Abstract
The current research plan was designed to investigate occurrence, source origin and association of heavy metals (HMs) in multi-matrix (road dust, soil and vegetation) samples along elevation gradients of Himalayan foothill, Pakistan. Therefore for this purpose, road dust (n = 28), soil (n = 42) and vegetation (n = 125) samples along different elevation gradients were collected and analyzed for HMs concentrations on flame atomic absorption spectrophotometer. Higher ∑HM concentrations were recorded in soil, dust and vegetation samples at the high elevation zones [Temperate (zone-3) and moist-temperate (zone-4)]. A clear elevation trends for cadmium (Cd), and manganese concentration in soil and Cd concentration in vegetation samples at zones were depicted but on site basis there was no clear elevation trends for the targeted metals. Stable Carbon isotopic method in soil reported that air-borne sources and geological rock outcropping are the main contributors and have shown strong positive relation with elevation, temperature, precipitation, sand, silt, and total organic carbon. Principal component analysis/multiple linear regression predicted multiple HMs pollution sources (long range atmospheric transport (LRAT), wet deposition, parent rock materials, vehicular emissions and to some extent air-borne sources) as contributor in soil, dust and vegetation samples. The geo-accumulation index (Igeo) in soil predicted that all four zones were confirmed as moderately to strongly polluted for Cd; unpolluted to moderately polluted for lead and designated as unpolluted zones for all other HMs. Further, the future in-depth studies regarding LRAT phenomenon is needed to study the fates, behaviors’ and deposition patterns of HMs in soil and vegetation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the current scenario developing countries are facing extreme challenges due to the lack of proper preventive and management options, and thereby permitting the release of heavy metals (HMs) into environment from different operational activities (like mining, traffics, natural (parent rock materials) and anthropogenic etc.) (Tian et al. 2019). The vehicles transportation on road has the potential to release HMs that may be either adsorbed on associated dust particle or shifted to the nearby soil and vegetation through air current. HMs occurrence has deep concerns in the elevation zones where their emission origins are suspected from different sources. Road transport provides plentiful benefits to society, but also serves as key source of HMs pollution (Guan et al. 2018). Mountainous ecosystem is recognized as one of the most sensitive ecosystem (Bing et al. 2016), and act as sink for HMs accumulation (Guan et al. 2018). Mountainous ecosystem has been mainly characterized by low annual temperature and negligible anthropogenic activities. They have the ability to accumulate HMs from atmosphere via long-range atmospheric transport (LRAT) phenomenon or may directly from the associated or nearby vehicles transportation (Bing et al. 2016; Chen et al. 2010; Guan et al. 2018). LRAT Phenomenon explains the transports of HMs via particulate phases of atmosphere for long distances from the point of their origin to the places of the destination (low temperature zones) and deposit HMs in the remote region (Kyllonen et al. 2009). Besides that, multiple factors viz., traffic and highway characteristics, distance, soil properties and climatic factors are also involved to influence HMs concentrations along the road side (Pagotto et al. 2001; Bai et al. 2009). HMs releases are reported from different types of vehicles through the consumption of fuel, engine oil, tire wear, brake wear and abrasion of road (Wilcke et al. 1998; Winther and Slento 2010). The consumption of engine oil by vehicles have been categorized as the main source for Cd; tire wear for Zn; and brake wear for Cu and Pb (Winther and Slento 2010). The asphalt road surfaces with bitumen and filler materials are also considered as the source origin for Cu, Zn, Cd and Pb (Winther and Slento 2010). At high-altitude areas, the combustion of fuel is typically less complete than in low-altitude areas because of the reduced oxygen concentration in air, resulting in greater fuel consumption and thus greater pollutant emissions. HMs after their release have the potency to accumulate in the soil along the road vicinity via runoff, geological parent materials and through atmospheric precipitation and deposition processes (Viard et al. 2004; Nabulo et al. 2006; Nagajyoti et al. 2010). The soil development, formation and vegetation distribution at high altitude zones could be determined by variation in elevation that forms a marked climatic gradient. The soils nearby highways are responsible to act as a sink of metals released from vehicles (Zhang et al. 2013; Khan and Kathi 2014). Such higher HMs concentrations in soil are threats for the entire ecosystem and with passage of time make possible its transfer to plants parts (Bakirdere and Yaman 2008). To assess the HMs risk in soil, geoaccumulation index (Igeo) is used as predictor to assess pollution status of any particular soil of an area and also as a tool to monitor the healthy status of soil for vegetation life. The stable isotope ratios of chemical elements in the environmental samples could be used as valuable tracer for information on processes and sources identification (Wiederhold, 2015). They are also used as a tracer to study carbonate formation and climate relation (Cerling 1984). Both organic and inorganic components in the soil environment could be assessed in terms of stable isotope ratios (Boutton et al. 1998). The carbon isotopic composition of soil CO2 is directly related to the carbon isotopic composition of the biomass which ultimately represents the proportion of plants that use the C3 or C4 photosynthetic pathway. CO2 is emitted as a byproduct, when organic matter finds its way to the forest floor and decomposes; enhancing microbial activity and degradation of soil organic matter (SOM) (Wang et al. 2015). The carbon dioxide has the potency to form carbonates with water under some conditions. The impact of climate on soil-formation and pollutant distribution in the soil can be traced via Carbon (δ13C) stable isotope in soil carbonates. Thus carbon isotopic composition of soil carbonate can be indicated by soil CO2.
Murree is one of the prime tourist places in Pakistan, where tourism and transportation activities have been increased recently. The murree road tract can be classified as the low altitude zones of Himalayan foothill with relatively hot and warm temperature than high altitude zone and has been used by the visitors for transportation purposes. Very limited literatures are available regarding HMs occurrence, distribution patterns and sources in road dust, soil and vegetation and moreover relation among the multi-matrix along the elevation gradient. Research based on the sources and distribution patterns of HMs in the elevation gradient of Himalayan foothill along the roadside tract and its associated factors influencing is of great significance. Because in Pakistan perspective, this is the first study of its nature to probe HMs concentration, distribution and sources in soil, dust and vegetation samples along elevation sites of murree road, Pakistan. This study further emphasizes the relation and impacts of HMs among different environmental matrixes (soil, dust and vegetation) along different elevation gradient and zones.
Materials and methods
Study area information
The study area lies between 33° 44′ 41″ to 33° 59′ 48″ N latitude and 73° 9′ 10″ to 73° 23′ 25″ E longitude in the Himalayan foothill, Pakistan. It covers an elevation gradient of 600–2575 m along the Murree road that start from Margalla foothill to Khuza galli having high mountains and deep valleys geo-morphology (Table 1). A total of 14 elevation sites were preferred for the collection of road dust, soil and vegetation samples along Murree road during December, 2016 (Table 1). The study area is bounded by the sub-mountainous regions of Rawalpindi tehsil in the south, river Jhelum in the east, and Khyber Pakhtunkhwa in the north-west (Hameed et al. 2012). It comprises sedimentary rocks, mainly originates from limestone, shale, conglomerates and sandstones. The soils of these areas have uneven proportion of coarse fragments with dominant loamy texture and with sharp variations in precipitation, temperature and physiographic features. An elevation differences and rainfall patterns have developed four main forests zones with different dominant vegetation species as described in Text S1. The selected sampling points present diverse altitudinal gradient variations within different topography, climatic conditions and vegetation zones.
Collection and processing of samples
Soil
Surface soil (n = 42) samples (1 kg) were collected at the depth of 0–10 cm from 14 sampling sites along the Murree road at different elevation gradients. From each site, 3 composite (3–5 sub-samples) soil samples were taken at the distance of 100 m from the road side. Soil samples were collected using hand spade and the sample was preserved in plastic zip lock bags and transferred to the laboratory for further analysis. All the soil samples were air dried until constant weight was achieved and sieved through 2 mm mesh sieve prior to analysis.
Dust
The road dust (n = 28) samples (50–100 g) were collected in duplicate manner from 14 sites. Each sample of road dust was further made as a composite of 3–5 sub-samples within the road curb. Road dust was whisked using a clean plastic brush and kept preserved in a clean plastic bag (Ahmed and Ishiga 2006). All the samples were air dried at room temperature to a constant mass, and then sieved through 2 mm mesh stainless-steel and stored at room temperature till further analysis.
Vegetation
A total of 125 dominant vegetation samples along the roadside vicinity from four distinct vegetation zones were collected (Text S1). Trees were randomly selected at each site by clipping their branches at the height of 3–4 m from ground level using a cutter (Luo et al. 2014). Scrub type vegetation’s were collected using the same method independent of height from above ground level. Marchantia and bryophytes were collected using a spade and knife to separate them from trees and stones to which they were adhered. Each sample was a composite of many sub-samples of each species that were collected 100 m distance apart from the road. Samples were collected separately (about 100 g for large species and 10–20 g for smaller species) in clean zip lock plastic bags followed by rinsing first with tap water and then with distilled water to remove coarse and adhering particles. After air drying, all the samples were oven dried at 80 °C to remove complete water content. Moreover, all vegetation samples were grounded in a mild steel mill, passed through 2 mm mesh and preserved till further analysis.
Analytical approach
Soil physicochemical properties
Soil texture was determined using dry sieve method for particle size distribution and % (sand, silts and clay). Soil moisture was calculated by weighing sample before and after drying in oven at 105 °C by American Society for Testing and Materials method (ASTM, 2005). The power of hydrogen ion concentration (pH) and electrical conductivity (EC) were measured by Crison pH meter and PCS testr 35 multi parameter EC meter and calibrated at pH of 4.01, 7 and 9.21 by standard solutions. Walkey-Black titration method was used for calculation of TOC contents.
Digestion of environmental samples
Soil and dust samples of about 1.0 g were weighed in a 100 mL Pyrex beaker with addition of 3 mL concentrated HNO3 and were placed on hotplate. Slowly increasing the heat of the hotplate till dryness of the sample extract and then add 4 mL of concentrated HClO4 and heated until dense white fumes was formed.
The HNO3–HClO4 digestion method was carried out for vegetation sample and about 1 g vegetation sample was added into a 100 mL Pyrex beaker. Further, 10 mL of 2:1 nitric-perchloric acid mixture was added and allowed to heat on hot plate until clear white dense fumes was formed.
The final extract of all samples (soil, dust and vegetation) were filtered through Whattman filter paper No. 42 and was raised up to 50 mL volume with de-ionized water (Ryan et al. 2007). Each batch of the samples contains at least one reagent blank sample (no sample included).
Stable carbon isotope (δ 13C) analyses
The stable carbon isotope (δ 13C) analyses for soil have been performed using a modified Varian Mat GD-150 Mass Spectrometer. For isotopic analysis on mass spectrometer, soil samples have been converted into gas phase. For the extraction of CO2 gas from total dissolved inorganic carbon of soil, 1 g soil sample was added in the Pyrex reaction flask. A small burette containing H3PO4 acid was attached to the reaction flask assembly, which was further connected to the vacuum line. Dewar flask containing liquid N2-acetone mixture (temperature: − 80 °C) was linked to the moisture traps in the vacuum system. Moreover, about 5 mL of H3PO4 acid was added to the pre-evacuated reaction flask. The CO2 gas was evolved as a result of reaction between inorganic carbon component/fraction of sample and the phosphoric acid according to the following equation:
The moisture produced during reaction was removed by cryogenic trap at − 80 °C. The CO2 gas was passed through vacuum line, purified by freezing in U-trap dipped in liquid nitrogen flask and other gases were pumped out. The liquid N2 flask from CO2 trap was removed to expand CO2 in vacuum line which was finally collected in an ampoule dipped in liquid nitrogen flask at the other end. The ampoule was labeled and removed from the line for isotope ratio measurement with Isotope ratio mass spectrometer (IRMS). The overall analytical errors are reported to be ± 0.01 ‰ for δ13C measurement. To ensure precision in each sample, standard deviation of the mass spectrometer was also computed that ensured to be within permissible limit.
Quality assurance and quality control
HMs was analyzed using Flame atomic absorption spectroscopy (FAAS) with the help of nitrous oxide/Acetylene gas. Strict quality control procedures were followed for each sample analysis. The 1000 ppm stock solution of HMs with three different concentrations (40 mg/L, 80 mg/L and 120 mg/L) were prepared. All the solvent blanks were analyzed to check any contamination occurred during lab processes. A solvent blank was run after every 10 samples to check any sort of interference and cross-contamination in any sample. A natural standard reference soil (JSI) material was used for recovery test to further estimate the accuracy and efficiency of the method. The recoveries calculated for all metals ranged between 89% and 104% and percentage error observed were 4–12%.
Calculation
Geo-accumulation index (Igeo) was used to assess the presence and intensity of anthropogenic contaminant deposition on surface soil and can be calculated by normalization of one metal concentration in the topsoil respect to the concentration of a reference element (Muller 1979). It was employed to evaluate the possible environmental risks by assessing the soil contamination (Iqbal and Shah 2011). Geo-accumulation (Igeo) index was used to assess the HMs pollution of sediments in stream but later on this was applied to soil (Zhiyuan et al. 2011) as expressed by the following equation:
where, Cn depicts concentration of HMs in soil, and Bn refers to average concentration of shale in geochemical background values of element n. Due to lithogenic effect, the background matrix correction is 1.5 in factor.
Statistical analysis
Detailed statistics summary (min–max, mean ± standard deviation) for road dust, soil, and vegetation samples were obtained. The statistical analysis of data were performed using Excel stat and SPSS (IBMS). Data was further subjected to ANOVA and correlation analysis to test variations among different elevations, climatic factors and the HMs concentrations. For matrix wise (soil, road dust and vegetation) source identification, Principal component analysis (PCA) followed by multiple linear regression (MLR) was applied on data set. The purpose of PCA was to obtain the total variability of the data into factors, which are orthogonal to each other and represent low possible covariance. Factor scores are then regressed against the normal standard deviation of the HMs values (Z score) (Pekey and Dogan 2013).
Results
Concentration and distribution of HMs in multi-matrix samples
Basic descriptive statistics of targeted HMs concentration in soil, dust and vegetation samples along the murree road at four (4) zones are summarized in Table 2. The total HMs concentrations were ranged from not detected (n.d) to 3152, n.d to 2394, and n.d to 537 (mg/kg) in soil, dust and vegetation samples, respectively. The higher summed HMs were accumulated in soil followed by dust and vegetation samples and the higher individual HMs concentration was recorded for Cd, Cr and Fe in soil; Mn in dust samples; and Cu, Pb, Zn and Ni in vegetation samples.
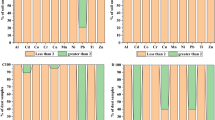
The overall increasing order of HMs concentration in soil along murree road was: Fe > Zn > Pb > Ni > Cu > Mn > Cr > Cd. The summed HMs concentrations in soil were recorded at zone-4 (3382.87 mg/kg) followed by zone-3 (2906.62 mg/kg), zone-1 (947.62 mg/kg) and zone-2 (921.5 mg/kg) (Table 1). On the other hand, site based variation in soil showed higher mean concentration for Cd at S-12; Cr at S-8; Cu at S-11; Ni at S-12; Mn at S-14; Zn at S-12; Pb at S-11 and Fe at S-8 (Fig. S1a). The concentration trends for ∑HM in road dust at different zones of Himalayan foothill are: zone-3 (2106.48 mg/kg) > zone-4 (1756.5 mg/kg) > zone-2 (642.42 mg/kg) > zone-1 (522.16 mg/kg) (Table 1). The increasing trends for overall HMs in dust were: Fe > Mn > Pb > Zn > Cu > Ni > Cr > Cd. On site base variation, the highest mean concentration was depicted for Cd at S-11; Cr at S-11; Cu at S-10; Ni at S-11; Mn at S-6; Zn at S-4; Pb at S-7 and Fe at S-14 (Fig. S1b). The HMs concentration in vegetation samples at different zones along the murree road showing the trend as: Fe > Pb > Mn > Zn > Ni > Cu > Cd > Cr. The ∑HM concentration was recorded in order of highest at Zone-3 (124.64 mg/kg) > zone-4 (123.14 mg/kg) > zone-1 (45.92 mg/kg) > zone-2 (43.10 mg/kg) (Table 1). The higher spatial distribution in vegetation samples was recorded for Cd at S-14; Cr at S-10; Cu at S-12; Ni at S-2; Mn at S-13; Zn at S-3; Pb at S-10 and Fe at S-14 (Fig. S1c).
The one way analysis of variance (ANOVA) showed significant spatial variations for Cd, Cu, Mn, Zn and Pb in soil; Zn in dust and Cd in vegetation samples among different zones along different elevation gradients (Table S1). The Pearson correlation analysis among studied HMs concentration and physico-chemical properties and environmental factors in multi-matrix (soil, dust and vegetation) samples at each zone along elevation gradients are summarized in Table 3.
HMs variation in multi-matrix samples along different elevation gradients
The HMs concentration variations in soil, dust and vegetation samples along different elevation sites and zones are depicted in Fig. S1a,b,c and Fig. S2a,b,c, respectively. There was not shown any clear increasing elevation trends for targeted HMs in soil, dust and vegetation samples on basis of sites Fig. S1a,b,c, but high variability of elevation trends for individual metal at different sites were recorded. But on the basis of zones, metal concentration like Cd, and Mn in soil samples; and Cd in vegetation samples, showing clear increasing concentration trend against the elevation (from low to high elevation), while for rest of HMs a clear elevation trends to HMs concentrations were not observed (Fig. S2a,b,c). The significant positive and negative correlation pairs in soil, dust and vegetation samples along elevation gradients are shown in Table 3.
Physicochemical properties/environmental factors of multi-matrix samples and its relation with HMs
The statistical descriptions (min–max and mean ± SD) for physicochemical properties in soil and dust at different zones are summarized in Table S2. The PH, EC, TOC, moisture, sand, silt and clay were ranged between 6.1 and 8.1, 116 and 289, 3.1 and 5.9%, 2.5 and 38%, 34 and 49, 45 and 58% and 5.2 and 12%, respectively in soil, while in dust moisture content was 0.2–2.3% (Table S2). The correlation pairs (+ ve and −ve) among HMs in soil, dust and vegetation samples at zone-1, zone-2, zone-3 and zone-4 are presented and showing the interaction among dust and soil; dust and vegetation; and soil and vegetation samples (Table S3). The correlation pairs (+ ve and −ve) among metals and edaphic and environmental factors in soil, dust and vegetation samples at different sites are presented in Table 3.
Relation of δ13C with climatic, edaphic factors and HMs
Figure 1 depicted the correlation of δ13C in soil carbonates with climatic (temperature, precipitation, elevation) and edaphic (TOC, sand, silt) factors. All the climatic (temperature, precipitation, elevation) and edaphic (TOC, sand, silt) factors are relatively positive correlated with δ13C in soil (Fig. 1). But on the other hand clay (r2 = 0.008), moisture (r2 = 0.173), wind speed (r2 = 0.144), pH (r2 = 0.083), and EC (r2 = 0.004) are weakly correlated (not shown in figure). The δ 13C was also regressed with targeted HMs but extreme weak correlation was observed.
PCA/MLR and stable carbon (δ 13C) isotope as source apportionment for HMs
The PCA components or factors were extracted for HMs in soil, dust and vegetation samples on the basis of eigenvalue > 1 and cumulatively explained 81%, 82% and 70% of total variance, respectively (Table 4). The PCA components were extracted by using varimax rotation. The MLR method was applied on factor values of PCA and Z score to obtained mass apportionment from each source. The % contributions of targeted HMs in soil, dust and vegetation samples by MLR are summarized in Table 4.
The δ13C values in the surface soil collected from four zones were ranged between − 9.06 and 0.66 ‰ [Pee dee delemnite (PDB)] as shown in Table S2.
Ecological risk of HMs in soil
The calculated Igeo values in soils for HMs (Cu, Zn, Ni, Pb, Cr, Cd, Mn and Fe) at different elevation gradients and its classifications are depicted in Table 5. The min–max Igeo values for Cd, Cr, Cu, Ni, Mn, Zn, Pb and Fe were ranged from 2.17 to 3.36, − 6.35 to − 4.37, − 5.13 to − 2.79, − 2.96 to − 2.50, − 10.88 to − 6.29, − 2.47 to − 1.38, 0.21 to 0.98, − 5.95 to − 5.41, respectively.
Discussions
The higher HMs concentration in soil samples than other matrix (dust and vegetation) samples may be the result of their high susceptibility from different sources such as road traffic pollution, atmospheric pollution (dry and wet deposition), native bed rock origin and the falling of twigs and leaves of the vegetation. The moisture condition of the soil due to higher vegetation covers further support the HMs sustainability. The strong +ve correlation of TOC with moisture and precipitation in soil and −ve with temperature support the HMs sustainability and its distribution. Precipitation has recorded significant association with metals (Cd, Mn, Zn, Fe) and moisture in soil and also with Cd in vegetation samples. The soil of the study area is loamy with dominancy of silt and sand percentage and very low percentage of clay that may favor the HMs accumulation. The sand texture of soil showing positive relationship with Cd and Mn. The significant HMs pairs (Cu and Cr; Fe and Cr; Zn and Fe) in soil and (Cr and Cd; Mn and Ni; Fe and Ni; Fe and Mn) in vegetation samples depicting its similar behavior and source origin. The pH has negative correlation with Cd that also affects Cd and other heavy metals’ mobility and solubility in soil under the influence of the cation exchange capacity (CEC) of clay minerals (Al-Khashman 2004). As neutral pH reduces the availability of Cd, as found in the soil in lowest amount (Abollino et al. 2003). On the other hand, it enhanced interaction and retention of heavy metals in soil under influence of organic carbon (Wang 2008). Guan et al. (2018) reported that traffics of road near eastern part (Gansu province) of Tibetan plateau are responsible for different HMs release in the soil. Bing et al. (2016) have also recorded higher HMs concentration in the soil of Mt. Gongga, Tibetan Plateau.
The elevated HMs concentration in the higher elevation zones might be due to extreme low temperature prevalence and the HMs may bring to these zones by multiple sources. The significant negative association of temperature was found with metals (Cd, Mn, Zn and Fe) and elevation showing their role in its deposition at high altitude. Bing et al. (2016) also stated that HMs accumulation in colder and high elevated zones could be attributed to wet deposition and long range atmospheric transport (LRAT) processes. The overall HMs accumulation at higher elevation zones (especially zone3 and 4) or sites may be due to the high bulk precipitation, higher moisture contents, higher TOC contents, higher silt and sand contents and prevalence of low temperature over there. The bulk precipitation plus high elevations contributes to higher level of particle deposition (Reiners et al. 2016). Zechmeister (1995) also reported that high precipitation levels at the higher altitude are responsible for HMs deposition and further stated that native particles are the source of different HMs origin. Bing et al. (2016) stated that higher elevation sites are more prone to high deposition rates of HMs that may appear to firmly retain in the soil. The higher elevation zones (especially zone 3 and 4) have higher vegetative cover that could effectively attenuate the elevated HMs concentration from atmospheric deposition (far-away sources) and nearby road vehicles to forest canopy (Liu et al. 2014). The vegetation may be influenced by atmospheric inversions up to 2100 m and after that slight decrease in metals concentration was observed. The canopy or forest filtering effect is another mechanism that affects the distribution of HMs in soil (Fantozzi et al. 2013). For forest filtering effect, LAI (area of leaf/area of ground surface) is very crucial and high LAI was observed in the mixed and coniferous forests (1050–2500 m). This suggests greater interception of Cd, Mn, Fe and Zn by plant uptake and adsorption onto the leaves surfaces that would return accumulated HMs in leaves to the soils through litter falls (Bing et al. 2016). The Pb, Ni, Cr and Cu from multiple deposition surveys showed that the soil contaminated with these metals were in the vicinity of point source mainly (Steinnes and Friedland 2006), but hence they do not show any significant correlation with elevation. The Pb and Zn both show higher concentration at zone 1 and zone 2 of the multi-matrix (soil, dust and vegetation) samples, representing as a hotspot, where road humps and high traffic density including heavy duty vehicles led to large consumption of gasoline. Such activities boosted the emission of Pb and abrasion of tires to release Zn (Wei et al. 2010). Temperature is positive correlated with Pb concentration and negatively correlated with elevation, and precipitation that may affect its concentration at different sites.
A spatial variation of Cd, Cu, Mn, Zn and Pb concentration in soil; Zn in dust and Cd in vegetation at sites were observed and that could be attributed to the multiple local and other foreign sources (Bing et al. 2016; Tang et al. 2014). But for other studied metals no spatial variations were recorded reflecting the same bed rock material and the similar local sources of their origin (Li et al. 2008). The physicochemical characteristics of the matrix itself, road structure, vehicles age and life span are the other factors for metal variation at sites. The significant spatial differences for pH, moisture, sand, silt, TOC, 13C was evident in the soil showing its spatial variability at the zones. There was no subsequent association recorded among road dust and associated soil and vegetation for each HMs concentration at each zone but different metal pairs have associated among different environmental matrices suggesting their multiple source origin. There is dearth of information’s among the HMs relation with multi-matrix samples (soil, dust and vegetation samples) along different elevation gradient.
Among the studied HMs, Fe, Zn, Pb, and Ni were the most abundant in the soil as compared to Cd, Cr, Mn and Cu. Considerable surface augmentation of Cd along high altitude was reported from France and Germany (Saur and Juste 1994; Heinrichs and Mayer 1980), but no such trend was apparent in Finland (Tamminen et al. 2004). High deposition fluxes were also shown by Zn and Mn in areas prone to LRAT, whereas, in later studies no such significant positive correlation between Zn and atmospheric deposition was observed (Schlesinger et al. 1974; Heinrichs and Mayer 1977; Billett et al. 1991; Johansson et al. 1995). Ni, Cr and Cu from multiple deposition surveys showed that the soil contaminated and deposited with them have their origin from many point sources in their vicinity (Steinnes and Friedland 2006) and these HMs concentration were similar to the concentration measured in different studies (Al-Khashman 2004; Hjortenkrans et al. 2006; Zheng et al. 2008). But the measured concentration by Zhang et al. (2013) at Qing yuan China showed relatively higher metals concentration as compared to our results. The results for Fe in this study was in agreement with other relevant studies (Tüzen 2003; Pagotto et al. 2001) and the highest Fe concentration recorded in our soil samples attributed mainly to their background natural contents (Nagajyoti et al. 2010) and also its release from the vehicles parts. An increasing elevation may enhances the tire rubbing of vehicles due to friction as well as the use of petrol/diesel by vehicles and these activities result in deposition of the metals-bound particles into dust. Pb concentration in dust samples are comparable with the previous studies carried in China (Wei et al. 2010), Turkey (Divrikli et al. 2003) and Sweden (Hjortenkrans et al. 2006). As concentrations for Cr and Ni are comparable to different studies results reported from Pakistan, Nigeria, Mexico and Africa (Faiz et al. 2009; Meza-Figueroa et al. 2007; Ferreira-Baptista and De Miguel 2005). On the other hand, Zn, Cd, Cu concentrations in the road dust are also comparable to those studies investigated in Jordan and Nigeria (Al-Khashman, 2004; Ndiokwere 1984), while studies reported from China have also high concentration for these metals due to higher traffic density (Shi et al. 2008; Wei et al. 2010; Zhang and Hao 2009). For Fe and Mn concentration, our results are in good agreement as measured in Jordan and Malaysia (Al-Khashman 2007; Ramlan and Badri 1989), whereas concentration reported by Herngren et al. (2006) have much lower concentration as compared to the current study results but China, France and USA have recorded higher concentration for Fe and Mn (Duzgoren-Aydin et al. 2006; Robertson et al. 2003; Apeagyei et al. 2011). The current study results for HMs in the vegetation samples showed their wide variation with different studies carried out in China, Ukraine, Finland,, Spain, Jordan, Iran and Africa (Shparyk and Parpan 2004; Jordanova et al. 2003; Luo et al. 2014; Kuang et al. 2007; Gandois and Probst 2012; Salemaa et al. 2004; Sun et al. 2009; Kord et al. 2010; Al-Alawi and Mandiwana 2007; Tang et al. 2014; Arjouni et al. 2015). Such metals concentration variation in vegetation to other studies may be due to the vegetation and site variation and its selection criteria.
There was a lack of elevation trends for targeted HMs concentration on site basis but for some metals viz., Cd and Mn in soil and Cd in vegetation showed a clear elevation trends on zone basis. Moreover, strong positive correlations of HMs with precipitation suggest wet deposition as the main source of its enrichment at higher elevation zones. The distribution patterns of targeted HMs in the soils, dust and vegetation would not increase with elevation if there were no or rather weak local pollution. Bing et al. (2016) also reported that constant trend of HMs accumulation with elevation has not been observed due to local or regional reasons. In all the multi-matrix samples (soil, dust and vegetation) elevation has a strong association with precipitation and negative relation with temperature attributing its key role in metals distribution at different elevation gradients. The strong associations of metals like Cd, Mn, Zn and Fe in soil were found with elevation. TOC, moisture, sand, clay and precipitation also have strong correlation with elevation that shows that they are responsible for metal distribution at different elevation gradient, while strong −ve correlation of elevation with silt were observed in soil. In dust samples, negative correlation of elevation with Pb was also recorded showing its low concentration at the top of elevation. The study findings suggest that environmental and physicochemical characteristics have their potential role to distribute HMs at different sites/ or zones along the elevation gradient.
PCA provide qualitative information’s and multiple linear regression (MLR) was applied to the PCA scores to determine the percent contribution of different sources for HMs. Therefore, the PCA/MLR was applied to different environmental matrix (soil, dust and vegetation) samples to assess the sources origin of HMs at different sites. In soil samples along different elevation gradients, Factor 1 accounts for 30% of variance with high factor loading of Cd, Mn and Zn. Strong positive correlation of Cd, Mn, Fe and Zn with elevation and precipitation showed that these metals are influenced by LRAT and consequently characterize wet deposition as the main source. Our results are also in support with study results of Bing et al. (2016). Second factor represents high factor loading for Cr, Cu and Fe to explain 27% of total variance and represents geological parent materials as the main source for these metals (Li et al. 2008). The third component explained 24% of total variance and have high factor loading for Cu, Ni, and Zn that are indicator of vehicular emissions. Mainly Zn and Cu are particularly associated with car components, tire abrasion and lubricants, whereas Ni is believed to release from car corrosion (Christoforidis and Stamatis 2009). The road dust is a good representative of current profile of region particularly in transportation sector. The first component of road dust accounts for 27% of total variance and is loaded with Cd, Cr, Ni and Pb, characterizing vehicular emissions as the main source. Cd and Cr are metals of concern from brake lining (Hjortenkrans et al. 2006), while Ni in road dust is believed to be released from corrosion of cars (Christoforidis and Stamatis 2009). Gasoline is the main source of Pb that being phased out in Pakistan in 2005 but still remain persistent along road side dust (Faiz et al. 2009). Factor 2 explained 20% of total variance and loaded with Cr, Cu, and Mn, reflecting miscellaneous sources. The third factor loaded with Ni and Zn explained 18% of the total variance and factor 4 contains Mn and Fe that further explain 18% of the total variance in dust. Most of the HMs loaded in the PCA component explains their origin from both natural sources and from vehicles different body parts. Along different elevation gradients in vegetation samples, Factors 1 explains 27% of the variance with high weightage for Cr, Ni and Fe and has their natural occurrence in soil from weathered parent rock material (Nagajyoti et al. 2010). Factor 2 contains Cd, Zn and Mn loading with 23% of total variance and probably this contaminant may be carried with aerosol or air masses to the plants surfaces, as earlier reported in the study of Tang et al. (2014). Factor 3 explains about 20% of the total variance containing Cu, Ni and Pb as indicator of vehicular emissions.
The % sources of HMs on basis of MLR predicted that LRAT and wet deposition contribute 45%, while parent rock material and vehicular emission contribute 42% and 13% of total HMs in soil samples, respectively. But on other hand in dust samples, the vehicular emissions contribute 10% of total metals followed by miscellaneous sources (11%), air borne emissions (0.5%) and geological parent material (79%). MLR prediction also indicates that natural sources contribute about 67% of total HMs in vegetation samples but in other case, air-borne sources and vehicular emission contribute 22% and 11% of total metals in vegetation, respectively. The findings of the current study concluded that multiple HMs pollution sources in dust, soil and vegetation samples are responsible for their sources.
The stable carbon isotopic compositions (variations of the C13/C12) of soil inorganic carbon (SIC) provides criteria for the evaluation of the depositional environment for samples of a wide range (Cuna et al. 2012). The difference in the signatures of stable Carbon isotope in SIC can be used as a tracer of soil C dynamics (Powers and Veldkamp 2005), which further elaborates heavy metals sources due to strong carbon-heavy metal affinity. The studied zones of the study area have been experienced influence of atmospheric mixing, and several values of δ13C were depicted as more depleted than the others and also in agreement with the results of the study of Stevenson et al. (2005). The δ13C values were − 3.81 to 0.66‰ in the zone-1 and zone-2, showing dissolved inorganic carbon (DIC) as the main source. Carbon in pedogenic carbonate originates from dissolved CO2 in soil solution. Zamanian et al. (2016) have studied that respiration of roots and microorganisms and the decomposition of litter and SOM are the CO2 sources in the soil. The values of δ13C in soil at zone 3 and 4 were − 9.06 to − 4.62‰ that attributed to the atmospheric mixing which is the main contributors and our findings is concurrent with the results of Stevenson et al. (2005). There are many studies which reported that soil carbonates as start leaching (high MAP), then the affinity of carbonate fraction for metals increases three times (Wang et al. 2015). Hence, from the results it was conceded that dominancy of carbonate fraction (calcite) in zone 1 and 2 showed that parent rock material is the main source of HMs in soil, whereas atmospheric mixing in zone 3 and 4 indicates air-borne particles which are the main contributor for HMs in these zones. Cuna et al. (2001) have studied that the depleted values of δ13C in the sites also indicates organic sources. Yu et al. (2001) have also stated that the results obtained from different studies imply that organic matter is more accessible to HMs than any other soil phase. Metals have the ability to bind with organic carbon, form ligands, complexes and travel through different media (Nagajyoti et al. 2010). The current findings revealed that δ13C is not responsible for targeted HMs distribution but it has great relation with some environmental factor and edaphic factors. This reflects that the atmospheric CO2 mixing increases into the soil as the precipitation decreases. Stevenson et al. (2005) also reported that temperature is the primary determinant of the isotopic composition of pedogenic carbonate. Our results confirm that temperature, precipitation, elevation, sand, silt and TOC were the main contributors for spatial variation of soil δ13C along the sites and were in agreement with the findings of Yang et al. (2015).
The geo-accumulation index can be used to evaluate the occurrence and intensity of HMs in surface soil (Barbieri 2016). The Cd showed an ascending trend in terms of contamination along the four altitudinal zones, designated as moderately to strongly pollute as shown in Igeo classification. The second contributor to soil contamination is Pb in the altitudinal zones and were categorized under the class of unpolluted to moderately polluted (0 < Igeo < 1). But, all other studied altitudinal zones were declared as unpolluted with respect to Cu, Zn, Ni, Cr, Mn and Fe concentrations, as their Igeo values were < 0. Zhiyuan et al. (2011) conducted a detailed study in soils of Jiangxi Province, China and on the basis of Igeo assessment, they concluded that Cd and Cu were the main contributing metals in the soil, while Zn, Ni, Pb, and Cr were the least contributor in a soil as their Igeo values were < 1.
Conclusion
The study results concluded that Cd and Mn concentration in soil while Cd concentration in vegetation samples showed zones based elevation trends. But on the other hand, the site base has no clear elevation trends for each HMs. Most of the HMs is notably enriched in the mid-elevation zones (approximately 1300 m) of the Himalayan foothills. This study finding showed that multiple pollution sources are culprit for HMs contamination in dust, soil and vegetation samples. The δ13C stable carbon (δ13C) isotope analyses in soil predicts that parent rock material in zone-1, and zone-2 whereas, atmospheric mixing at zone-3, and zone-4 were the main contributors of HMs at these zones and furthermore the δ13C showed significantly positive correlation with elevation, temperature, precipitation and TOC and confirm as the main contributors for spatial variation of soil δ13C. Further, on the basis of Igeo assessment, it was concluded that Pb and Cd were the main contributing metals in soil, while other HMs were the least contributor in a soil as their Igeo values were < 1. This study provides ample information from the risks posed by LRAT of HMs but further in depth studies are needed to probe the long range transport phenomenon for different HMs fates; behavior and their modeling approach in future research studies.
References
Abollino O, Aceto M, Malandrino M, Sarzanini C, Mentasti E (2003) Adsorption of heavy metals on Na-montmorillonite. Effect of pH and organic substances. Water Res 37(7):1619–1627
Ahmed F, Ishiga H (2006) Trace metal concentrations in street dusts of Dhaka city, Bangladesh. Atmos Environ 40:3835–3844
Al-Alawi MM, Mandiwana KL (2007) The use of Aleppo pine needles as a bio-monitor of heavy metals in the atmosphere. J Hazard Mater 148:43–46
Al-Khashman OA (2004) Heavy metal distribution in dust, street dust and soils from the work place in Karak Industrial Estate, Jordan. Atmos Environ 38:6803–6812
Al-Khashman OA (2007) Determination of metal accumulation in deposited street dusts in Amman, Jordan. Environ Geochem Health 29:1–10
Apeagyei E, Bank MS, Spengler JD (2011) Distribution of heavy metals in road dust along an urban-rural gradient in Massachusetts. Atmos Environ 45:2310–2323
Arjouni MY, Bennouna MA, El Alaoui EL, Fels MA, Romane A (2015) Assessment of mineral elements and heavy metals in leaves of indigenous cypress of High Atlas Mountains. Nat Prod Res 29:764–767
ASTM-D-2216-98 (1998) Standard test method for laboratory determination of water (moisture) content of soil and rock by mass. ASTM Int. https://doi.org/10.1520/D2216-19
Bai J, Cui B, Wang Q, Gao H, Ding Q (2009) Assessment of heavy metal contamination of roadside soils in Southwest China. Stoch Environ Res Risk Assess 23:341–347
Bakirdere S, Yaman M (2008) Determination of lead, cadmium and copper in roadside soil and plants in Elazig, Turkey. Environ Monit Assess 136:401–410
Barbieri M (2016) The importance of enrichment factor (EF) and geoaccumulation index (Igeo) to evaluate the soil contamination. J Geol Geophys 5:1–4
Billett M, Fitzpatrick E, Cresser M (1991) Long-term changes in the Cu, Pb, and Zn content of forest soil organic horizons from north-east Scotland. Water Air Soil Pollut 59(1–2):179–191
Bing H, Wu Y, Zhou J, Li R, Luo J, Yu D (2016) Vegetation and cold trapping modulating elevation-dependent distribution of trace metals in soils of a high mountain in Eastern Tibetan Plateau. Sci Rep 6:1–14
Boutton TW, Archer SR, Midwood AJ, Zitzer SF, Bol R (1998) δ13C values of soil organic carbon and their use in documenting vegetation change in a subtropical savanna ecosystem. Geoderma 82:5–41
Cerling TE (1984) The stable isotopic composition of modern soil carbonate and its relationship to climate. Earth Planet Sci Lett 71(2):229–240
Chen X, Xia X, Zhao Y, Zhang P (2010) Heavy metal concentrations in roadside soils and correlation with urban traffic in Beijing, China. J Hazard Mater 181:640–646
Christoforidis A, Stamatis N (2009) Heavy metal contamination in street dust and roadside soil along the major national road in Kavala’s region, Greece. Geoderma 151:257–263
Chuan MC, Shu GY, Liu JC (1996) Solubility of heavy metals in a contaminated soil: effects of redox potential and pH. Water Air Soil Pollut 90:543–556
Cuna S, Pop D, Hosu A (2001) Carbon and oxygen isotope ratios in rona limestone, Romania. Stud Univ Babes-Bolyai Geol 46:139–152
Divrikli U, Soylak M, Elci L, Dogan M (2003) Trace heavy metal levels in street dust samples from Yozgat city center, Turkey. J Trace Microprobe Tech 21:351–361
Duzgoren-Aydin NS, Wong CSC, Aydin A, Song Z, You M, Li XD (2006) Heavy metal contamination and distribution in the urban environment of Guangzhou, SE China. Environ Geochem Health 28:375–391
Faiz Y, Tufail M, Javed MT, Chaudhry MM (2009) Road dust pollution of Cd, Cu, Ni, Pb and Zn along Islamabad Expressway, Pakistan. Microchem J 92:186–192
Fantozzi F, Monaci F, Blanusa T, Bargagli R (2013) Holm Oak (Quercusilex L.) canopy as interceptor of airborne trace elements and their accumulation in the litter and topsoil. Environ Pollut 183:89–95
Ferreira-Baptista L, De Miguel E (2005) Geochemistry and risk assessment of street dust in Luanda, Angola: a tropical urban environment. Atmos Environ 39:4501–4512
Gandois L, Probst A (2012) Localisation and mobility of trace metal in silver fir needles. Chemosphere 87:204–210
Gerdol R, Bragazza L, Marchesini R (2002) Element concentrations in the forest moss Hylocomiumsplendens: variation associated with altitude, net primary production and soil chemistry. Environ Pollut 116:129–135
Guan Z, Li XG, Wang L (2018) Heavy metal enrichment in roadside soils in the eastern Tibetan Plateau. Environ Sci Pollut Res 25:7625–7637
Hameed M, Nawaz T, Ashraf M, Ahmad F, Ahmad KS, Ahmad MSA, Raza SH, Hussain M, Ahmad I (2012) Floral biodiversity and conservation status of the himalayan foothill region, Punjab. Pakistan J Bot 44:143–149
Heinrichs H, Mayer R (1977) Distribution and cycling of major and trace elements in two central european forest ecosystems. J Environ Qual 6:402–407
Heavy Metal Emissions for Danish Road Transport, National Environmental Research Institute, Aarhus University
Heinrichs H, Mayer R (1980) The role of forest vegetation in the biogeochemical cycle of heavy metals. J Environ Qual 9:111–118
Herngren L, Goonetilleke A, Ayoko GA (2006) Analysis of heavy metals in road-deposited sediments. Anal Chim Acta 571:270–278
Hjortenkrans D, Bergbäck B, Häggerud A (2006) New metal emission patterns in road traffic environments. Environ Monit Assess 117:85–98
Iqbal J, Shah MH (2011) Distribution, correlation and risk assessment of selected metals in urban soils from Islamabad, Pakistan. J Hazard Mater 192(2):887–898
Johansson K, Andersson A, Andersson T (1995) Regional accumulation pattern of heavy metals in lake sediments and forest soils in Sweden. Sci Total Environ 160–161:373–380
Jordanova NV, Jordanova DV, Veneva L, Yorova K, Petrovsky E (2003) Magnetic response of soils and vegetation to heavy metal pollution—a case study. Environ Sci Technol 37:4417–4424
Khan AB, Kathi S (2014) Evaluation of heavy metal and total petroleum hydrocarbon contamination of roadside surface soil. Int J Environ Sci Technol 11:2259–2270
Kord B, Mataji A, Babaie S (2010) Pine (Pinus Eldarica Medw.) needles as indicator for heavy metals pollution. Int J Environ Sci Technol 7:79–84
Kuang YW, Wen DZ, Zhou GY, Liu SZ (2007) Distribution of elements in needles of Pinusmassoniana (Lamb.) was uneven and affected by needle age. Environ Pollut 145:730–737
Kyllonen K, Karlsson V, Ruoho-Airola T (2009) Trace element deposition and trends during a ten year period in Finland. Sci Total Environ 407:2260–2269
Li C, Kang S, Wang X, Ajmone-Marsan F, Zhang Q (2008) Heavy metals and rare earth elements (REEs) in soil from the Nam Co Basin, Tibetan Plateau. Environ Geol 53:1433–1440
Liu X, Li J, Zheng Q, Bing H, Zhang R, Wang Y, Luo C, Liu X, Wu Y, Pan S, Zhang G (2014) Forest filter effect versus cold trapping effect on the altitudinal distribution of PCBs: a case study of Mt. Gongga, Eastern Tibetan Plateau. Environ Sci Technol 48:14377–14385
Luo J, She J, Yang P, Sun S, Li W, Gong Y, Tang R (2014) Heavy metal concentrations in timberline trees of eastern Tibetan Plateau. Ecotoxicology 23:1086–1098
Malandrino M, Abollino O, Giacomino A, Aceto M, Mentasti E (2006) Adsorption of heavy metals on vermiculite: influence of pH and organic ligands. J Colloid Interface Sci 299:537–546
Meza-Figueroa D, De la O-Villanueva M, De la Parra ML (2007) Heavy metal distribution in dust from elementary schools in Hermosillo, Sonora, México. Atmos Environ 41:276–288
Müller G (1979) Schwermetalle in den sedimenten des RheinseVeranderungen seitt 1971. Umschau 79:778–783
Nabulo G, Oryem-Origa H, Diamond M (2006) Assessment of lead, cadmium, and zinc contamination of roadside soils, surface films, and vegetables in Kampala City, Uganda. Environ Res 101:42–52
Nagajyoti PC, Lee KD, Sreekanth TVM (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8:199–216
Ndiokwere CL (1984) A study of heavy metal pollution from motor vehicle emissions and its effect on roadside soil, vegetation and crops in Nigeria. Environ Pollut Ser B Chem Phys 7:35–42
Pagotto C, Rémy N, Legret M, Legret M (2001) Heavy metal pollution of road dust and roadside soil near a major rural highway. Environ Technol (United Kingdom) 22:307–319
Pekey H, Doǧan G (2013) Application of positive matrix factorisation for the source apportionment of heavy metals in sediments: a comparison with a previous factor analysis study. Microchem J 106:233–237
Powers JS, Veldkamp E (2005) Regional variation in soil carbon and δ13C in forests and pastures of northeastern Costa Rica. Biogeochemistry 72:315–336
Ramlan MN, Badri MA (1989) Heavy metals in tropical city street dust and roadside soils: a case of Kuala Lumpur, Malaysia. Environ Technol Lett 10:435–444
Reiners AWA, Marks RH, Vitousek PM (2016) Nordic Society Oikos heavy metals in subalpine and alpine soils of New Hampshire Published by : Wiley on behalf of Nordic Society Oikos Stable https://www.jstor.org/stable/3543497 REFERENCES Linked references are available on JSTOR for this article : You may need to log in to JSTOR to access the linked references . Your use of the JSTOR archive indicates your acceptance of the Terms and Conditions of Use , available at 26, pp 264–275
Robertson DJ, Taylor KG, Hoon SR (2003) Geochemical and mineral magnetic characterisation of urban sediment particulates, Manchester, UK. Appl Geochem 18:269–282
Ryan J, Estefan G, Rashid A (2007) Soil and plant analysis laboratory manual: ICARDA
Sahu V, Nath V, Asthana AK, Yunus M (2014) Marchantia paleacea Bertol. as quantitative biomonitor of atmospheric heavy metals deposition. J Recent Adv Appl Sci 29:22–27
Salemaa M, Derome J, Helmisaari HS, Nieminen T, Vanha-Majamaa I (2004) Element accumulation in boreal bryophytes, lichens and vascular plants exposed to heavy metal and sulfur deposition in Finland. Sci Total Environ 324:141–160
Saur E, Juste C (1994) Enrichment of trace elements from long-range aerosol transport in sandy podzolic soils of southwest France. Water Air Soil Pollut 73:235–246
Schlesinger WH, Reiners WA, Knopman DS (1974) Heavy metal concentrations and deposition in bulk precipitation in Montane ecosystems of New Hampshire, USA. Environ Pollut 6:39–47
Shi G, Chen Z, Xu S, Zhang J, Wang L, Bi C, Teng J (2008) Potentially toxic metal contamination of urban soils and roadside dust in Shanghai, China. Environ Pollut 156:251–260
Shparyk YS, Parpan VI (2004) Heavy metal pollution and forest health in the Ukrainian Carpathians. Environ Pollut 130:55–63
Steinnes E, Friedland AJ (2006) Metal contamination of natural surface soils from long-range atmospheric transport: Existing and missing knowledge. Environ Rev 14:169–186
Stevenson BA, Kelly EF, McDonald EV, Busacca AJ (2005) The stable carbon isotope composition of soil organic carbon and pedogenic carbonates along a bioclimatic gradient in the Palouse region, Washington State, USA. Geoderma 124:37–47
Sun SQ, Wang DY, He M, Zhang C (2009) Monitoring of atmospheric heavy metal deposition in Chongqing, China-based on moss bag technique. Environ Monit Assess 148:1–9
Tamminen P, Starr M, Kubin E (2004) Element concentrations in boreal, coniferous forest humus layers in relation to moss chemistry and soil factors. Plant Soil 259:51–58
Tang R, Luo J, Yang P, She J, Chen Y, Gong Y, Zhou J (2014) Trace metals of needles and litter in timberline forests in the Eastern of Tibetan Plateau, China. Ecol Indic 45:669–676
Tian S, Liang T, Li K (2019) Fine road dust contamination in a mining area presents a likely air pollution hotspot and threat to human health. Environ Int 128:201–209
Tüzen M (2003) Determination of heavy metals in soil, mushroom and plant samples by atomic absorption spectrometry. Microchem J 74:289–297
Vega FA, Covelo EF, Andrade ML, Marcet P (2004) Relationships between heavy metals content and soil properties in mine soils. Anal Chim Acta 524:141–150
Viard B, Pihan F, Promeyrat S, Pihan JC (2004) Integrated assessment of heavy metal (Pb, Zn, Cd) highway pollution: bioaccumulation in soil, Graminaceae and land snails. Chemosphere 55:1349–1359
Walk M (1991) (Received June 4, 1990; revised February 5, 1991), pp 179–191
Wang XS (2008) Correlations between heavy metals and organic carbon extracted by dry oxidation Cuna procedure in urban roadside soils. Environ Geol 54(2):269–273
Wang C, Li W, Yang Z, Chen Y, Shao W, Ji J (2015) An invisible soil acidification: critical role of soil carbonate and its impact on heavy metal bioavailability. Sci Rep 5:1–9
Wei B, Jiang F, Li X, Mu S (2010) Heavy metal induced ecological risk in the city of Urumqi, NW China. Environ Monit Assess 160:33–45
Wei Z, Wang D, Zhou H, Qi Z (2011) Assessment of soil heavy metal pollution with Principal component analysis and Geoaccumulation index. Procedia Environ Sci 10:1946–1952
Wiederhold JG (2015) Metal stable isotope signatures as tracers in environmental geochemistry. Environ Sci Technol 49:2606–2624
Wilcke W, Müller S, Kanchanakool N, Zech W (1998) Urban soil contamination in Bangkok: heavy metal and aluminium partitioning in topsoils. Geoderma 86:211–228
Yang Y, Ji C, Chen L, Ding J, Cheng X, Robinson D (2015) Edaphic rather than climatic controls over 13C enrichment between soil and vegetation in alpine grasslands on the Tibetan Plateau. Funct Ecol 29:839–848
Yu KC, Tsai LJ, Chen SH, Ho ST (2001) Correlation analyses on binding behavior of heavy metals with sediment matrices. Water Res 35:2417–2428
Zamanian K, Pustovoytov K, Kuzyakov Y (2016) Pedogenic carbonates: forms and formation processes. Earth Sci Rev 157:1–17
Zechmeister HG (1995) Correlation between altitude and heavy metal deposition in the Alps. Environ Pollut 89(1):73–80
Zehetner F, Rosenfellner U, Mentler A, Gerzabek MH (2009) Distribution of road salt residues, heavy metals and polycyclic aromatic hydrocarbons across a highway-forest interface. Water Air Soil Pollut 198:125–132
Zhang M, Hao W (2009) Concentrations and chemical forms of potentially toxic metals in road-deposited sediments from different zones of Hangzhou, China. J Environ Sci 21(5):625–631
Zhang HM, Zhang H, Song A, Qin J, Song M (2013) Evaluation of ecological risk on soil heavy metals pollution of Qingyuan. Adv Mater Res 610–613:928–931
Zhang M, Wang H (2009) Concentrations and chemical forms of potentially toxic metals in road-deposited sediments from different zones of Hangzhou, China. J Environ Sci 21:625–631
Zheng YM, Chen TB, He JZ (2008) Multivariate geostatistical analysis of heavy metals in topsoils from Beijing, China. J Soils Sedim 8:51–58
Zhiyuan W, Dengfeng W, Huiping Z, Zhiping Q (2011) Assessment of soil heavy metal pollution with principal component analysis and geoaccumulation index. Procedia Environ Sci 10:1946–1952
Acknowledgements
This work was supported by the Pakistan institute of nuclear sciences and technology (PINSTECH), Islamabad and department of biochemistry, Quaid-i-Azam University, Islamabad especially in regards of metal and physico-chemical analysis and stable carbon isotope determination. The authors are thankful to the members of the Environmental biology and Applied Ecotoxicology Laboratory, Department of Environmental Sciences, Faculty of Biological Sciences, Quaid-i-Azam University, Islamabad for their support during sampling. The authors are highly encouraged the efforts of Dr. Mushtaq Ahmed and Dr. Zaffar for the plant species identification.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All the authors of the manuscript have declared that they have no competent interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Salim, Z., Khan, M.U. & Malik, R.N. Concentration, distribution and association of heavy metals in Multi-matrix samples of Himalayan foothill along elevation gradients. Environ Earth Sci 79, 479 (2020). https://doi.org/10.1007/s12665-020-09218-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-020-09218-6