Abstract
The present work aimed to compare the effects of coal mining and agricultural land uses on the quality of surface water and groundwater in the Handan region in China. For this purpose, the natural water samples were collected from both a mining area and an area without mining in a similar geological setting. The results showed that the contents of total hardness (TH), NO3−, SO42−, Ca, K, Co, Se, Rb, Fe and Ni in groundwater of the mining area (MGW) were significantly higher (1.6–6.1 times) than those in non-mining area (AGW), exhibiting a decreasing trend with the increasing distance from the mining areas. Most elements in surface water from the mining areas (MSW) were higher (1.1–30.8 times) than the non-mining areas surface water (AGW). The medium and high-level pollution in mining area groundwater samples were 16.7% and 50%, while in non-mining area samples were 9.5% and 4.8%, respectively. The lithology of the aquifer [including coal and rock (Permian, Carboniferous, Triassic, Ordovician, and Quaternary)] and watercourses are the main controlling factor of the element variance in groundwater of the Handan area. The Fe and SO42− in the Handan water are derived from sulfide mineral oxidation and the evaporative concentration of sulfate in the aquifer, while the coal mining induced higher NO3− in the mining area (MGW) as compared to the non-mining area (AGW). Interestingly, several samples from the mining areas have met the requirement of Se-supplementation in the water (average = 3.9 μg/L), which is higher than the Hebei groundwater (0.8 μg/L). The high Se in water is associated with the oxidation and leaching of selenium-rich coal and sedimentation of the coal ash. The concentration of Se, Sr, Rb, K, and NO3− generally show a large decline with the distance from mining areas, while Co, Ni and Ca trend are less clear. Unexpectedly, the elevated concentrations of chloride caused a non-carcinogenic risk for potable groundwater use in both the areas, while Pb caused a carcinogenic health risk in the mining areas. The spatial distribution of chemical constituents declines with the distance from mining areas. Noticeably, the elemental enriched coal-rock has caused the pollution of groundwater by rock weathering and percolation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
China is the largest coal producer and consumer in the world. Coal is the primary energy source in China and is also a source of useful elements that are used for a variety of purposes (Dai et al. 2012). Over recent decades, coal production and consumption (mainly for combustion) in China has increased rapidly. Almost, 70% of the coal that is mined is directly used for energy production, while 5% is used in households (Ewing et al. 2010; Dai et al. 2012; Hussain et al. 2018).
During the mining and processing of coal, large amounts of hazardous and trace elements are released to the environment which may cause serious environmental and health problems (Wang et al. 2010; Dai et al. 2012). Among the environmental factors, water quality is an important factor which is closely related to human health, is also inevitably affected through mining, i.e., coal and Cu-mining (Keller 1979; Flaten 2001; Liu et al. 2003; Cao et al. 2014; Rzymski et al. 2017). Mining causes water pollution, including contamination of potential sources of drinking water. The water quality and pathways of the natural water might be affected in the mining area due to the mining and dumping of wastes (Rzymski et al. 2017).
However, some studies pointed out that the discharging of mine drainage and leachate of water from the coalmine debris can cause the degradation of water quality, especially through increases in acidity and hardness and the dissolution of trace elements (Tiwary 2001; Dang et al. 2002; Bhuiyan et al. 2010; Sun et al. 2009). However, the extensive mining and coal combustion also cause the degradation of the quality of surface water and shallow groundwater in areas used for stockpiling. However, the disposal of coal and coal combustion products, potentially increasing the health risks for local inhabitants who may use the water for domestic uses (Huang et al. 2009).
Although there is a large body of research that has focused on the extent and severity of heavy metals contamination in natural water around the mining areas (Tiwary 2001; Bhuiyan et al. 2010), much less attention has been made to the impacts of mining on other major-ion and trace-element concentrations which are important for human health (Mertz 2012; WHO 1996). These changes in chemical composition in natural waters may occur at a regional scale from the effects of mining in general, while regulatory monitoring is usually only concerned with assessing the impacts of a particular coalmine on its surrounding water environment.
Consequently, this study was undertaken to assess both the potential beneficial and adverse effects of coal mining on water quality in a mining area. Coal seams and their host rocks have the potential to increase the level of some beneficial elements in the ambient environment, which may have positive effects on the surrounding areas to overcome the deficiency of useful trace elements in water and daily diet (Dreher and Finkelman 1992; Lussier et al. 2003; Huang et al. 2009; GSIH 2009). Conversely, the same seams and host rocks have the potential to introduce high concentrations of extremely toxic elements like thallium and arsenic into the surrounding environment, potentially causing severe health impacts.
In the present study, typical samples of surface water and groundwater were collected from mining areas and non-mining areas at various locations to compare the concentration characteristics and distribution differences of a range of elements. Additionally, an integrated assessment of water quality was undertaken using the water pollution index (WPI) to assess the local water contamination level. Furthermore, factor analysis (FA) and cluster analysis (CA) techniques were used to provide insights into associations of elements and of the factors that may influence their concentrations in water samples (Jolliffe 2002; Farnham et al. 2003; Panda et al. 2006). Therefore, the FA and CA analysis were applied to identify the similarities and differences in the element sources in two areas. Finally, a risk assessment of drinking groundwater was conducted based on the trace element and major ion contents of the water in mining and non-mining areas. The purpose of the investigation was to provide the information to help to develop a more in-depth understanding of the complex impacts of mining and related activities on the water environment and of the potential public health impacts of mines on nearby communities.
Coal mining has been carried out in the Handan area for more than 130 years and this area has a mining history that is typical in the occurrence of the coal seams, characteristics of surface disturbances and of the living conditions of residents in North China. Due to the prolonged period of coal mining, it is hypothesized that the mining and related activities in the Handan mining areas has caused significant changes in the quality of both shallow groundwater and surface water bodies. However, few comparative studies have been conducted on the pollution intensity and potential health risks related to coal wastes, and soil (Fang 2014; Appleton et al. 2006).
Consequently, the aim of this study was to assess the water quality in Handan area to determine the mode of occurrence of trace elements in water and their potential environmental and health impacts. For this purpose, the study selected the mining areas and non-mining area/natural areas of the Handan, Hebei Province in China. A total of 51 representative samples were collected and analyzed for 44 parameters (Tables 1, 2, 3).
Materials and methods
Characteristics of the study area
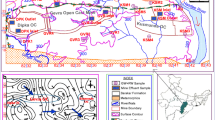
Handan Prefecture (113°28ʹ–115°28ʹE, 36°04ʹ–37°01ʹN) is located in southern Hebei Province in northern China, a region that has a warm temperate continental monsoonal climate. The study areas are situated between the Taihang Mountains in the west and the North China Plain in the east. The western part of Handan has a hilly topography with an altitude of 33–1900 m, which decreases in elevation from the west to the east. The region is an important source of coking and steam coal. Mining in Handan mainly takes place in the central and western parts of the prefecture, specifically from the Fengfeng coalmine and associated regions. The eastern boundary of the mining areas is roughly in line with the Beijing–Guangzhou railway transit (Fig. 1).
Mining and agriculture areas coexist in an area of about 957 km2. The region is underlain by sediments of Ordovician, Permian, Triassic, Tertiary, and Quaternary age, but the mineable coal seams occur in sediments of Carboniferous and Permian age (Fang 2014). In 2016, the annual output of raw coal, washing coal and coke in Handan were 22.4, 29.5 and 14.6 million tons from 20 mines, respectively. Mining does not take place to the west of the Fengfeng area, or in the vast alluvial plain in the east of this area, in which the Quaternary sediments are widely distributed (Fig. 1) (Fang 2014). This plain is a traditional agricultural area where the staple crops of wheat, maize, millet, and other local food crops are grown.
The major waterways in the region are the Fuyang River, the Ming River, and a branch of the Zhang River, which flows through the study areas. The principal aquifers in the mining areas occur in the Permian–Tertiary sandstones and in Quaternary alluvial fans, while groundwater in the non-mining area is mostly withdrawn from Quaternary alluvium. All the domestic and irrigation water is mainly from deep groundwater (from depths of 60–500 m).
Sampling and preparation of samples
A field visit was carried out in September 2018 in the study areas under the supervision of a qualified geoscientist. The following water samples were collected from the study areas: 24 groundwater samples from the mining area (the MGW series of samples); 4 surface water samples from the mining area (the MSW series of samples); 21 groundwater samples from an area not used for mining (the AGW series of samples); and 2 surface water samples from an area not used for mining (the ASW series of samples) (Fig. 1). Each collection point was recorded using a handheld GPS (GARMIN Rino 530HCx) to get an accurate position. The main purposes of GPS data were to show the sampling points on maps and their distribution pattern. Some of the parameters, i.e., pH, electrical conductivity (EC), total dissolved solids (TDS), salinity (SAL) and resistivity (RES) of the water samples were determined in situ using a pH tester (SevenGo SG2, Switzerland Mettler Toledo) and EC tester (SevenGo SG3, Switzerland Mettler Toledo). All the water samples were stored in polyethylene bottles that had been pre-cleaned with deionized water and kept at 4 °C for further analysis (Table S1 and Table S2). All the chemical analyses were undertaken using standard procedures (MEPPRC 2002b; Eaton et al. 2005).
Analytical methods and quality control
All the water samples were analyzed in the Institute of Geographic Sciences and Natural Resources Research, Chinese Academy of Sciences (IGSNRR, CAS). The chloride (Cl−) concentration was measured by the chloride ion-selective electrode method (Hirokazu et al. 1985). Nitrate (NO3−) concentration was determined through UV spectrophotometry (MEPPRC 2002b). The alkalinity (HCO3− and CO32−) was detected using the acid–base titration method within 3 days of sampling (MHPRC 1985; MEPPRC 2002b; Hussain et al. 2014). The concentrations of major cations (Ca2+, K+, Mg2+, Na+, P, and SiO2) and anion (SO42−) were measured by inductively coupled plasma optical emission spectrometry (ICP-OES, Optima 5300 DV, PerkinElmer) with a detection limit of 0.001 mg/L. The total hardness (TH) was calculated by summation of the concentrations of Ca2+ and Mg2+. The major and trace element (Al, Ag, B, Ba, Be, Cd, Co, Cs, Cr, Cu, Fe, Ga, Hg, Li, Mn, Mo, Ni, Pb, Rb, Sb, Sr, Ti, Tl, U, V, and Zn) concentrations in water were determined through inductively coupled plasma mass spectrometry (ICP-MS, DRC-e, PerkinElmer) directly. Selenium and arsenic concentrations were determined through hydride generation atomic fluorescence spectrometry (HG-AFS, AFS-9780, Beijing Haiguang) (MHPRC 1985; MEPPRC 2002b; Hussain et al. 2018).
For quality control, blank samples (2 distilled water), parallel samples (after every 10 samples), and repeated measurements (select one sample and repeated after every 15th sample) were used with each batch of samples (Table S3). A balance of ionic charge of water samples is shown in Fig. 2. The error percentage in the water samples ranged from ± 0.01 to ± 7.60%, indicating the high reliability of the analytical data (Shen et al. 1993). The correlation coefficients of all the element standard curves reached 0.999. In all analyses, the relative standard deviation (RSD) was relatively low, and mostly less than 8%.
Degree of contamination
The degree of contamination (Cf) summarizes the combined effects of the water quality parameters and their impacts on human health and was determined through Eqs. 1 and 2 (Bhuiyan et al. 2010; Hussain et al. 2015, 2019):
where Cf is the contamination factor, CAi and CNi represent the analytical value and the upper permissible concentration indicated in the guidelines provided by Ministry of Health People Republic of China (MHPRC), respectively (MHPRC 2006). The output Cf values must be assessed through varying contamination levels, i.e., low contamination (Cf < 1), medium contamination (Cf = 1–3), and high contamination (Cf > 3).
Identification of elemental sources
Factor analysis (FA)
To find out the source difference of the elements in between the groundwater of mining and non-mining area, a factor analysis (FA) was applied to the results of the chemical analyses. Factor analysis was measure through the principal component method. This assessment was undertaken to determine associations between chemical parameters and other variables and explain the significance of variation among the groups (Jolliffe 2002; Everitt and Dunn 2001). As the concentrations of chemical constituents measured in water samples varied over several orders of magnitude, each variable was normalized to ensure they were comparable in the FA assessment (Chen et al. 2007; Davis 1986). Finally, only factors with an eigenvalue greater than 1 were taken into account in the assessment of the FA results (Dragović et al. 2008; Franco-Uría et al. 2009).
Cluster analysis (CA)
Cluster analysis is often used to identify groups of samples on the basis of similarities within a class and may produce similar groupings to those produced by factor analysis (Panda et al. 2006). The levels of similar observations were merged to construct a dendrogram (Chen et al. 2007). In this study, Ward’s method was applied (linkage between groups), using a standardized m-space Euclidian distance as a similarity measure to produce dendrograms (Davis 1986). All statistical analyses in this study, including independent sample t tests, FA, CA and Pearson’s correlation coefficient analysis were performed using the statistical package IBM SPSS 22.0 software for Windows (SPSS Inc., USA). The sampling sites and concentration distribution maps were drawn by using ArcGIS software version 10.0.
Health risk assessment models
Exposure assessment
In the study areas, groundwater is the main source of household water. From this source, excessive inputs of chemical constituents may enter into the human body either through ingestion of water or by dermal contact (Wu et al. 2009), while the exposure dose of other intake pathways can be ignored (Hussain and Luo 2018). In this study, the dermal contact exposure pathway was neglected and only the ingestion of drinking water was considered to estimate the inhabitant’s exposure to specific chemical constituents in the water supply. The dose received through an individual ingestion pathway was calculated through Eq. 3 (USEPA 1989; Phan et al. 2010; USEPA 2004):
where ADD is the average daily dose from ingestion (mg/kg day), Cw is the concentration of contaminants in water (mg/L), IR is the ingestion rate of water (L/day), ABSg is the absorption factor of oral ingestion (no dimension), EF is the exposure frequency (day/year), ED exposure duration (year), BW average body weight (kg), and AT average time/life expectancy (day). The standard values for each variable are found in the USEPA (2004, 2011; Du et al. 2018) such as IR = 2 L/day, ABSg 100%, EF = 365 day/year, ED = 30 year, BW = 60 kg, and AT 25,550 days (70 years).
Non-carcinogenic risk
The non-carcinogenic risk was characterized and quantified through hazard quotients (HQs) and was calculated through Eq. 4 as suggested by USEPA (1989):
where HQ is the hazard quotient (dimensionless), the RfD is the oral reference dose (mg/kg day), which represents an estimate of the daily intake and adverse effects (USEPA 2010, 2019). If the HQ is equal to or higher than 1, there will be a potential health risk (Wang et al. 2005), and related protective measurements and interventions should be taken (Ahmed et al. 2015).
For the integrated health risk, the hazard index (HI) was introduced to evaluate the total non-carcinogenic risks and was determined through Eq. 5 (Li and Zhang 2010):
Carcinogenic risk
Carcinogen risk is an estimated incremental probability of an individual to develop cancer over a lifetime exposure to carcinogenic metals and was determined through Eq. 6 (USEPA 1989):
where CR represents the carcinogenic risks over a lifetime and SF is a slope factor (mg/kg day) (USEPA 2010). The acceptable or tolerable risk levels for carcinogens suggested by the US-EPA range from 10−4 (1 in 10,000) to 10−6 (1 in 1,000,000) (USEPA 2004).
Results
Hydrochemical characteristics of water
The physicochemical/hydrochemical properties of the water in the study areas are presented in Tables 1, 2, and 3. The water samples (both groundwater and surface water samples) in the mining areas and non-mining areas showed near neutral to slightly alkaline pH values (7–8.4 and 6.96–7.9, respectively), suggesting the presence of carbonate minerals in aquifer materials. The TDS of water samples in mining areas ranged from 219 to 1740 mg/L, varying from freshwater (TDS < 1000 mg/L) to brackish water (1000 < TDS < 5000 mg/L). The TDS in the non-mining areas was ranged 445–1836 mg/L, indicating that some samples exceeded the recommended WHO potable limit for this parameter (WHO 2011).
The total hardness (TH) of water in the mining area was 22.7–1521 mg/L, ranging from very soft water (TH < 75 mg/L) to very hard water (TH > 450 mg/L). However, most of the samples are relatively uniformly distributed with high TH values. Similarly, the hardness of the non-mining area ranged 55.8 mg/L (very soft water) to 1106 mg/L (very hard water), indicating that there is a decreasing trend in TH values from the mining areas to the northeastern part of the non-mining areas. A similar west to the east trend was also observed (Fig. 6f). Furthermore, the hardness of water in the mining area was significantly higher than that of non-mining areas, while no significant differences were observed in other physicochemical parameters (pH, EC, TDS, and SAL) between the two areas (p < 0.05). Overall, the natural water in mining areas was mainly fresh-hard water (82.2%) and brackish-hard water (14.3%), while in non-mining areas was fresh-hard water (60.9%) and fresh-soft water (34.8%) (Fig. 3).
The hydrogeochemical characteristics of water samples were also different in the two areas. A Piper diagram (Fig. 4) (Piper 1944) showed that the water types in the mining areas were relatively diverse, with Ca, Ca–Mg, Ca–Na, HCO3, HCO3–SO4, and SO4 (65%) as the main composition-types, while Na–HCO3–Cl was the most prominent chemical composition in the non-mining areas (34.8%). The order of relative abundance of major cations in mining areas was Ca2+ > Na+ > Mg2+ > K+, while the anions were SO42− > HCO3− > Cl− > NO3−. The mean cation and anion levels in the non-mining areas were Na+ > Ca2+ > Mg2+ > K+, HCO3− > Cl− > SO42− > NO3− (Table 1).
The hydrochemical differences presented in the two regions can also be seen in a Gibbs plot (Fig. 5). This plot suggests that the primary factors that control the anion and cation composition in both the mining and non-mining areas are the rock weathering and evaporation (Fig. 5).
This is also consistent with what is known about the climate, terrain, and the lithology of aquifer materials in the Handan region. It was mentioned before that the western mining area occurs in the Taihang Mountains and on an alluvial plain. Infiltrating rainfall from the mountainous area flows through extensive limestone deposits that contain sulfide minerals (such as pyrite), and the reaction of groundwater with these materials can increase the dissolved concentrations of Ca2+, SO42− and HCO3− due to karstification (Fang 2014; Winograd and Thordarson 1975). The effects of evaporative concentration are revealed by the high TDS levels in unconfined aquifers (surface water) relative to the confined groundwater flow-system with a high concentration of sodium and chloride ions (due to the precipitation of CaCO3). The evaporation dominance is also possible due to the effects of flood irrigation for agriculture, a process that increases the elevation of the water table and increases evaporation and induces soil salinization (Wu et al. 2015). This is especially the case in the alluvial fan and alluvial-plain aquifers in the eastern part of the area where water infiltration and evaporation has induced change the chemical composition of groundwater (Na+ and Cl−) due to cation exchange processes in the subsurface (Fang 2014).
Variations among the chemical constituents in the waters of mining and non-mining areas
The vast majority of elemental and ionic concentrations, especially Al, Rb, Se, Mn, SO42−, and NO3− in the surface water samples from the mining area (MSW) are much higher (3.7–30.8 times) than those from the surface water of non-mining area (ASW) except for concentrations of Ga, Ba, U and Cr (Fig. 6, Tables 1, 2, 3).
In groundwater, there are significant differences in the concentrations of Cl−, HCO3−, NO3−, SO42−, Ca, K, Na, Li, B, Ti, V, Co, Se, Rb, Cr, Fe, Ni, Cu, As, and Mo (at p < 0.05) between the mining and non-mining areas. To be specific, the concentrations (range) of chemical constituents in groundwater of the mining area (i.e., for NO3−, SO42−, Ca, K, Co, Se, Rb, Fe, and Ni for the MGW series of samples) were significantly higher (1.61–6.07 times) than those in non-mining area (the AGW series of samples). An assessment of the spatial distribution of concentrations revealed that the concentrations of many chemical constituents decline from mining areas to the adjacent non-mining areas (Fig. 6). The highest concentrations of Se, Sr, Rb, and K are mainly concentrated in the central–northern part of the mining area. Nitrate concentrations, in particular, generally show large declines in water samples with distance from the mining area, while trends for Co, Ni and Ca are less clear. In contrast, the concentrations of Cl−, HCO3−, Na, Li, B, Ti, V, Cr, Cu, As, and Mo in the mining area groundwater were significantly lower (0.14–0.8 times, p < 0.05) than those in the non-mining area (AGW). The highest concentrations of many chemical constituents (i.e., Na, B, V, Cr, Cu, As, and Mo) were occurred in the northeastern part of the non-mining area, whereas the highest concentrations of HCO3− occurred in the central part of the non-mining area. Concentrations of Cl−, Li, and Ti are relatively evenly distributed in the non-mining area.
The concentrations of Se (9.3–25.8 μg/L) in several natural water samples (MSW1, 2 and MGW1, 3, 4, 9) from the mining area were close to or exceeded the Chinese National Standards for Mineral water (for drinking) (CNSM ≥ 10 μg/L) (GAQS 2008) (Fig. 6a). The maximum level of MSW1 and 2 were 1.6 and 2.6 times higher than the Chinese national standards (CNSM). The concentrations of Sr (0.14–7.7 mg/L) in water from both areas were far higher than the CNSM standard (≥ 0.2 mg/L) (Fig. 6b). The maximum concentrations of Sr in the mining area (7.7 mg/L) and non-mining area (2.9 mg/L) were 38.5 and 14.5 times higher than the national standard.
Overall, the above results show there were large variations of most hydrochemical properties and of trace element concentrations in water in the region along an east–west transect. Concentrations of TH, NO3−, SO42−, Ca, K, Co, Se, Rb, Fe and Ni in the groundwater of the western part of the mining area were higher than the water in the eastern part of a non-mining area, most likely due to a combination of geogenic and anthropogenic factors.
Drinking water quality assessment
Water samples MSW3, 4 and ASW1 satisfied the Grade I, II and III, water quality (CNSS) limits (MEPPRC 2002a) for all measured parameters. By contrast, samples MSW1 and ASW2 exceeded the phosphorus water quality standard (2.8 and 0.4 mg/L, respectively) and selenium levels in MSW2 (26 μg/L) were worse than the Grade V criterion (Se ≤ 20 μg/L, P ≤ 0.4 mg/L). The high P in the surface water may be because of particulate matter.
Groundwater is the main source of drinking water in the region. In the mining area groundwater, the concentrations of TDS, TH, Cl−, NO3−, Na+, and SO42− exceeded the CNSD limits (MHPRC 2006) (Table 4) by up to a factor of 5. In the non-mining area, the concentration of TDS, TH, Cl−, NO3−, Na+, and SO42− were also higher than the standard limits. However, the concentrations of heavy metals in both the areas generally met the CNSD criterion (MHPRC 2006) and WHO drinking water quality standards with the exception of the high iron concentrations (Table 4, Fig. 6c).
The order of exceedances of water quality parameters was NO3− > Fe > TH > SO42−, which mostly occurred in waters in the central part of the mining area. The order of concentration exceedances of water quality parameters in the non-mining area (AGW) was Na+ > TH > Fe > NO3− > Cl−, however, most of the exceedances occurred in waters in the southwestern part of the non-mining area near the mining area. Overall, the quality of drinking water in both study areas is poor.
Classification of water samples using a Ficklin plot
A Ficklin plot (Fig. 7; Ficklin et al. 1992; Caboi et al. 1999; Bhuiyan et al. 2010) indicated that most water samples (79%) from the mining area contained elevated metal concentrations under near-neutral pH conditions. By contrast, water samples from the non-mining area had near-neutral pH values and highly variable metal concentrations, with some concentrations being at levels of concern for both irrigation and potable uses.
Discussion
Water pollution evaluation
The degree of contamination (Cf) was used as a reference to estimate the extent of water pollution (Al-Ani et al. 1987; Hussain et al. 2018). In this study, the Cf index was computed using the Chinese national standards (MHPRC 2006), and the distribution map is shown in Fig. 8.
It can be seen that the Cf values in surface and groundwater of non-mining areas were obviously lower than those in the western part of the mining areas. Based on the Cf classification (Edet and Offiong 2002; Backman et al. 1998), all the mining area samples (MSW series) could be considered to have a “medium” to a “high” level of contamination. By contrast, 16.7% and 50% of the mining area groundwater samples are classified as having a “moderate” and “high” level of contamination, respectively, while 33.3% would be classified as either being not contaminated or having a “low” level of contamination (Table 5). The samples with high Cf values are distributed throughout the mining area but are especially concentrated in the central parts of this area (Fig. 8). Similarly, in both ASW and AGW (non-mining area) samples, 85.7% are either “non-contamination” or have a “low” level of contamination, 9.5% have a “medium” level of contamination and 4.8% are highly contaminated (Table 5, Fig. 8).
Taking into account the results of the water quality and pollution evaluations together, the pollution situation of the natural water in both mining and non-mining area are not optimistic. However, the water quality of the non-mining area is generally better than that in the mining area. This is probably due to the presence of an aquitard that limits the infiltration of contamination into the confined aquifer in this area (Fang 2014).
Source identification
Factor analysis
The results produced by applying factor analysis to the water quality data are listed in Table 6. Five independent factors with eigenvalues > 1 were extracted for the mining area groundwater and non-mining area groundwater areas by a reduction of the initial dimensions of the data sets, which account for about 80% and 83% of the total variation, respectively. However, the small sample size (< 1000) may generate an error of up to 30% in the assessment (Osborne and Costello 2004).
For the mining area (MGW), factors F1, F2, and F3 accounted for 35, 19 and 10%, respectively (Table 6). F1 was heavily loaded with TDS, SAL, TH, Cl−, NO3−, Ca, Mg, Na, SO42−, Fe, Co, Ni, and HCO3−, while their distribution was relatively centralized (Fig. S1a). These ions and related parameters with higher concentrations in the groundwater are generally controlled by the recharge, lithology of the aquifer, and hydrodynamic conditions in the aquifer (Drever 1988; Omo-Irabor et al. 2008). The groundwater samples (i.e., MGW 7, 9, 11, 20, 21 and 22), which had the highest positive scores on F1 (Fig. S1c), had chemical compositions that belonged to the Ca + Na–SO4–Cl and Ca–SO4 hydrochemical facies and have a high permanent hardness (Fig. 4). This facies may reflect the mixing and movement of water from carbonate rocks to magmatic rocks (Stetzenbach et al. 2001).
By contrast, the mining area groundwater (MGW series) samples 8, 14, 16 and 24 had the greatest negative F1 scores (Fig. S1c). These waters were of the Ca + Mg–HCO3 facies, which causes temporary hardness (Fig. 4). This characteristic suggests that groundwater flowed through the aquifer primarily composed of carbonate rocks (Winograd and Thordarson 1975). Meanwhile, the elevated concentrations of SO42− and Fe in mining areas (308.7 mg/L and 466.2 μg/L, respectively) are probably associated with the coal-rock aquifer, where the water undergoes redox reactions in the sulfide-rich coal seam (Bhuiyan et al. 2010; Fang 2014). However, the release and oxidation of ammonium ions from clays in this area increased the concentration of NO3− in water in the mining area (Luo et al. 2015; Le et al. 2019) (Fig. 6d), which could further enhance sulfide oxidation (Böhlke 2002). Additionally, a large amount of limestone exists in the mining areas (Fang 2014) in which the concentrations of some trace elements such as Ni are typically higher than many other lithologies as indicated by Drever (1988) and this factor could account for the higher concentrations of Ni and Co that were observed in F1 (mining areas) (Table 6). Overall, F1 can be attributed to the natural hydrogeochemical conditions within mining areas, which are closely related to the interaction of groundwater with the lithology of the aquifer (Farnham et al. 2003; Omo-Irabor et al. 2008).
In the mining area (MGW), the major elements in F2 are Sr, Se, As, Al, Ti, V, Cr, Cd, Cs, Ba, U, and Mo which are positively correlated with each other, while Mn and Zn are negatively correlated (Table 6, Fig. 9a). The elements As, V, Cr, Mo, Cd, U, and Se typically occur as soluble oxyanions in oxidizing waters, whereas Mn, Co, and Zn are generally more soluble under reducing conditions in groundwater (Hem 1985). Vanadium can exist as the oxyanion HVO42− under oxidizing conditions, but is present in solution as the oxycation V(OH)2+ under reducing conditions which can be strongly adsorbed by the aquifer materials under near-neutral pH conditions (Collier 1984; Jeandel et al. 1987; Domagalski et al. 1990). The redox-sensitive elements such as As, and Se occur as AsO42−, H2AsO4− and SeO32−, HSeO3, while Cr exists as CrO42− (soluble) to the insoluble ion of Cr3+ (Langmuir 1997; Welch and Lico 1998; Welch et al. 1988; Gui 2005). Uranium (UVI) generally occurs in groundwater as uranyl ions (UO22+) and uranyl-carbonate complexes (such as UO2(CO3) 2−2 , UO2(CO3) 4−3 ) (Langmuir 1978; Farnham et al. 2003).
These factors suggest that the factor F2 is associated with redox conditions within groundwater (Farnham et al. 2003; Chen et al. 2007). Correspondingly, combined with the factor scores (Table 6, Fig. S1c), the groundwater of MGW 1, 3 and 4 were relatively oxidized compared to the more reducing water from MGW 8, 22 and 24.
F3 contains high loadings of pH, Na, SiO2, Ti, and Cu, while F4 contains high loadings of NO3−, SO42−, Co, and Zn, which were significantly higher than those in the non-mining area (Table 6, Fig. 9b), particularly in samples MGW 5, 11, 14 and 16 (Fig. 9d). Dissolved SiO2 is likely to have originated from the leaching of silicate minerals in the upper soil, which was closely related to the pH of the solution (Zeng 1999). Simultaneously, the moderate negative F5 loadings (Table 6) also indicated that the considerable NO3− in this area was not from the agricultural activities. The positive NO3−, SO42−, Co and Zn in mining area groundwater (F4) suggest that coal-mining activities (such as the effects of mine drainage) are the source of the elevated nitrate levels in the water in this area. The current study results are consistent with Azcue (2012), Tiwary (2001).
In the non-mining areas, the factors F1–5 account for 42, 18, 11, 7 and 5% of the total variance, respectively (Table 6, Fig. S2c). In a similar manner to the mining area, the factor F1 of the non-mining area (AGW) also had high values of TDS, SAL, TH, Cl−, HCO3−, Ca, Mg, SO42−, Sr, Li, Mn, Fe, Co, Ni, and U (Fig. S2a), which occurred as important parameters in AGW1, 3, 4, 10 and 17 (Fig. S2c). This factor could also be attributed to the variation in elemental composition of the aquifer materials through which groundwater flowed (Drever 1988; Farnham et al. 2003), while the negative F1 (in AGW) classified as Na–HCO3–Cl waters (Fig. 4), indicate significant salinity variations across the area (Fang 2014). F2 was moderately loaded with Na, As, V, Mn, Cu, Mo, Cd, and U, which were significantly distributed in AGW5, 6, 7, 11, 17 and 19 (non-mining area) (Fig. S2a). F3 had a strong loading of NO3−, K, SiO2, As, and Li (Fig. S2b) which may be associated with anthropogenic pollution from agricultural and domestic sources (Omo-Irabor et al. 2008). The factor F4 was dominated by positive loadings of Al, Ti, and Cs, while F5 had positive loadings of Se and Cr (Table 6) which may be due to leaching from overlying soils, especially from the clay minerals to the aquifer (Fig. S2).
Fuyang River and other losing streams flow through the non-mining areas, which, in addition to precipitation, are also important sources of recharge for the shallow groundwater in the eastern alluvial plain (Niu 2007). NO3−, K, and SiO2 are common pollutants in the agricultural areas. These pollutants may be from agricultural practices, whereas the elevated SiO2 concentrations in water are likely to have been derived from the use of silica fertilizer, which contains soluble silica and is more prone to leaching into groundwater than solid silicate minerals. Arsenic is a common impurity associated with phosphate fertilizers (Zheng et al. 2007; Omo-Irabor et al. 2008) and could locally be a source of elevated As concentrations in groundwater. However, it is likely that the elevated Al and Ti concentrations are derived from leaching from clay minerals (Merritts et al. 1998; Zeng 1996). These elements could transport into groundwater through the eluviation of soil sediments and rock–water interaction.
The non-mining area is located in the downstream of Handan urban areas where industrial sewage discharge or sewage irrigation may be one of the sources of Se and Cr in groundwater (Huang et al. 2009; Zhang et al. 2013). The high concentrations of iron in groundwater in the non-mining area (32–557 mg/L) (Fig. 6c) are attributed to the leaching of iron-containing nodules occur abundantly in the Quaternary sediment. Additionally, commercial sewage may also enhance the ion exchange capacity between iron in soil and groundwater (Zhang et al. 2013).
Cluster analysis
The hierarchical cluster analysis was also performed to visualize elemental groupings in the two different groundwater datasets, and the results were illustrated by the use of dendrograms (Fig. 9). Elements in the same group were expected to have either originated from a common source or to have been released into the water by similar processes (Lee et al. 2006).
In the mining area groundwater, five distinct grouping of parameters could be identified by cluster analysis (Fig. 9a). Cluster 1 contained TDS, SAL, SO42−, Cl−, Ca, Fe, Ni, TH, Co, Mg, NO3−, Na, and Cu, and is likely to reflect the processes of rock–groundwater interaction dominated by F1. Cluster 2 consists of Se, U, Al, Ba, As, V, and Sr, and corresponds with the oxidizing/reducing conditions reflected in F2. Cluster 3 consists of K, Cs, HCO3−, Cr, and Li, which may reflect agricultural pollution (in a similar manner to F5). Cluster 4 contained Mn and Zn and is possibly associated with the oxidation of sulfide minerals due to underground mining activities (Bhuiyan et al. 2010), an association that is consistent with F4. Cluster 5 consisted of SiO2, Ti, Mo, Cd, pH, and RES, and is likely to indicate the influence of leaching chemical constituents from soil horizons to the aquifer as reflected in F3.
The non-mining area (AGW) samples were also grouped into 5 clusters (Fig. 9b). Cluster 1 contained all the elements attributed to F1 loadings in the factor analysis, which may be associated with water–rock hydrogeochemical processes. Cluster 2 consisted of NO3−, Se, Cr, SiO2, Li, K, Ti, and Zn, and appeared to be associated with areas where there is likely to be a large influence of domestic and agricultural pollution (in a similar way to F3 from the FA assessment of the non-mining area). Cluster 3 included Na, As and Cu, and possibly indicates the influence of industrial activities in the area. Cluster 4 (Al and Cs) and cluster 5 (Mo, Cd, V, pH, RES, and Ba) may indicate the leaching of soils and recharge to the aquifer from surface water, respectively. However, there were some differences between elemental groupings obtained from factor analysis and cluster analysis, which is possibly due to the effects of multiple sources and to the small sample size, which could lead to the formation of statistical artifacts as also suggested by Osborne and Costello (2004).
Sources of selenium-rich water in mining areas
As previously stated, elevated selenium concentrations were observed in the mining area water samples (Fig. 6a) although Se concentrations in some sites in this area met the CNSM standard criteria (< 10 μg/L). Additionally, the average content of Se in the mining area was 4 μg/L and was much higher than the average concentration in groundwater (0.8 μg/L) of Hebei Province (GSIH 2009). Although Se is known to be an essential trace element for human health, small excesses or deficiencies of this element may cause severe disease (Tan et al. 2002; Rayman 2000; Selinus et al. 2013; Fordyce et al. 1996). When ingested at optimal levels, selenium is an important antioxidant and can protect the body from Keshan and Kashin–Beck disease as well as strengthen the body’s immune system (WHO 2004; Fordyce 2007; Hussain et al. 2018).
Drinking water is also an important exposure pathway for the intake of selenium into the human body. Consequently, both low and elevated concentrations of Se in natural waters have received much research attention (Luo et al. 2002, 2004; Zhao et al. 2017). However, the Se concentration in the mining area water is generally higher (Table 2) than the average concentration in global river waters (0.2 μg/L) (Selinus et al. 2013), while concentrations higher than 10 μg/L Se are of particular health concern. Some studies suggest that the Se in natural water in the region is mainly derived from the weathering and leaching of bedrock, especially from the Se-enriched strata (6–20 μg/g) of the Lower Cambrian and Silurian systems of southern China (Chen and Luo 1996; Li et al. 2005; Luo 2011). In northern China, the Cambrian outcrop to the west of Handan mining area (limestone) has a comparatively low level of Se (0.07–0.11 μg/g) by comparison with the average crustal concentration (0.08 μg/g) (Yan and Chi 2005).
However, the average Se content in coal in the mining area is 59.2, 1.9 and 3.6 times higher than average crustal content, the average Chinese coal, and the average world coal contents, respectively (Dai et al. 2003, 2012). The content of selenium in coal in the study area is significantly correlated with Sr (r = 0.4, p < 0.05), As (r = 0.6, p < 0.01), V (r = 0.5, p < 0.05), Ag (r = 0.5, p < 0.05) and U (r = 0.7, p < 0.01) (Table 7). Elevated concentrations of selenium also occur in sulfide, carbonate, and aluminosilicate minerals in the host-rocks for the coal seams (Dai et al. 2003; Ren et al. 2006). The high concentrations of NO3− in the mining area groundwater may have also contributed to the oxidation of sulfide minerals and the release of Se into solution/water (Böhlke 2002). In contrast, Se in reducing waters tend to be incorporated in insoluble minerals and precipitated as a solid phase, resulting in the lower Se content in AGW (non-mining area).
The elevated concentrations and widespread distribution of selenium in surface water in the mining areas may also be influenced by the leaching of this element from coal waste-rocks of mining and fly-ash dumps in the area (Huang et al. 2009). Although Se concentrations in the mining area groundwater were elevated, most samples (with the exception of MGW 9) met the Chinese water quality standard for potable use. This suggests that leachate from coal waste materials does not have a large influence on selenium concentrations in water in this area. Additionally, the release of selenium into waterways is of environmental concern due to its ability to be biomagnified in food webs and to cause impacts on fish and bird populations. However, the optimum level could reduce the risk of Keshan and Kashin–Beck disease, Selenosis and prevent cardiac toxicity (Tacyildiz et al. 2012).
Health risk associated with water in Handan
Non-carcinogenic risks
Increasing the concentrations of pollutants in drinking water has the potential to increase public health risks if contaminated water is used for potable supply. The hazard quotients (HQ) of the selected elements from drinking water consumption are presented in Table 8. The non-carcinogenic risk caused by the elements/ions in the mining area (MGW) are Cl− > P > NO3− > Li > As > Sr, while in the non-mining area (AGW) are Cl− > Li > P > As > NO3− > Mo, respectively. The non-carcinogenic risk of most selected elements was less than the standard level (HQ = 1), with the exception of Cl− (average 15.3) and P (average 2.9) (Table 8), which could be a significant health hazard to the local residents, especially in the Fengfeng mining areas. Further, the integrated health risk [hazard index (HI)] also exceeds the safety threshold and indicates a significant non-carcinogenic risk in both the mining areas (1.9–65.3) and non-mining areas (4.6–79.3) (Table 8). These factors suggest that there is an increased health risk in both the mining and non-mining areas of Handan, but especially to the inhabitants living nearby the Fengfeng mining areas (Wang et al. 2016).
Carcinogenic risks
The lifetime carcinogenic risk (CR) of As, Cr, Cd and Pb due to exposure from the ingestion of groundwater in the two areas is listed in Table 9. The average carcinogenic risk for all four heavy metals in both mining areas and non-mining areas was generally within the “acceptable” range of 10−6–10−4 or the “negligible” range of less that than 10−6, although Pb levels in samples MGW13, 20 and AGW14 were above the acceptable value (10−4) (Table 9). Therefore, the potential carcinogenic risk over a long lifetime exposure to groundwater should not be ignored. It is notable that NO3− was not clearly identified as a carcinogen. The concentration of NO3− in drinking groundwater of the western mining areas (especially Fengfeng mining areas) was significantly higher than the eastern non-mining areas (Fig. 6d), which may have contributed to the incidence and mortality associated with local esophageal cancer (Fang 2014; Appleton et al. 2006), however, their medical investigation is necessary to identify the exact cause of esophageal cancer in this area.
Conclusions
The quality of surface water bodies and of groundwater in areas with and without mining was compared in the Handan region in China. The following conclusions can be drawn from this water quality investigation:
-
1.
In groundwater in the mining area, the concentrations of TH, NO3−, SO42−, Ca, K, Co, Se, Rb, Fe, and Ni were significantly higher (1.6–6.1 times, p < 0.05) than those in the area without mining and there was a trend of decreasing concentrations of these chemical constituents with distance from the mining area. By contrast, the concentrations of Cl−, HCO3−, Na, Li, B, Ti, V, Cr, Cu, As, and Mo in groundwater in the mining area were 0.14–0.8 times significantly lower (p < 0.05) than those in the area without mining. In surface water in the mining area, NO3− concentrations were more than 30 times higher than in surface water in the area without mining. Selenium concentrations in the mining area water were close to or exceeded the Chinese water quality standard (10 μg/L), and the average content of Se (3.9 μg/L) in all groundwater samples in the mining area was much higher than the average for groundwater of the Hebei Province (0.84 μg/L), possibly due to leaching from selenium-rich coal wastes and fly-ash dumps in the area. The elevated levels of Sr and other elements/ions in both mining and non-mining area water samples may be from the leaching of coal and calcium-rich minerals in both areas.
-
2.
The quality of natural water in both the mining area and the area without mining is poor. The lower pollution level in the non-mining area is likely due to the presence of an aquitard that limits the infiltration of contaminants into the unconfined aquifer in this area.
-
3.
The use of factor analysis and hierarchical cluster analysis suggested that the hydrogeochemical conditions of the aquifer, including rock–groundwater interaction with coal are the main factors that control the concentrations of chemical constituents in groundwater in the area. These assessments have indicated that mining activities have affected the water quality in the mining area, whereas the water quality of the area without mining has been affected by agricultural and domestic pollution. The elevated concentrations of NO3− in the mining areas may be caused by the release and oxidation of ammonium ions from clay minerals and subsequent mine drainage, while nitrate concentrations may be lower in the non-mining areas due to the effects of denitrification under reducing conditions that are present in groundwater in these areas. The elevated levels of Fe and SO42− in the mining area are probably derived from the oxidation of sulfide minerals in coal and dissolution of sulfate minerals, while lower SO42− in the area without mining may be due to the lack of sulfide mineral (gypsum) or may be due sulfate oxidation (the process is associated with the oxidation of ammonia).
-
4.
The non-carcinogenic risk of chloride in the mining area (HQ = 15) and non-mining area (HQ = 25) and phosphorus in mining area groundwater (HQ = 3) exceed the safety threshold. However, the health risk assessment indicated that there is a potential carcinogenic risk for Pb in groundwater as the level of risk associated with this metal exceeded the 10−4 cancer risk threshold in groundwater in the mining area.
-
5.
Concentrations of Co, Fe, Ni, Se, and Rb in water were found to be higher in the mining area than the area without mining. Additionally, a number of other chemical parameters including TH, NO3−, SO42− and Cl− were higher in water in the mining than the non-mining area and are considered to be potential health hazards for water users in the mining area. These chemical constituents are likely to have been introduced into the water in the mining area by the interaction of groundwater with the lithology of an aquifer. In particular, coal seams and their host rocks in the mining area contain very high levels of selenium, and the leaching of this element from these materials is likely to have caused higher concentrations of Se in the water in this area than elsewhere in Hebei Province.
-
6.
Elevated concentrations of Se, Sr, Rb, and K in groundwater and surface water bodies are mostly found in the central–northern part of the mining area. Nitrate concentrations in particular generally show large declines in water samples with distance from the mining area, while trends for Co, Ni and Ca are less clear. The spatial distribution revealed that the concentrations of many chemical constituents decline from mining areas to the adjacent non-mining areas. Furthermore, the study recommended prohibiting coal mining and coal dumping near natural water bodies to limit potential impacts on human health and on environmental receptors.
References
Ahmed MK, Shaheen N, Islam MS, Habibullah-al-Mamun M, Islam S, Mohiduzzaman M, Bhattacharjee L (2015) Dietary intake of trace elements from highly consumed cultured fish (Labeo rohita, Pangasius pangasius and Oreochromis mossambicus) and human health risk implications in Bangladesh. Chemosphere 128:284–292
Al-Ani MY, Al-Nakib SM, Ritha NM, Nouri AH (1987) Water quality index applied to the classification and zoning of Al-Jaysh canal, Baghdad-Iraq. J Environ Sci Health Part A/B/C 22(4):305–319
Appleton J, Zhang QL, Green KA, Zhang GD, Ge XL, Liu XP, Li JX (2006) Selenium in soil, grain, human hair and drinking water in relation to esophageal cancer in the Cixian area, Hebei Province, People’s Republic of China. Appl Geochem 21(4):684–700
Azcue JM (2012) Environmental impacts of mining activities: emphasis on mitigation and remedial measures. Springer, New York
Backman B, Bodiš D, Lahermo P, Rapant S, Tarvainen T (1998) Application of a groundwater contamination index in Finland and Slovakia. Environ Geol 36(1–2):55–64
Bhuiyan MA, Islam MA, Dampare SB, Parvez L, Suzuki S (2010) Evaluation of hazardous metal pollution in irrigation and drinking water systems in the vicinity of a coal mine area of northwestern Bangladesh. J Hazard Mater 179(1):1065–1077
Böhlke JK (2002) Groundwater recharge and agricultural contamination. Hydrogeol J 10(1):153–179
Caboi R, Cidu R, Fanfani L, Lattanzi P, Zuddas P (1999) Environmental mineralogy and geochemistry of the abandoned Pb–Zn Montevecchio-Ingurtosu mining district, Sardinia, Italy. Chron Rech Min 534:21–28
Cao SZ, Duan XL, Zhao XG, Ma J, Dong T, Huang N, Sun CY, He B, Wei FS (2014) Health risks from the exposure of children to As, Se, Pb and other heavy metals near the largest coking plant in China. Sci Total Environ 472:1001–1009
Chen DL, Luo KL (1996) The selenium content and its environment effect in Early Paleozoic in south of Shaanxi Province. Shaanxi Environment 32(2):29–31 (in Chinese)
Chen KP, Jiao JJ, Huang JM, Huang RQ (2007) Multivariate statistical evaluation of trace elements in groundwater in a coastal area in Shenzhen, China. Environ Pollut 147(3):771–780
Collier R (1984) Particulate and dissolved vanadium in the North Pacific Ocean. Nature 309(5967):441–444
Dai SF, Ren DY, Liu JR, Li SS (2003) Occurrence and distribution of minor toxic elements in coals of Fengfeng Coalfield, Hebei Province, North China. J Chin Univ Mining Technol 32(4):358–362
Dai SF, Ren DY, Chou CL, Finkelman RB, Seredin VV, Zhou YP (2012) Geochemistry of trace elements in Chinese coals: a review of abundances, genetic types, impacts on human health, and industrial utilization. Int J Coal Geol 94:3–21
Dang Z, Liu CQ, Haigh MJ (2002) Mobility of heavy metals associated with the natural weathering of coal mine spoils. Environ Pollut 118(3):419–426
Davis JC (1986) Statistics and data analysis in geology, 2nd edn. Wiley, New York
Domagalski JL, Eugster HP, Jones BF (1990) Trace metal geochemistry of Walker, Mono, and Great salt lakes; Fluid–mineral interactions: a tribute to H.P. Eugster, Vol 2, The Geochemical Society Special Publication, New York
Dragović S, Mihailović N, Gajić B (2008) Heavy metals in soils: distribution, relationship with soil characteristics and radionuclides and multivariate assessment of contamination sources. Chemosphere 72(3):491–495
Dreher GB, Finkelman RB (1992) Selenium mobilization in a surface coal mine, Powder River Basin, Wyoming, USA. Environ Geol Water Sci 19(3):155–167
Drever JI (1988) The geochemistry of natural waters, vol 437, 2nd edn. Prentice Hall, Englewood Cliffs
Du Y, Luo K, Ni R, Hussain R (2018) Selenium and hazardous elements distribution in plant–soil–water system and human health risk assessment of Lower Cambrian, Southern Shaanxi, China. Environ Geochem Health 40(5):2049–2069
Eaton AD, Clesceri LS, Greenberg AE, Franson MAH (2005) Standard methods for the examination of water and wastewater. Am Public Health Assoc 21:1600
Edet AE, Offiong OE (2002) Evaluation of water quality pollution indices for heavy metal contamination monitoring: a study case from Akpabuyo-Odukpani area, Lower Cross River Basin (southeastern Nigeria). GeoJournal 57(4):295–304
Everitt BS, Dunn G (2001) Applied multivariate data analysis. Oxford University Press, Oxford
Ewing SA, Christensen JN, Brown ST, Vancuren RA, Cliff SS, Depaolo DJ (2010) Pb isotopes as an indicator of the Asian contribution to particulate air pollution in urban California. Environ Sci Technol 44(23):8911–8916
Fang JJ (2014) Use of multi-isotope and microbial technology (E. coli) for evaluating sources and transformation of nitrate in the groundwater in Handan area in the North China plain. Ph.D thesis, China University of Geosciences
Farnham IM, Johannesson KH, Singh AK, Hodge VF, Stetzenbach KJ (2003) Factor analytical approaches for evaluating groundwater trace element chemistry data. Anal Chim Acta 490(1):123–138
Ficklin WH, Plumlee GS, Smith KS, McHugh JB (1992) Geochemical classification of mine drainages and natural drainages in mineralized areas. Water Rock Interact 7:381–384
Flaten TP (2001) Aluminium as a risk factor in Alzheimer’s disease, with emphasis on drinking water. Brain Res Bull 55(2):187–196
Fordyce FM (2007) Selenium geochemistry and health. Afr J Hum Environ 36(1):94–97
Fordyce FM, Zhang G, Green K, Liu X (1996) Soil, grain and water chemistry and human selenium imbalances in Enshi District, Hubei Province, China. Appl Geochem 15(1):117–132
Franco-Uría A, López-Mateo C, Roca E, Fernández-Marcos ML (2009) Source identification of heavy metals in pastureland by multivariate analysis in NW Spain. J Hazard Mater 165(1):1008–1015
GAQS (2008) Inspection and Quarantine of the People’s Republic of China Drinking Natural Mineral Water, General Administration of Quality Supervision. GB 8537-2008
GSIH (2009) The report of multi-targets regional geochemical survey: plain and coastal area of Hebei province (unpublished). Geological Survey Institute of Hebei Province
Gui HR (2005) The hydro-geochemical characteristics and discrimination model of mining area groundwater in Northern Anhui. University of Science and Technology of China (in Chinese)
Hem JD (1985) Study and interpretation of the chemical characteristics of natural water, vol 2254, 3rd edn. New York, U.S. Geological Survey Water-Supply Paper
Hirokazu H, Yoshiki W, Satoshi O (1985) Silver chloride pre-treatment for the determination of chloride in stream chloride ion-selective electrode. Analyst 110(9):1087–1090
Huang SS, Hua M, Feng JS, Zhong XY, Jin Y, Zhu B, Lu H (2009) Assessment of selenium pollution in agricultural soils in the Xuzhou District, Northwest Jiangsu, China. J Environ Sci 21(4):481–487
Hussain R, Luo K (2018) Geochemical valuation and intake of F, As, and Se in coal wastes contaminated areas and their potential impacts on local inhabitants, Shaanxi China. Environ Geochem Health 40(6):2667–2683
Hussain R, Ahmad W, Nafees M, Hussain A (2014) Optimization of wastewater treatment process in industry “a case study of Hattar Industrial Estate Haripur”. Pak J Anal Environ Chem 15(1):28–34
Hussain R, Khattak SA, Shah MT, Ali L (2015) Multistatistical approaches for environmental geochemical assessment of pollutants in soils of Gadoon Amazai Industrial Estate, Pakistan. J Soils Sed 15:1119–1129. https://doi.org/10.1007/s11368-015-1075-9
Hussain R, Luo K, Chao Z, Xiaofeng Z (2018) Trace elements concentration and distributions in coal and coal mining wastes and their environmental and health impacts in Shaanxi, China. Environ Sci Pollut Res 25(20):19566–19584. https://doi.org/10.1007/s11356-018-2148-2
Hussain R, Luo K, Liang H, Hong X (2019) Impact of the coal mining-contaminated soil on the food safety in Shaanxi, China. Environ Geochem Health 10:1–24. https://doi.org/10.1007/s10653-018-0233-6
Jeandel C, Caisso M, Minster JF (1987) Vanadium behaviour in the global ocean and in the Mediterranean Sea. Mar Chem 21(1):51–74
Jolliffe I (2002) Principal component analysis. Wiley, Amsterdam
Keller EA (1979) Environmental geology, 2nd edn. Charles E. Merrill Publishing, Columbus
Langmuir D (1978) Uranium solution-mineral equilibria at low temperatures with applications to sedimentary ore deposits. Geochim Cosmochim Acta 42(6):547–569
Langmuir D (1997) Aqueous environmental geochemistry, vol 549. Prentice Hall, New Jersey
Le TTH, Fettig J, Meon G (2019) Kinetics and simulation of nitrification at various pH values of a polluted river in the tropics. Ecohydrol Hydrobiol 19(1):54–65
Lee CS, Li XD, Shi WZ, Cheung SC, Thornton I (2006) Metal contamination in urban, suburban, and country park soils of Hong Kong: a study based on GIS and multivariate statistics. Sci Total Environ 356(1):45–61
Li S, Zhang Q (2010) Risk assessment and seasonal variations of dissolved trace elements and heavy metals in the Upper Han River, China. J Hazard Mater 181(1):1051–1058
Li YH, Wang WY, Yang LS, Luo KL, Li HR (2005) Concentration and environmental significance of water soluble-Se and water soluble-F in soils of south Shaanxi Province. Environ Chem 24(3):279–283 (in Chinese)
Liu CW, Lin KH, Kuo YM (2003) Application of factor analysis in the assessment of groundwater quality in a blackfoot disease area in Taiwan. Sci Total Environ 313(1):77–89
Luo KL (2011) Arsenic and fluorine contents and distribution patterns of early Paleozoic stonelike coal in the Daba Fold Zone and Yangtze Plate, China. Energy Fuels 25(10):4479–4487
Luo KL, Xu LR, Xiang LH (2002) Selenium content in the water of the rivers, springs and wells in the Daba Mountain Area of the south Qinling Mountains. Acta Geol Sin 76(3):389–394 (in Chinese)
Luo KL, Xu LR, Tan JA, Wang DH, Xiang LH (2004) Selenium source in the selenosis area of the Daba region, South Qinling Mountain, China. Environ Geol 45(3):426–432
Luo X, Yan Q, Wang C, Luo C, Zhou N, Jian C (2015) Treatment of ammonia nitrogen wastewater in low concentration by two-stage ozonization. Int J Environ Res Public Health 12(9):11975–11987
Lussier C, Veiga V, Baldwin S (2003) The geochemistry of selenium associated with coal waste in the Elk River Valley, Canada. Environ Geol 44(8):905–913
MEPPRC (2002a) Chinese national standards for surface water quality. Ministry of Environmental Protection of the People’s Republic of China. GB 3838-2002
MEPPRC (2002b) Methods for chemical analysis of water and waste water, Ministry of Environmental Protection of the People’s Republic of China, 4th edn. Environmental Science Press, Beijing
Merritts D, De Wet A, Menking K (1998) Environmental geology: an earth system science approach. Macmillan, London
Mertz W (2012) Trace elements in human and animal nutrition, vol 2. Elsevier, Underwood E
MHPRC (1985) Standards for drinking water test. Ministry of Health of the People’s Republic of China, GB, p 575085
MHPRC (2006) Chinese national standards for drinking water quality, Ministry of Health of the People’s Republic of China. GB 5749-2006
Niu CX (2007) Quaternary sedimentary environment analysis in Handan. China University of Geoseience, Beijing (in Chinese)
Omo-Irabor OO, Olobaniyi SB, Oduyemi K, Akunna J (2008) Surface and groundwater water quality assessment using multivariate analytical methods: a case study of the Western Niger Delta, Nigeria. Phys Chem Earth Parts A/B/C 33(8–13):666–673
Osborne JW, Costello AB (2004) Sample size and subject to item ratio in principal components analysis. Pract Assess Res Eval 9(11):8–17
Panda UC, Sundaray SK, Rath P, Nayak BB, Bhatta D (2006) Application of factor and cluster analysis for characterization of river and estuarine water systems–a case study: Mahanadi River (India). J Hydrol 331(3):434–445
Phan K, Sthiannopkao S, Kim K-W, Wong MH, Sao V, Hashim JH, Yasin MSM, Aljunid SM (2010) Health risk assessment of inorganic arsenic intake of Cambodia residents through groundwater drinking pathway. Water Res 44(19):5777–5788
Piper AM (1944) A graphic procedure in the geochemical interpretation of water-analyses. EOS Trans Am Geophys Union 25(6):914–928
Rayman MP (2000) The importance of selenium to human health. The Lancet 356(9225):233–241
Ren D, Zhao Y, Hua F, Dai SF, Zhang JY, Luo KL (2006) Geochemistry of trace elements in coal. Science Press, Beijing (in Chinese)
Rzymski P, Klimaszyk P, Marszelewski W, Borowiak D, Mleczek M, Nowiński K, Pius B, Niedzielski P, Poniedziałek B (2017) The chemistry and toxicity of discharge waters from copper mine tailing impoundment in the valley of the Apuseni Mountains in Romania. Environ Sci and Poll Res 24(26):21445–21458. https://doi.org/10.1007/s11356-017-9782-y
Selinus O, Alloway BJ, Centeno JA, Finkelman RB, Fuge R, Lindh U, Smedley P (2013) Essentials of medical geology. Springer, New York
Shen ZL, Zhu WH, Zhong ZS (1993) Hydrogeochemical basis. Geological Publishing House, Beijing (in Chinese)
Stetzenbach KJ, Hodge VF, Guo C, Farnham IM, Johannesson KH (2001) Geochemical and statistical evidence of deep carbonate groundwater within overlying volcanic rock aquifers/aquitards of southern Nevada, USA. J Hydrol 243(3):254–271
Sun YZ, Fan JS, Qin P, Niu HY (2009) Pollution extents of organic substances from a coal gangue dump of Jiulong Coal Mine, China. Environ Geochem Health 31(1):81–89
Tacyildiz N, Ozyoruk D, Ozelci Kavas G, Yavuz G, Unal E, Dincaslan H, Atalay S, Ucar T, Ikinciogullari A, Doganay B, Oktay G, Cavdar A, Kucuk O (2012) Selenium in the prevention of anthracycline-induced cardiac toxicity in children with cancer. J Oncol 2012:651630. https://doi.org/10.1155/2012/651630
Tan JA, Zhu WY, Wang WY, Li RB, Hou SF, Wang DC, Yang LS (2002) Selenium in soil and endemic diseases in China. Sci Total Environ 284(1):227–235
Tiwary RK (2001) Environmental impact of coal mining on water regime and its management. Water Air Soil Pollut 132(1–2):185–199
USEPA (1989) Risk assessment guidance for superfund. Volume I: human health evaluation manual (part A) EPA/540/1-89/002. Office of Emergency and Remedial Response, U.S. Environmental Protection Agency, Washington, DC
USEPA (2004) Risk assessment guidance for superfund. Volume I: human health evaluation manual (Part E, Supplemental Guidance for Dermal Risk Assessment) EPA/540/R/99. Office of Superfund Remediation and Technology Innovation, U.S. Environmental Protection Agency, Washington DC
USEPA (2010) Risk-based concentration table. Environmental Protection Agency, National Center for Environmental Assessment. https://www.epa.gov/risk/regional-screening-levels-rsls-users-guide. Accessed 17 May 2010
USEPA (2011) Exposure factors handbook: 2011 Edition. EPA/600/R-09/052F. National Center for Environmental Assessment, Office of Research and Development, U.S. Environmental Protection Agency, Washington, DC
USEPA (2019) Ris Assessment, Regional Screening Levels (RSLs) - Generic Tables, May, 2019. Environmental Protection Agency, Office of Research and Development, National Center for Environmental Assessment. https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables
Wang XL, Sato T, Xing BS, Tao S (2005) Health risks of heavy metals to the general public in Tianjin, China via consumption of vegetables and fish. Sci Total Environ 350(1):28–37
Wang PJ, Liu L, Li FS, Tian YJ, Hu ZQ, Yang CH (2010) Analysis on pollutant occurred from coking process. Coal Sci Technol 38:114–118
Wang X, Zhang D, Sun C, Wang Y (2016) Surface subsidence control during bag filling mining of super high-water content material in the Handan mining area. Int J Oil Gas Coal Technol 13(1):87–102
Welch AH, Lico MS (1998) Factors controlling As and U in shallow ground water, southern Carson Desert, Nevada. Appl Geochem 13(4):521–539
Welch AH, Lico MS, Hughes JL (1988) Arsenic in ground water of the western United States. Groundwater 26(3):333–347
WHO (1996) Trace elements in human nutrition and health. World Health Organization, Geneva
WHO (2004) Vitamin and Mineral Requirements in human nutrition. World Health Organization and Agriculture Organization of the United Nations, 2nd edn. WHO Press, Switzerland
WHO (2011) World Health Organization guidelines for drinking-water quality, vol 4. WHO, Geneva
Winograd IJ, Thordarson W (1975) Hydrogeologic and hydrochemical framework, south-central Great Basin, Nevada-California, with special reference to the Nevada Test Site. Geological Survey Professional Paper 712-C. U.S. Department of the Interior, Washington, DC
Wu B, Zhao DY, Jia HY, Zhang Y, Zhang XX, Cheng SP (2009) Preliminary risk assessment of trace metal pollution in surface water from Yangtze River in Nanjing Section, China. Bull Environ Contam Toxicol 82(4):405–409
Wu H, Chen J, Qian H, Zhang X (2015) Chemical characteristics and quality assessment of groundwater of exploited aquifers in Beijiao water source of Yinchuan, China: a case study for drinking, irrigation, and industrial purposes. J Chem 2015:14. https://doi.org/10.1155/2015/726340
Yan MC, Chi QH (2005) The chemical compositions of the continental crust and rocks in the eastern part of China. Science Press, Beijing
Zeng ZH (1996) The background features and formation of chemical elements of groundwater in the area of the middle and lower beaches of the Yangtze River. Acta Geol Sin 70(3):262–269 (in Chinese)
Zeng ZH (1999) The distribution feature and development assessment of SiO2 in groundwater in the area of Poyang lake, Jiangxi Province. Geol Prosp 35(2):37–40 (in Chinese)
Zhang Y, Li FD, Ouyang Z, Zhao GS, Li J, Liu Q (2013) Distribution and health risk assessment of heavy metals of groundwaters in the irrigation district of the lower reaches of Yellow River. Environ Sci 34(1):121–128 (in Chinese)
Zhao C, Luo KL, Du YJ, Tian Y, Long J, Zhao XF, Zhang SX (2017) Hydrochemical characteristics of natural water and selenium-rich water resources in the Northern Daba Mountains, China. J Water Health 15(2):273–287
Zheng D, Tan JA, Wang WY (2007) Introduction to environmental geoscience. Higher Education Press, Beijing (in Chinese)
Acknowledgements
The National Natural Sciences Foundation (Grant Nos. 41877299, and 41472322); The National Basic Research Program of China (Grant No. 2014CB238906); Science and Technology major project of Guangxi (Grant No. AA17202026-1), and CAS-TWAS PhD fellowship [1st author] supported this study. The authors thank Ma Jialiang for providing assistance in fieldwork and sampling. Dr. Wang Shaobin and Dr. Pu Haixia are also acknowledged for their suggestions and language modifications in this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declared no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hussain, R., Wei, C. & Luo, K. Hydrogeochemical characteristics, source identification and health risks of surface water and groundwater in mining and non-mining areas of Handan, China. Environ Earth Sci 78, 402 (2019). https://doi.org/10.1007/s12665-019-8350-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-019-8350-9