Abstract
Characterization of the groundwater flow systems can be used in conjunction with the geochemical evolution of groundwater to assess water–rock interaction processes, time of residence, and the regional hydrogeochemical evolution system. To identify the regime of groundwater flow, we consider it is essential to localize the geographic components of the hydrologic cycle, where the geologic environment determines the functional areas such as the recharge and discharge areas and the dynamics of water availability of the aquifer, as well as water quality. In this paper, we analyzed the groundwater flow systems on a section of chalky rocks, known as Plataforma Valle-San Luis Potosí (PVSL), located in the state of Tamaulipas, Northeast Mexico. The groundwater from the study area has dissolved Ca2+ and HCO3− as dominant ions; average concentrations are 118.9 and 253.9 mg/L, respectively. Thought to be derived from calcite dissolution, basically under the influence of the massif of the Sierra Madre Oriental, a karst Quaternary environment denominated El Abra Formation, a sedimentary rocks geology that dominates the regional geology. We used the cluster analysis to classify the primary element concentrations in two groups and three subgroups, where we could identify a local, intermediate, and regional flow. Defining the evolution of groundwater from recent waters on Ca2+ to HCO3− and dominance of SO4−2 to waters with more residence period, associated with the dissolution of gypsum from the Guaxcama Formation. That's was corroborate by utilization of δD and δ18O data which allow the identification of the recharge areas with mean values from − 7.94 δ18O (‰) and 53.84 δD (‰) on recharge areas, and − 8.19 ‰ δ18O and 57.83 ‰ δD on intermediate flow. This result contributes to describe the geochemical process of groundwater and how it interacts with geological formations with El Abra Formation, the most crucial geological-water unity for the study area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater quality evolution along flow paths involves a series of processes that allow the characterization of geochemical evolution of the water, from input sources (rainfall) to mixing at discharge resurgences, as well as rock/water interaction with multiple lithologies along the flow path (Carrillo-Rivera and Cardona 2012). This process was defined as Flow Systems Theory (FST) (Tóth 1999, 2000), and according to those authors, in local systems, the attributes of the flow system is defined by cold temperatures; pH less than 7; high dissolved oxygen (DO); positive values of Eh; low salinity; low trace element concentrations; and dominance of bicarbonate anions (HCO3−). Conversely, the water in regional systems is characterized by relatively warmer temperatures; pH greater than 7; low or no measurable DO; negative values of Eh; higher concentrations of minor and trace constituents; dominance of SO42− and Cl− anions; relatively high salinity, although this is not always present if the regional flow system, it does not encounter evaporites or connate water. The inclusion of items 5–7 explains why major and minor ions can be beneficial for the identification of groundwater mixtures or water–rock interactions, as well as a consideration in identifying groundwater flows (Alconada et al. 2011; Carrillo-Rivera et al. 2013; Huizar-Alvarez et al. 2016).
The FST uses tools based on the physicochemical analysis of natural waters, with changes that occur in response to residence time and geological variations (Tóth 1999). In addition to the physicochemical analysis described above, the evaluation of the δD and δ18O stable-isotopic abundance in groundwater has proved to be useful as tracers in the characterization of the hydrological cycle (IAEA 2006; Girmay et al. 2015), specifically the elevation of recharge sources. This method offers the possibility to follow the geochemical evolution of the water from precipitation, to water infiltration, to water–rock interaction along the groundwater flowpath, to mixing of different waters, and finally, to resurgence.
The study of the dynamics of FST has been became a useful tool in the water-management programs development, where the understanding of evolution of groundwater and this specifics geochemical interactions allow a better knowledge of the environment in general and the better ways to approach to sustainability criteria in the use of natural resources (Carrillo-Rivera and Cardona 2012). Karst environment an important source of water in many large arid and semiarid areas (Afrasiabian 2007), where the availability of groundwater cannot be accurately determined from classical groundwater hydrology principles (Bonacci 1987; Ford and Williams 2007). By this, the karst becomes extremely vulnerable to over exploration and pollution, so water resources protection is one of the most important measures to be observed in karst resources management (Keqiang et al. 2011).
Sedimentary karst rocks typically have been classified into two main groups, carbonates and evaporites. Dominant mineral forms of carbonates are calcite (CaCO3) and dolomite (CaMg(CO3)2), which typically yield dissolved water-quality parameters Ca+2 and HCO−3 and Ca+2, Mg+2, HCO−3, respectively during the karstification process. Evaporite rocks most commonly are gypsum (CaSO4 × 2 H2O), anhydrite (CaSO4), which provide Ca+2 and SO4−2 ions dissolved in the groundwater, and halite (NaCl), and less commonly, sylvite (KCl), which provide Na+, K+, and Cl− during karstification. Owing to their greater solubility, dissolution of evaporites typically creates large E.C. values of groundwater compared to E.C. of carbonate waters, commonly greater than hundreds to thousands of µS/cm (Ford and Williams 2007; Goldscheider and Drew 2007; Reicher et al. 2015; Stevanovic 2015; Pracny et al. 2017).
The principal objective of this work was to identify the characteristics of the groundwater flow evolution in the southwestern semi-arid state of Tamaulipas, Northeast Mexico. We did all this through hydrogeochemical characterization and assessment of the δD and δ18O stable isotopes.
Study area
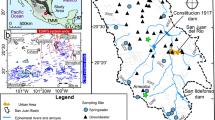
The study area is in the state of Tamaulipas, Northeast Mexico (Fig. 1). Physiographically, the study area is in the folds in the highly deformed regions of the Sierra Madre Oriental (SMO) basins, with mountainous massifs surrounding lower intermountain plains and land-surface altitudes from massifs to plains range between 2500 and 1000 m above sea level (m. a. s. l.). In general, the area is characterized by subtropical climate, with average annual rainfall less than 400 mm/year. Average annual temperatures range between 8 and 24 °C (CONAGUA 2015).
Setting: geology and hydrogeology
The region in formally know as Plataforma Valles–San Luis Potosí (PVSLP), described by Carrillo (1971) as a large segment of Precambrian and rocks, folded and faulted, where a thin sequence of marine sediments from Upper Jurassic were deposited, and then, a thicker section of evaporitic and limestone rocks of reef and post-feer origin were deposited during the Lower and Upper Cretaceous (Lopéz-Doncel Rubén 2003). The basalts do not appear to have contributed to dominant water–rock dissolution. In the Lower Paleocene to Upper Eocene period, the compressive efforts of the Laramide Orogeny create a folded and ridged front (Armas et al. 2004). These efforts are in Fig. 1, where the most of geological structures (faults, fractures, anticlines, synclines, etc.) has a preferential orientation from North to the South.
The oldest rocks outcropping in the study area are Precambrian-age Granjeno Formation, predominantly shale, located in small, isolated outcrops within the Bustamante and Miquihuana municipalities. According to Barboza-Gudiño et al. (2011), the Granjeno Formation contains metavolcanic and metasedimentary rocks and metamorphosed green shales, including phyllites, quartzites, and serpentines. Within the same region and resting discordantly on the Granjeno Formation, there are small outcrops of Huizachal Formation (belonging to the Late Triassic) consisting of an alternation of sandstones, conglomerates, shales and siltstone (López Infanzon 1986; Barboza-Gudiño et al. 2004).
Rocks older than Cretaceous (Fig. 1) likewise do not appear to be involved in the dissolution process. The Guaxcamá Formation was developed in an evaporite environment, whose thickness is greater than 2000 m; it underlies the El Abra Formation, which is a marine transgression product with evaporite and carbonate sequences (Lopez-Doncel 2003). As a predominant lithology in the study area is the calcareous complex type platform El Abra Formation, which by its biohermal type reef character, irregularities and conditions provided by the seabed deposition. Furthermore, this formation has lithological differences, as well as variable age and depositional environments. Recent deposits include thin siliciclastics with layers of intercalated limestones, deposited in shallow waters at the end of the Upper Cretaceous. Overlying the El Abra Formation is the Cárdenas Formation, consisting of a predominating limestones basis, as well as stratified sequences with a predominance of clays-biosparite, shale-sandstone-limestone, and sandstone-shales, respectively, in layers from the base of the formations to the top surface (de Antuñano 2011; CONAGUA 2015; Guevara et al. 2017).
Some aquifers in the study area appear to have regional flow paths, wherein the groundwater follows a longer flow path (> 10′s of km) and moves downgradient from recharge areas to discharge springs, which emerge from the confined karst system. Discharge points are controlled by interconnected fissures, fractures, faults, and conduits that allow artesian flow to mix with local groundwater in the PVSL area.In the study area, groundwater follows general flow directions from the highlands in the north to the PVSL in the south (CONAGUA 2015). Both areas have flows of western and eastern origin; the elevation of the static levels measured in November 2014, fluctuated from 977 and 980 m. a. s. l. (Guevara et al. 2017).
The conglomerate formed during the Neogene period is formed by continental sediments with grain sizes that vary from gravel to clays. This formation is composed of limestone, sandstone, flint, igneous rocks, and other fragments, encompassed in a clay matrix and carbonates cemented; their thickness varies from 10 to 30 m (SGM 2004). Padilla (1978) described the initiation of volcanic activity in the study area occurring in the early Quaternary Period. This volcanism was associated with normal faults and resulted in outcrops of basaltic rocks. During the Quaternary, alluvial material composed of sedimentary rocks of variable grain sizes were deposited, some as large as boulders, as much as 20 cm in diameter. These are distributed in large regions primarily near the margins of streams and down-gradient from the deltas formed on the less-steep plains areas. These sediments were derived from the physical and chemical weathering of preexisting rocks from the surrounding uplands (de Antuñano 2011; Guevara et al. 2017).
The limestone of the El Abra Formation, which underlies most of the existing mountain ranges in the study area, has a primary porosity that allows recharge to infiltrate and circulate into deeper rocks. The low-permeability rocks include the Cárdenas, Mendez, and San Felipe Formations, as well as the overlying Quaternary conglomerate and alluvium. The formations mentioned above are constituted of limestones constitutes but occasionally are fractured; therefore, they include some aquifers. On the other hand, the aquifers located in conglomerates and alluvial deposits are contained by clastic filling materials, that is, a granular medium and function as unconfined aquifers. The hydraulic characteristics, transmissivity, and storage coefficients are currently unknown (CONAGUA 2015). According to CONAGUA (2017), administratively, the study area is referred to as the 2814 Tula-Bustamante aquifer, in which the groundwater availability is about 54 M m3/year. Current groundwater extraction is slightly greater than 15.8 m3/year, most of which is for agriculture.
Methodology
A total of 25 groundwater-sampling sites were selected and sampled between February and June of 2016 (Fig. 2), including drilled wells (wells), springs, and dug wells. Parameters measured included temperature (T), electrical conductivity (E.C.) by portable potentiometers, calibrated, and measured in situ (Table 1). Field determination of alkalinity (and concurrent calculation of HCO3− concentration) was determined with a digital titration kit Hatch© AL-DT brand, using the H2SO4 0.16 N reaction with phenolphthalein and bromocresol green.
We collected water samples from each site in three high-density plastic containers and sent it to appropriate labs within 5 days of sampling. For preservation of cation samples, we filtered the water using 0.45 μm filter, and acidified it with high-purity nitric acid. Samples to be analyzed for anions were filtered, but not acidified. We neither filtered nor acidified samples for stable isotopes, but stored in an ice bath at 4 °C temperature and sent the samples collected for the cation and anion analyses to the Environmental Geochemistry Laboratory, Geosciences Center of the National Autonomous University of Mexico, where the cations and strontium were analyzed by the optical emission spectrometry method (ICP-Optical), and the anions by high-performance liquid chromatography (HPLC). The Isotope Hydrology Laboratory of the Mexican Institute of Water Technology analyzed stable isotopes. We performed hydrogeochemical specialized analyses through the 10.1 version of ARCgis® software (ESRI) and the 6.51 version of Diagrammes© software (Laboratoire d'Hydrogéologie d'Avignon). For the database management and multivariate analysis development were Excel software was used (Microsoft) and the 1.0.0.800 version of SPSS® (IBM).
We verified accuracy of the water samples by following the protocols of conservation and storage of water recommended by Bartram and Ballance (1996). The storage and transporting of the samples to the laboratory did not exceed 5 days. We calculated the ionic balance of cations with anions to assess the accuracy of the laboratory analysis and the holding time of the samples, and ten of the 25 samples were within the acceptable range of error (≤ ± 10%), that's is allowable for regional hydrogeochemistry studies (Sirshendu and Abhijit 2012; David and Pyne 2017). The source of such errors was unexpected and not immediately apparent, it could be: the type of aquifer, the speed of groundwater transport or the mix and turbulence caused by the extraction equipment. All they could generated some variations when water has collected or measure. On springs and open dug well, the source of problems, it can be the null care of said water sources, with activities of domestic animals and anthropogenic activity around them. The set of these circumstances agreed with errors typical of the sampling campaigns such as: too long a holding time, field and laboratory handle of samples, influenced the quality of the data so the error may be greater than the recommended 5%. Unfortunately, the study area does not have an environment that allows the implementation of all the integrity mechanisms of samples, a large part of the wells are very old, with limited maintenance, and in an unfavorable environment to access information such as usage, technical specifications and other essential information for a job like the one presented (Appelo and Postuma 2005).
\(\mathrm{EB }(\mathrm{\%}) =\frac{\sum \mathrm{cations}+ \sum \mathrm{ anions }}{\sum \mathrm{cations}- \sum \mathrm{ anions}}\)×100.
Statistical analysis
We treated statistically the groundwater data results were statistically treated utilizing a hierarchical cluster analysis, which allowed the grouping of similar water qualities and attributes. Hierarchical clustering analysis is a function used to group the multiple parameters of cluster water chemistry and produces a structured tree dendrogram that is used in the hierarchical clustering calculation (Ogunmola et al. 2017). We used Ward's method in the selection of hierarchical methods, a technique commonly applied in water chemistry research. Ward’s method utilizes an analysis of variance approach to evaluate the distances between the groups by minimizing the sum of squares of two hypothetical groups l that can be formed in each step. The result is a dendrogram (tree diagram) that provides a visual summary of the grouping process (Güler and Thyne 2004). For this study, we grouped data from the water geochemistry parameters through the similarity of samples collected from different places. According to the groups formed from this analysis, these served to group similar water quality that indicated sites might be related based on the geochemical interaction processes.
Results and discussion
Table 1 shows the dissolved major constituents from the groundwater collected. In these results, the E.C. values, which ranged from 420 to 2069 μS/cm2, marked a wide range for the salt concentration in the study area. Regarding the significant components, the highest average concentrations of cations were Ca2+, Mg2+, Na+, and K+ with averages levels of 121., 33.24, 32.45, and 3.58 mg/L, respectively. The dominant anions were HCO3− with 260 mg/L and SO42− with 211.9 mg/L, followed by Cl− and NO3− with averages of 35.25 and 10.86 mg/L, respectively.
According to the results from the Piper diagram (Fig. 3), dissolved constituents that dominated the analyses were the following ions: Ca2+, Mg2+, Na+, HCO3−, SO42−, and Cl−. These observations indicate that dissolution of limestone and evaporates, coupled with the mixing of these two predominant water types which has been facilitated by the intense faulting and fracturing in the study area. The high concentration Ca2+, Mg2+ and SO42− ions in the groundwater, can be explained by dedolomitization processes (Appelo and Postma 2005). In Fig. 4a, b, the dedolomitization was predicting with the relationship between the Ca2+ and Mg2+ ion concentration vs the SO42− concentration. The figure display that the springs and dug wells do not have a correlation between the dissolution of Ca2+, Mg2+ and SO42− ions. In the other hand, the samples that were taken from wells, increases their ion concentration in the groundwater direction North–South, probably due to the dedolomitization processes, that is an indicator of the interaction between the limestone and dolostone of the El Abra Formation, and the gypsum from the Guaxcamá Formation. Two things can be assumed from this data: (1) the structural direction in the study zone, largely defines the groundwater flow direction in the wells; and (2) the geological structures have a deep enough to make the groundwater, that circulating in a gypsum environment, interact with the groundwater in the limestone aquifer, probably due to the water pumping (López-Chicano et al. 2001). The dissolution of evaporite is water who comes from deepest sections on the karst aquifer, with influence of Guaxcamá Formation that is the reason why this water present more ionic charge and dominion of SO42− facie on the water (Fig. 3). In the case of Mg+2 the Dolomite dissolution is described in Fig. 4c, where in the relation Mg+2 vs Ca2+, all samples are above the 1: 1 ratio, which indicates dedolomitization process (Díaz-Puga et al. 2016) on regional hydrogeochemistry. According to (Guevara et al. 2017), the Cárdenas Formation, located in the intermontane valleys, was the probable source of Na+ and Cl−, product of mixing prosses on fractured limestone (Fig. 3) (Table 1) (Diaz-Puga et al. 2016).
Generalized hydrogeochemical facies based on Table 1 plotted on a Piper diagram
The cluster analysis formed two groups (A and B) and further segmented into three subgroups, each one of (Fig. 5). The A Group formed by three subgroups presents waters with mineralization, where the average E.C. was 406.69 μS/cm, characterized by the Ca2+ and HCO3− ions dominance; this might be associated to the calcite dissolution (Stevanovic 2015; Krienen et al. 2017). While the B group is composed of 3 subgroups, doubles the mean E.C. (1,075.89 μS/cm) compared to A group, B group is characterized by the Ca2+ and SO42− ions dominance; according to this values, this might raise the effect of minerals dissolution in evaporite rocks such as gypsum (Figs. 1, 3) (Stevanovic 2015; Pracny et al. 2017).
Regarding the analysis of the subgroups, Group A divisions were classified in A1, A2, and A3 (Fig. 3). It seems to be related by an upward increase in the concentration of SO42− and Mg2+, due to these increases in subgroups A2 and A3; respecting to A1, it looks a calcite solution dominance in recent infiltration waters, of local flow. Waters of subgroups A2 and A3 have more residence time than A1. In the subgroups of A2 and A3, the SO42− and Sr concentrations suggest waters with the presence of gypsum and dolomite dissolution, although, in these subgroups, there was a calcite dissolution dominance (Diaz-Puga et al. 2016).
Group B is characterized by more highly mineralization waters than Group A, due to the parameter values (Table 1) with average of E.C. registered at (1075 μS/cm) compared to (688 μS/cm) of group mean A. In the subgroups B1 and B3, the SO42− dominates the water concentration (Table 1), the differentiation between these seems to be caused by the average E.C. registered (1056.71–217 μS/cm). The distinction in subgroup B2 with the other subgroups (B1 and B3) seems to be because of mineralization effect, since this subgroup (B2) was formed by a unique sample with high concentrations of Cl− (260.40 mg/L), this could be because groundwater comes from aquifer with dissimilar characteristics compared to the other samples collected. This aquifer is a shallow aquitard, composed of shales and sandstones (Cárdenas Formation), which contains and transmits highly mineralized water (Helstrup et al. 2007).
The Cluster analysis characterized mineralization processes and flow systems, as analyzed by Toth (1999,2000), Subgroup A1, where the division of subgroups depends on the specific water–rock relationship, in a foreground dominated by the limestone rocks in A1 and indicates areas near the recharge zone. These waters evolve towards A2 and A3 in which an effect of the dissolution of gypsum is manifested and, to a lesser extent, of dolomites that we can describe as transition water between local to intermediate flow. The dominance of the dissolution of limestone is the primary geochemical process, producing waters of group A.
Group B represents the evolution of water quality from mixing and water/rock interaction along with the regional flow system, Subgroup B1, according to its hydrogeochemical characteristics, it presents an evolution towards intermediate or regional flows from group A, since subgroup B1 water–rock reflects the dissolution of gypsum.
Subgroups B2 and B3, on the other hand, present different characteristics to the system of evolution of Ca2+ ions dominance in recent waters and increase of SO42− in waters with longer residence processes in the karst of the study area. These groups were differentiated from the rest of the samples by a marked increase in E.C., which, under the analysis of the laboratory data, the water from these sampling points are extracted from an aquifer other than the fractured aquifer.
In general, group A and subgroups B1 delineated by cluster analysis have characteristics that indicate a geochemical evolution of groundwater from karst zones in semi-arid zones (Stevanovic 2015; Houatmia et al. 2016). The dissolution of calcite in waters recharged by local infiltration, also manifested in multiple natural springs throughout the mountainous area of the SMO, the groundwater flow continues increasing the residence time, taking distance from the recharge zones, where they encounter the dissolution of gypsum (Carrillo-Rivera et al. 2013; Vallejos et al. 2015; Diaz-Puga et al. 2016; Ray et al. 2017). According to the stratigraphy of the study area (López-Doncel 2003; Rocha Rocha 2008), there is no evidence of the presence of gypsum in the El Abra Formation, so the likely flow path of these waters likely includes flow within the Guaxcamá Formation, which underlies the El Abra Formation.
The evolution of groundwater in the study area suggests that the water that infiltrates the outcrops of the El Abra Formation in SMO flows through the fractures of the karst and is stored in the same structure, following the path of rocks fractures on the low section of the rock formation, filling the cavities on the karst.
Isotope analysis
The environmental isotopes from 21 samples of groundwater presented in Table 1 are also in Fig. 6, where we compare the data with the Global World Meteoric Line (GWML) (Rozanski et al. 1993) and the Local Meteoric Water Line from San Luis Potosí (LMWL SLP) (Carrillo-Rivera et al. 2007). The general average of all the isotope data obtained in the present study is located at − 8.16 ‰ of δ18O and − 56.37 ‰ δD, which can be associated to the representation of the groups generated by the cluster analysis, where group A presents mean values − 7.94 δ18O (‰) and 53.84 δD (‰), whereas group B − 8.19 ‰ δ18O and 57.83 ‰ δD (Fig. 6).
The isotopic signature of groundwater is distributed slightly below the local meteoric water line, which is consistent with other studies conducted in the SMO (Aguilar-Ramirez et al. 2017). This isotopic behavior suggests that subsurface infiltration is carried out rapidly with few losses by evaporation, as these losses would result in significant isotopic excursions to the right of the meteoric water line (Fig. 6) (Clark and Fritz 1997).
The waters samples with more enriched isotopic signatures are classified with surface water characteristics, which in this case represents the water of recent infiltration in recharge zones (Figs. 2, 5). The samples with these characteristics are the numbers 6, 13, 24 and 25, and are on the limestones from SMO, a recharge area for the aquifer of the region.
While the waters located around − 59 ‰ of δD, and − 8.1 ‰ of δ18O (water samples 23, 22 and 16), are consistent with those that have infiltrated rapidly through fractures in the upland areas of the study area (Fig. 1), at altitudes around at 1,500 m a.s.l., mixed with more recent flows (Huizar-Álvarez et al. 2014). Also, the isotopic distribution of the samples suggests a water–rock interaction, which goes according to the direction of the groundwater flow that is North–South.
The most isotopically impoverished waters of the sample grouped around − 65 ‰ of δD, and − 9.3 ‰ of δ18O are over the LMWL SLP, so these samples are associated with others of fast infiltration in fractured limestones at elevations of 2500 m.a.s.l. (Fig. 6). In this group of water can be found the groundwater with influence of evaporite dissolution, and longer residence time, that has quickly infiltrated from the outcrops of El Abra Formation. There is another group consisting of three samples (from sites 10, 11, and 21), which are out of linearity, indicating waters that show evaporation effects (Clark and Fritz 1997; Edmunds et al. 2002). It should be noted that these samples are in a shallow aquifer formed by shales from the Cárdenas Formation, which has an intermediate permeability (Krienen et al. 2017). Therefore, the circulation of underground water is slower, and probably mixing with fresh water from lower fractured aquifer, and the effect of evaporation effects are be attenuated.
All this explains the origin of the processes of the residence time of the groundwater (Fig. 7), since the high contents of Cl− in these waters cannot be induced by the evaporation process but may be derived from mixing and different kinds of water–rock interaction (Yangui et al. 2011; Ray et al. 2017), This indicates that the recharge of the aquifer formed by shale and sandstones of the Cárdenas Formation, and it is likely not recharged by local rainwater if it presents a communication with the fractured aquifer of El Abra Formation, through which it is recharged vertically and horizontally..
Conclusions
Evidence suggests that the major geochemical mechanism affecting groundwater quality in the study area is dissolution of limestone and gypsum in the aquifers contained in the El Abra and Guaxcamá Formations. The hydrogeochemical and statistical analysis of the cluster defines the groundwater flow systems at the regional level, coinciding in that the waters of recent infiltration that are dominated by the dissolution of calcite. As the water flow moves downgradient the faults and fractures contribute to mixing of recent and more residence time waters, where the dissolution of gypsum plays an essential role. The δ18O and δ2H stable isotopes contribute to the theoretical approach to the functioning of the karst system analyzed, where water flows through fractures, in rapid infiltration processes associated with the mountainous flow system of the SMO. The most crucial geological system for its aquifer characteristics at the regional level is El Abra Formation that functions as a recharging area and storage of groundwater.
References
Aguilar-Ramírez CF, Camprubí A, Fitz-Díaz E, Cienfuegos-Alvarado E, Morales-Puente P (2017) Variación en la composición isotópica del agua meteórica a lo largo de la sección centro-noreste de la Sierra Madre Oriental. Bol Soc Geol Mex 69(2):447–463
Afrasiabian A (2007) The importance of protection and management of Karst wateras drinking water resources in Iran. Environ Geol 2007(52):673–677. https://doi.org/10.1007/s00254-006-0502-z
Alconada M, Rayneiro J, Castillo F, Carrillo RJJ (2011) La definición de flujos de agua subterránea en la elección de prácticas de manejo del suelo. IX Congreso Cubano De Geología. Taller sobre Aguas subterráneas y Contaminación. ISBN 978-959-7117-30-8
Appelo CAJ, Postma D (2005) Geochemistry, groundwater and pollution. A. A. Balkema, Rotterdam, Brookfield, p xvi+536
Armas ZJM (2004) Cartografía Geológica Estructural del Valle de Huizachal, Como Base para el Análisis de Estabilidad de Taludes de La Carretera Rumbo Nuevo, Tamaulipas, México, p 134
Barboza-Gudiño JR, Hoppe M, Gómez-Anguiano M, Martínez-Macías PR (2004) Aportaciones para la interpretación estratigráfica yestructural de la porción noroccidental de la Sierra de Catorce, San Luis Potosí, México. Revista Mexicana de Ciencias Geologicas 21(3):299–319
Barboza-Gudiño JR, Ramírez-Fernández JA, Torres-Sánchez SA, Valencia VA (2011) Geocronología de circones detríticos de diferentes localidades del Esquisto Granjeno en el noreste de México. Boletin de La Sociedad Geologica Mexicana 63(2):201–206
Bartram J, Ballance R, World Health Organization & United Nations Environment Programme (1996) Water quality monitoring: a practical guide to the design and implementation of freshwater quality studies and monitoring programs / edited by Jamie Bartram and Richard Ballance. E & FN Spon, London
Bonacci O. 1987. Karst hydrology. Springer-Verlag. ISBN: 978-3-642-83167-6. https://doi.org/10.1007/978-3-642-83165
Carrillo-Bravo J (1971) La Plataforma Valles-San Luis Potosí. Boletín de la Asociación Mexicana de Geólogos Petroleros
Carrillo-Rivera JJ, Cardona A (2012) Groundwater flow systems and their response to climate change: a need for a water-system view approach. Am J Environ Sci 8(3):220–235 ((ISSN 1553-345X))
Carrillo-Rivera JJ, Varsányi I, Kovács LÓ, Cardona A (2007) Tracing groundwater flow systems with hydrogeochemistry in contrasting geological environments. Water Air Soil Pollut 184(1–4):77–103. https://doi.org/10.1007/s11270-007-9400-6
Carrillo-Rivera JJ, Ouysse S, Hernández-Garcia GJ (2013) Integrative approach for studying water sources and their vulnerability to climate change in semi-arid regions (Drâa Basin, Morocco). Int J Water Resour Arid Environ 2(1): 26–36. ISSN 2079–7079© PSIPW
Clark ID, Fritz P (1997) Environmental isotopes in hydrogeology. Lewis Publishers, New York, p 328
Comisión Nacional del Agua (CONAGUA) (2015) Actualización de la disponibilidad media anual de agua en el acuífero Tula—Bustamante, estado de Tamaulipas
Comisión Nacional del Agua (CONAGUA) (2017) Actualización de la disponibilidad media anual de agua en el acuífero Tula - Bustamante, estado de Tamaulipas
Cortes A, Durazo J, Farvolden RN (1997) Studies of isotopic hydrology of the basin of Mexico and vicinity: annotated bibliography and interpretation. J Hydrol 198(1–4):346–376
David R, Pyne G (2017) Groundwater recharge and wells. Routledge, Abingdon, p 400
de Antuñano ES (2011) Sinopsis geológica de la Cuenca de Burgos, noreste de México: producción y recursos petroleros. Boletín de la Sociedad Geológica Mexicana 63(2):323–332
Derek F, Paul W (2007) Wiley, The atrium. ISBN 978-0-470-084996-5
Díaz-Puga MA, Vallejos A, Sola F, Daniele L, Molina L, Pulido-Bosch A (2016) Groundwater flow and residence time in a karst aquifer using ion and isotope characterization. Int J Environ Sci Technol 13:2579–2596. https://doi.org/10.1007/s13762-016-1094-0
Edmunds WM, Carrillo-Rivera JJ, Cardona A (2002) Geochemical evolution of groundwater beneath Mexico City. J Hydrol 258(1–4):1–24
Eguiluz de Antuñano S, Aranda García Mario y Marrett R (2000) Tectónica de la Sierra Madre Oriental, México. Boletín de la Sociedad Geología Mexicana. v. LIII, 1–26. ISSN 14053322
Girmay E, Tenalem A, Seifu K, Mulugeta A, Stefan W, Frank W (2015) Conceptual groundwater flow model of the Mekelle Paleozoic-Mesozoic sedimentary outlier and surroundings (northern Ethiopia) using environmental isotopes and dissolved ions. Hydrogeol J 2015(23):649–672. https://doi.org/10.1007/s10040-015-1243-4
Goldscheider N, Drew D (2007) Methods in karst hydrogeology. Taylor & Francis Group, London UK ((e-book: 978-0-20393462-3))
Guevara MÓ, René Ventura H, Elizabeth del Carmen AL (2017) Uso de sondeos electromagnéticos en la caracterización hidrogeológica del acuífero del altiplano de Tula, Tamaulipas. Investigación y Ciencia 25(70):23–30
Guler C, Thyne GD (2004) Hydrologic and geologic factors controlling surface and groundwater chemistry in Indian Wells-Owens Valley area, southeastern California, USA. J Hydrol 285:177–198
Helstrup T, Jørgensen NO, Banoeng-Yakubo B (2007) Investigation of hydrochemical characteristics of groundwater from the Cretaceous-Eocene limestone aquifer in southern Ghana and southern Togo using hierarchical cluster analysis. Hydrogeol J 2007(15):977–989
Houatmia F, Rim A, Abdelkrim C, Mourad B (2016) Assessment of groundwater quality for irrigation and drinking purposes and identification of hydrogeochemical mechanisms evolution in Northeastern, Tunisia. Environ Earth Sci 75:746. https://doi.org/10.1007/s12665-016-5441-8
Huizar-Álvarez R, Varela-González GC, Espinoza-Jaramillo M (2014) Sistemas de flujo subterráneo y contenido de fluoruro en el agua de Tenextepango Morelos, México. Revista Mexicana de Ciencias Geológicas 31(2):238–247
Huizar-Alvarez R, Ouysse S, Espinoza-Jaramillo MM, Carrillo-Rivera JJ, Mendoza-Archundia E (2016) The effects of water use on Tothian flow systems in the Mexico City conurbation determined from the geochemical and isotopic characteristics of groundwater. Environ Earth Sci 75:1060. https://doi.org/10.1007/s12665-016-5843-7
International Atomic Energy Agency (IAEA) (2006) Global network of isotopes in precipitation. International Atomic Energy Agency, Vienna
Keqiang H, Yuyue J, Wang Lu, Yaoru L (2011) Overview of karst geo-environments and karst water resources in north and south China. Environ Earth Sci 2011(64):1865–1873. https://doi.org/10.1007/s12665-011-0998-8
Krienen L, Heuser M, Höbig N, Mares Ochoa ME, Rüde TR, Cardona Benavides A (2017) Hydrogeological and hydrochemical characterization of two karstic discharge areas in San Luis Potosí, Mexico. Environ Earth Sci 76(24):1–18. https://doi.org/10.1007/s12665-017-7166-8
López-Chicano M, Bouamama M, Vallejos A, Pulido-Bosch A (2001) Factors which determine the hydrogeochemical behaviour of karstic springs. A case study from the Betic Cordilleras, Spain. Appl Geochem 16:1179–1192
Lopéz-Doncel Rubén (2003) La formación Tamabra del Cretácico medio en la porción central del margen occidental de la Plataforma Valles-San Luis Potosí. Centro Noreste de México. Revista Mexicana de Ciencias Geológicas 20(1):1–19
López Infanzon M (1986) Estudio petrogenético de las rocas ígneas en las formaciones Huizachal y Nazas. Boletin de La Sociedad Geologica Mexicana 47(2):1–41
Mádl-Szőnyi J, Tóth Á (2015) Basin-scale conceptual groundwater flow model for an unconfined and confined thick carbonate region. Hydrogeol J 2015(23):1359–1380. https://doi.org/10.1007/s10040-015-1274-x
Ogunmola FJ, Adetola SO, Hammed OS, Igboama WW (2017) The use of multivariate statistical analysis in the assessment of groundwater hydrochemistry in some parts of southwestern Nigeria. Arab J Geosci 10:328. https://doi.org/10.1007/s12517-017-3125-7
Padilla JR (1978) Dialnet BosquejoGeologicoestructuralDeLaSierraMadreOrienta-281994.pdf. Universidad Nacional Autónoma de México, Instituto de Geologia, 2, 45–54
Pérez-Quezadas J, Cortés-Silva A, Salas-Ortega MR, Araguás-Araguás L, Morales-Puente P, Carrillo-Chávez A (2017) Evidencias hidrogeoquímicas e isotópicas sobre el origen del agua subterránea en la cuenca hidrográfica Río Actopan, Estado de Veracruz. Revista Mexicana de Ciencias Geológicas 34(1):25–37
Pracny P, Jirı FD, Vsiansky LK (2017) Evolution of Mg/Ca ratios during limestone dissolution under epikarstic conditions. Aquat Geochem 2017(23):119–139. https://doi.org/10.1007/s10498-017-9313-y
Ray RK, Syed TH, Saha D, Sarkar BC, Reddy DV (2017) Recharge mechanism and processes controlling groundwater chemistry in a Precambrian sedimentary terrain: a case study from Central India. Environ Earth Sci 76:136. https://doi.org/10.1007/s12665-017-6435-x
Reischer M, Bichler B, Spötl C, Höfer-Öllinger G, Wyhlidal S (2015) Karst hydrogeology of the Untersberg massif and its interaction with the porous aquifer in the adjacent Salzburg Basin. Aust J Earth Sci 108(2):68–81. https://doi.org/10.17738/ajes.2015.0014
Rocha Rocha M (2008) Yacimientos de Celestina en la Plataforma Valles-San Luis Potosí. Tesis de Maestria. Universidad Autonoma de San Luis Potosi
Rozanski K, Araguas-Araguas L, Gonfiantini R (1993) Isotopic patterns in modern global precipitation, climate change in isotopic records, 78, Geophysical Monographic Series. ISBN: 9781118664025. DOI: https://doi.org/10.1029/GM078
SGM Servicio Geologico Mexicano (2004) Carta Geologico-Minero. F 14-2 (Ciudad Victoria), F 14-5 (Ciudad Mante). Pachuca Hgo. Mexico
Sirshendu D, Abhijit M (2012) Arsenic removal from contaminated groundwater. The Energy and Resources Institute (TERI), pp 304
Stevanovic Z, Igor J, Sasha M (2007) Management of karst aquifers in Serbia for water supply. Environ Geol 2007(51):743–748. https://doi.org/10.1007/s00254-006-0393-z
Stevanovic Z (2015) Karst Aquifers – Characterization and Engineering. Professional Practice in Earth Sciences. Springer, Switzerland. ISBN 978-3-319-12850-4
Tóth J (1999) Groundwater as a geologic agent: an overview of the causes, processes, and manifestations. Hydrogeol J 7:1–14
Tóth J (2000) Las aguas subterráneas como agente geológico: causas, procesos y manifestaciones. Boletín Geológico y Minero 111(4):9–26
Vallejos A, Andreu JM, Sola F, Pulido-Bosch A (2015) The anthropogenic impact on Mediterranean karst aquifers: cases of some Spanish aquifers. Environ Earth Sci 2015(74):185–198. https://doi.org/10.1007/s12665-014-3994-y
Ward JH (1963) Hierarchical grouping to optimize an objective function. J Am Stat Assoc 58:236–244
Wassenaar LI, Van Wilgenburg SL, Larson K, Hobson KA (2009) A groundwater isoscape (δD, δ18O) for Mexico. J Geochem Explor 102(2009):123–136
Yangui H, Kame Z, Rim T, Kazimierz R (2010) Recharge mode and mineralization of groundwater in a semi-arid region: Sidi Bouzid plain (central Tunisia). Environ Earth Sci 2011(63):969–979. https://doi.org/10.1007/s12665-010-0771-4
Yangui H, Kame Z, Rim T, Kazimierz R (2011) Recharge mode and mineralization of groundwater in a semi-arid region: Sidi Bouzid plain (central Tunisia). Environ Earth Sci. https://doi.org/10.1007/s12665-010-0771-4
Yangui H, Kamel Z, Kazimierz R (2012) Hydrochemical and isotopic study of groundwater in Wadi El Hechim-Garaa Hamra basin. Central Tunisia Environ Earth Sci 2012(66):1359–1370. https://doi.org/10.1007/s12665-011-1346-8
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is a part of a Topical Collection in Environmental Earth Sciences on Sustainable Management of Karst Natural Resources, guest edited by Drs. Sasa Malinovic and Zoran Stevanovic.
Rights and permissions
About this article
Cite this article
Ventura-Houle, R., Guevara-Mansilla, O., Requena-Lara, G. et al. Hydrochemistry, δD and δ18O to explain the distribution of water quality in a karst setting in the semi-arid region of Northeast Mexico. Environ Earth Sci 80, 6 (2021). https://doi.org/10.1007/s12665-020-09310-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-020-09310-x