prieto García F (2009) Contaminación y fitotoxicidad en plantas por metales pesados provenientes de suelos y agua. Trop Subtrop Agroecosyst 10(1):29–44
Pozo W, Santafeliu T, Carrera G (2011) Metales pesados en humedades de arroz en la Cuenca baja del Río Guayas. Masakana 2(1):17–30
RAIS (1998) The risk assessment information system. https://rais.ornl.gov/tools/tox_profiles.html
RAIS (2011) The risk assessment information system. https://rais.ornl.gov/tutorials/toxvals.html
Ruiz D, Martínez Idrobo J, Otero Sarmiento J, Figueroa Casas A (2017) Effects of productive activities on the water quality for human consumption in an Andean basin, a case study. Rev Int Contam Ambient 33(3):361–375. https://doi.org/10.20937/RICA.2017.33.03.01
Sainz A, Grande JA, De la Torre ML (2004) Characterisation of heavy metal discharge into the Ria of Huelva. Environ Int 30(4):557–566. https://doi.org/10.1016/j.envint.2003.10.013
San Sebastián M, Armstrong B, Cordoba JA, Stephens C (2001) Exposures and cancer incidence near oil fields in the Amazon basin of Ecuador. Occup Environ Med 58(8):517–522. https://doi.org/10.1136/oem.58.8.517
Sovacool BK, Scarpaci J (2016) Energy justice and the contested petroleum politics of stranded assets: policy insights from the Yasuní-ITT Initiative in Ecuador. Energy Policy 95:158–171. https://doi.org/10.1016/j.enpol.2016.04.045
Tarras-Wahlberg NH, Flachier A, Lane SN, Sangfors O (2001) Environmental impacts and metal exposure of aquatic ecosystems in rivers contaminated by small scale gold mining: the Puyango River basin, southern Ecuador. Sci Total Environ 278:238–261. https://doi.org/10.1016/S0048-9697(01)00655-6
Tavakoly B, Sulaiman AH, Monazami GH, Salleh A (2011) Assessment of sediment quality according to heavy metal status in the West Port of Malaysia. World Acad Sci Eng Technol 3(2):633–637. https://repository.um.edu.my/id/eprint/19842
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metals toxicity and the environment. Mol Clin Environ Toxicol. 101:133–164. https://doi.org/10.1007/978-3-7643-8340-4_6
Tóth G, Hermann T, Da Silva MR, Montanarella L (2016) Heavy metals in agricultural soils of the European Union with implications for food safety. Environ Int 88:299–309. https://doi.org/10.1016/j.envint.2015.12.017
TULSMA (2013) Texto Unificado de Legislación Secundaria Medio Ambiental. Libro VI, Anexo 1, Norma de Calidad Ambiental y de Descargas de Efluente: Recurso Agua, pp 286–339. https://extwprlegs1.fao.org/docs/pdf/ecu112180.pdf
Turer DG, Maynard BJ (2003) Heavy metal contamination in highway soils. Comparison of Corpus Christi, Texas and Cincinnati, Ohio shows organic matter is key to mobility. Clean Technol Environ Policy 4(4):235–245. https://doi.org/10.1007/s10098-002-0159-6
USEPA (1989) Risk assessment guidance for superfund: volume I—Human Health Evaluation Manual (Part A) Interim Final. Office of Emergency and Remedial Response U.S. Environmental Protection Agency Washington, D.C. https://www.epa.gov/sites/production/files/2015-09/documents/rags_a.pdf
USEPA (1991a) Risk assessment guidance for superfund: volume I—Human Health Evaluation Manual (Part B, Development of Risk-based Preliminary Remediation Goals) Interim Office of Emergency and Remedial Response U.S. Environmental Protection Agency Washington, DC. https://hwbdocuments.env.nm.gov/Los%20Alamos%20National%20Labs/References/9540.PDF
USEPA (1991b) Risk assessment guidance for superfund: volume I—human health evaluation manual supplemental guidance "standard default exposure factors” interim final. United States environmental protection agency Washington, DC. https://rais.ornl.gov/documents/OSWERdirective9285.6-03.pdf
USEPA (2004) Risk assessment guidance for superfund: volume I—Human Health Evaluation Manual (Part E, Supplemental Guidance for Dermal Risk Assessment) Final. Office of Superfund Remediation and Technology Innovation U.S. Environmental Protection Agency Washington, DC. https://www.epa.gov/sites/production/files/2015-09/documents/part_e_final_revision_10-03-07.pdf
USEPA (2009) Risk assessment guidance for superfund: Volume I—Human Health Evaluation Manual (Part F, Supplemental Guidance for Inhalation Risk Assessment) Final. Office of Superfund Remediation and Technology Innovation Environmental Protection Agency Washington, D.C. https://www.epa.gov/sites/production/files/2015-09/documents/partf_200901_final.pdf
Valderas J, Mejías ME, Riquelme J, Aedo K, Aros S, Barrera F (2013) Intoxicación familiar por mercurio elemental: Caso clínico. Rev Chil Pediatr. 84(1):72–79. https://doi.org/10.4067/S0370-41062013000100009
Villamarín C, Rieradevall M, Paul MJ, Barbour MT, Prat N (2013) A tool to assess the ecological condition of tropical high Andean streams in Ecuador and Peru: the IMEERA index. Ecol Indic 29:79–92. https://doi.org/10.1016/j.ecolind.2012.12.006
WHO (2007) Health risks of heavy metals from long-range transboundary air pollution. Joint WHO/Convention Task Force on the Health Aspects of Air Pollution. Europe, pp 1–144. https://www.who.int/iris/handle/10665/107872
WHO (2010) Health impacts of chemicals. Ten chemicals of major public health concern. https://www.who.int/ipcs/assessment/public_health/chemicals_phc/en/
Wu B, Zhao DY, Jia HY, Zhang Y, Zhang XX, Cheng SP (2009) Preliminary risk assessment of trace metal pollution in surface water from Yangtze River in Nanjing Section, China. Bull Environ Contam Toxicol 82(4):405–409. https://doi.org/10.1007/s00128-008-9497-3
Zeng X, Liu Y, You S, Zeng G, Tan X, Hu X, Hu X, Huang L, Li F (2015) Spatial distribution, health risk assessment and statistical source identification of the trace elements in surface water from the Xiangjiang River, China. Environ Sci Pollut Res 22(12):9400–9412. https://doi.org/10.1007/s11356-014-4064-4
Acknowledgements
This work has been carried out as part of Project 1370-CU-P-2014, funded by the Technical University of Ambato, and the Prometeo Scholarship Program of the National Secretariat of Higher Education, Science, Technology and Innovation of Ecuador. The authors would like to thank the CORLABEC laboratories for water sample analysis and MAGAP for information on the irrigation canal and maps. The authors would like to thank MSc. Eloisa Le Riverend, Janet Lynn and Darren Clarke for their review of English.
Funding
This work has been carried out as part of Project 1370-CU-P-2014, funded by the Research Directorate of the Technical University of Ambato, and the 0932-CU-P-2016 Project financed by the Technical University of Ambato; in addition to the Prometeo Scholarship Program of the National Secretariat of Higher Education, Science, Technology and Innovation of Ecuador all granted to SS-M.
Author information
Authors and Affiliations
Contributions
SS-M, LVP, and MACS participated in the collection of samples; SS-M, LVP, MACS and DACR participated in the critical review; SS-M drafted and approved its final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sánchez-Mateos, S., Pérez, L.V., Córdova Suárez, M.A. et al. Heavy metal contamination in the Cotopaxi and Tungurahua rivers: a health risk. Environ Earth Sci 79, 144 (2020). https://doi.org/10.1007/s12665-020-8869-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-020-8869-9