Abstract
Alachlor interaction with soil has been evaluated by batch equilibrium method on nine soils, geographically distant, from hilly to desert areas. Different soil samples have been investigated for sorption and removal mechanism of Alachlor. Linear and Freundlich soil–pesticide sorption isotherms were used to study the adsorption phenomena. Distribution coefficient (Kd) shifted extraordinarily from 94.9 to 6.15 µg mL−1. Statistical analysis showed a negative correlation between soil pH and Kd (R2 = − 0.70) and a positive relationship with organic matter content (R2 = 0.80). The adsorption results gave a C-type isotherm. The results were additionally investigated by univariate ANOVA and their accuracy was checked through residual plots. Activated carbon prepared from walnut shells (Juglans regia) was utilized for green remediation of Alachlor-contaminated soils, proved to be a cost-effective absorbent. The influence of two parameters including pesticide concentration and contact time for the abatement of Alachlor were investigated. Most prominent removal in 5 ppm Alachlor concentration was 84% while in 7.5 ppm most astounding removal was 72% from soils. The utilization of Juglans regia shells for decontamination of soils makes this technique environmental friendly, economical and easily applicable.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The unchecked and extensive application of pesticides has caused some deep and stern apprehensions for the environmental stability (Agrawal et al. 2010). Thus, it is highly imperative to assess the fate of pesticides in soils and water (Tiryaki and Temur 2010). Evaluation of the sorption phenomena is important to predict the persistence and toxicity caused by the pesticides (Ahmad 2017a). The persistency of pesticides in turn determines its migratory behavior and leaching potential. To avert pesticide contamination in soils, several remediation strategies have been proposed. Greener remediation routes have gained recognition in this aspect that are conveniently adaptable, economical and sustainable for the environment (Reddy and Chirakkara 2013). The pesticide adsorption process is influenced by the physiochemical properties of soil as well as the pesticide being applied (Sondhia and Khare 2014; Hall et al. 2015; Ahmad 2017b). Furthermore, sorption is also directed by number of intermolecular forces such as Van der Waals forces, hydrogen bonding, ligand exchange, charge transfer, induced dipole and dipole–dipole interaction and chemisorption (Sizmur et al. 2016). Soil organic matter plays a major role in this mechanism as higher organic matter content can retain more pesticides and increase adsorption which is further enhanced by clay and loamy texture of soil (Afolabi et al. 2016). The major route by which Alachlor dissipates is through runoff and leaching (El-Nahhal et al. 1999).
Alachlor (2-chloro-N-(2,6-diethylphenyl)-N-(methoxymethyl)acetamide) is a chloroacetanilide herbicide, having pre-emergent application for the regulation of broad leave weeds and grasses in corn, soya beans and many other crops (Dehghani et al. 2013; Szewczyk et al. 2015) (Fig. 1). Alachlor is a moderately toxic pesticide (Class II) which is classified as a carcinogenic, an endocrine disruptor and also shows xenoestrogenic effect by disrupting human and animal hormonal system (WHO 2010; Słaba et al. 2013; Germany 2016). Other chronic effects of Alachlor include liver toxicity and eye irritation. High solubility (200 mg L−1) and low mineralization rate makes it more probable to leaching, consequently causing contamination of ground water (El-Nahhal et al. 1998; Caldas et al. 2010). The molar mass of Alachlor is 269.767 g mol−1 with a melting point of 40–41 °C.
Presence of pesticides in soil and water causes contamination that leads to environmental pollution and serious health problems; hence its removal is essential from both media. Biomass-based activated carbon can be used as an environmental friendly adsorbent for removal instead of commercially available expensive activated carbon. The effectiveness of activated carbon prepared indigenously from biomass depends upon the percent of carbon content along with its high internal surface area, resistance to degradation and negative surface charge (Yang and Qiu 2010; Tao et al. 2015). Biomass from nuts such as peanuts and walnut shells (Janaki et al. 2012; Nazari et al. 2016), date stones (Bouhamed et al. 2016) and many other seeds with a high carbon content can efficiently be used as a raw material for making activated carbon (Liu et al. 2010a, b; El-Nahhal and Hamdona 2017).
The aim of the current research work is to evaluate the adsorption behavior of herbicide Alachlor in nine Pakistani soils from distinct geographical locations with specific physiochemical properties. Pakistan is an agrarian country whose economy is commodiously based on agricultural productivity. Capacious utilization of pesticides without proper knowledge of their environmental fate and toxicity impels it essentially to evaluate the pesticide–soil interaction, mobility and subsequent remediation. The study further investigates Alachlor green removal by activated carbon prepared from walnuts shells (Juglans regia) as an environmental friendly remediation technique.
Experiment
Chemicals
Alachlor, analytical standard, is purchased from Augsburg, Germany (99.9% pure). Acetone and methanol utilized were 99.9% pure and anhydrous sodium chloride was utilized. Standard stock arrangement of the pesticide was set-up in distilled water. Sulfuric acid and sodium bicarbonate were utilized.
Soil sampling and preparation
Four to five kilograms of each soil sample was gathered from nine distinct territories of Pakistan (Table 1). Sample collection was done between January and February 2017 with the mean temperature of 25 °C over all regions. Samples were collected from specific points for each location through random sampling and stored in clean polythene bags. The soil samples were air-dried in the greenhouse for 2 days and sieved through a 2-mm mesh sieve to remove debris and large particles. Processed soil samples were stored in sterile Petri dishes to be examined for its physiochemical properties and further experimentation through standard test methods (OECD 2005; Martin and MacDonald 2009). Physiochemical analysis of soil samples was performed in Soil and water testing lab, Rawalpindi, Pakistan. Following instruments were used in analysis: atomic spectrophotometer (Varian company-220), oven (Memmert), octagonal sieve shaker (Endecotts company), pH meter (WTW Ino Labs company) and EC meter (Crison company).
Adsorption experiment
All adsorption experiments were carried out in duplicates using a standard batch equilibrium method. The stock solution of Alachlor was prepared by dissolving it initially in a few drops of acetone and later adding distilled water, and as per standard protocol stock solution of 10 ppm of Alachlor was prepared (Janaki et al. 2012). Alachlor is highly soluble in acetone (≥ 1000 µg mL−1) while only moderately soluble in water (240 µg mL−1). 10.3 mg of Alachlor was taken [Weighing balance (Shimadzu company-ATx224)] to make the stock solution. Diluted solutions (0, 0.25, 0.5, 0.75, 1.0, 2.5, 5.0 and 7.5 ppm) were prepared from the stock solution (Ahmad et al. 2015). 10 mL of 0.1 M sodium chloride solution was added to each dilution to maintain the ionic strength of soil as it acts as a background electrolyte.
Adsorption of Alachlor was carried out using batch equilibrium method (Ahmad et al. 2014; OECD 2005). All experiments were done at laboratory isothermal and ambient conditions 24–26 °C. 0.5 g of air-dried soil samples [weighed by weighting machine (Shimadzu company-AUX220)] were equilibrated with eight different concentrations. 10 mL of each Alachlor concentration was added to the soil sample in a 15 mL falcon tube. All samples along with duplicates plus blanks (no pesticide) and control were shaken on a rotary shaker [(orbital shaker (Irmeco Gmbh Germany)] at 150 rpm for a period of 48 h. The vials were centrifuged [Hettich Company (Sigma 26-E)] for 20 min at 3500 rpm (25 °C). One milliliter of the clear supernatant was removed, filtered through 0.2-µm nylon filters and analyzed under UV–Vis spectrophotometer for the pesticide concentration.
Utilizing UV–Vis spectrophotometer (Model: BMS-1602), the concentration of Alachlor in soil solution was analyzed. At UV–Vis spectrophotometer, the solutions were run at detection wavelength of 200–300 nm with the aid of taking water as the reference standard and the wavelength of Alachlor was determined at 240 nm.
Preparation of activated carbon (AC)
Shells of Juglans regia were initially dried in the sunlight for 2–3 days. Dried materials were kept inside the oven at 150 °C for 24 h for removal of moisture and other volatile impurities. After that it was crushed with mortar and pestle followed by electric grinder. Chemical activation of the powdered precursor was done with sulfuric acid to make the impregnation ratio 1:1. The slurry form of powder precursor was properly mixed and kept for 24 h for proper soaking in a fume hood. After that it was washed with cold distilled water and with normal distilled water to neutralize the pH of activated carbon and remove excess acid. It was then soaked in a 5% sodium bicarbonate solution and left for 24 h. Then it was finally washed with cold distilled water till the solution became neutral. Finally, the activated powder was kept inside the oven at 110 °C for 24 h and packed in an airtight container (Fig. 2) (Manocha et al. 2013; Tao et al. 2015).
FTIR characterization
FTIR-8400 (Schimadzu) was utilized for the FTIR measurements of the activated carbon. The instrument ranged from 400 to 4000 cm− 1. Pellets of Juglans regia shell powder and activated carbon were prepared. Pre-heated KBr in powdered form was used to form pellets. Prepared powder was well mixed with KBr with the help of mortar and pestle and ground. The mixture then was subsequently given high pressure through the hydraulic pump (Nabiyouni and Ghanbari 2012). Activated carbon was analyzed for active functional groups in the range of 500–4000 cm−1 that can act as an active site for the attachment of pesticide. A FTIR spectrum was obtained before preparation of activated carbon, i.e. raw material (Juglans regia shell powder) and after preparation of activated carbon.
Alachlor removal using activated carbon
Dilutions of 5 and 7.5 ppm had been prepared from 10 ppm stock solution. 10 mL of each dilution was added in 15 mL of centrifuge tubes, along with 0.5 gram of each soil sample and their absorbance was measured through UV spectrophotometer. First, UV was taken before the addition of activated carbon. After that 0.5 g of activated carbon was weighed and added in each vial, shaken and left for 3 h before measuring absorbance via UV spectrometer. Same process was repeated for 6 h duration (Liu et al. 2010a, b). All experiments were performed in duplicates.
Data Analysis
The concentration of Alachlor adsorbed in selected soil samples was calculated with the aid of difference and equilibrium concentration by the following equation (OECD 2005):
where Cs is the concentration of Alachlor adsorbed (µg/g), V is the volume of the solution, m is the amount of soil taken in grams, Ca is the equilibrium concentration of the supernatant and Cb is the equilibrium concentration of blank. From the above equation values of linear isotherm can be written as follows:
where Kd is the linear sorption coefficient in µg mL−1 and Ce is the concentration of Alachlor at equilibrium in µg mL−1 (OECD 2005). The Freundlich equation for all the soil samples can be represented by Eq. (3):
where Cs is the concentration of Alachlor adsorbed, Ce is the equilibrium concentration in µg mL−1, Kf and 1/n are Freundlich constant obtained using the Freundlich equation in linear form. Kfoc can be calculated by the equation given below:
Gibbs free energy is calculated by the following equation:
where R is the universal gas constant and T is the temperature in Kelvin. Its value can be used to determine the nature of adsorption and pesticide binding to soil; its value ≤ − 40 kJ mol−1 represents the physical binding of pesticide with soil (Ahmad 2017c).
The results were further evaluated statistically to assess their significance. The regression and correlation analysis was performed between the adsorption coefficient and soil physicochemical properties in Excel 2013 (Microsoft, USA) and univariate ANOVA and the residual plots were obtained in Minitab 17 (statistical package).
Results
Physicochemical analysis of soils
The physiochemical analysis of soil samples is presented in Table 2. On the basis of the investigation, pH of selected soils stretched from 7.32 to 8.08. The total organic carbon content (TOC) ranged from 0.35 to 1.02% in all soil samples. OM in the collected soil samples ranged from 0.6 to 1.9%. On the basis of textural assessment, soils were classified into loam, sandy loam and clay loam classes. Soil S1 and S6 were categorized as sandy loam due to the presence of large percentage of sand content, 40 and 82%, respectively. The textural assessment of soils revealed that most of the Soil S7 was categorized as clay loam due to abundant clay content percentage (50%) (Rowell 2014). The soil sample with highest pH 8.08 was sandy loam in texture with the second lowest OM (0.7%) percentage among all samples. The remaining soils were identified as loamy. The salinity hazard in the soil samples has been investigated by the electrical conductivity (EC). The EC values ranged from 131 to 6240 µS cm−1.
Adsorption isotherms
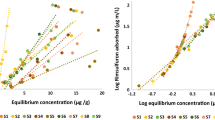
Selected soils exhibited variant adsorption of herbicide Alachlor, due to changes in their physicochemical make-up. These variations influenced the surface attachment of Alachlor onto them. The Alachlor adsorption on soils was studied using UV–Vis spectrophotometer and linear isotherms were plotted (Fig. 3). Following linear adsorption parameters were studied in the selected soils: adsorption distribution coefficient (Kd), Kom and Koc which signify the adsorptive interaction of pesticide (Table 3). The Kd values of Alachlor adsorption to soils ranged between 6.15 and 94.9 µg mL−1 with the regression coefficient R2 value ranging from 0.71 to 0.96. The highest value of adsorption coefficient belonged to soil sample S9 while the lowest to S3 due to the distinct physiochemical makeup of soils. The Koc values of the present work ranged from 960 to 6546 µg mL−1. All soils exhibited very high Koc values greater than 500. These soils interred in the low mobility class indicating very less probability of groundwater contamination. The values for the distribution coefficient normalized for organic matter (Kom) were also studied. In some researches, the Kom values have been given precedence over Kd as it considers the differences in soil organic matter and thus curtails variances. The Kom values ranged from 899 to 3103 µg mL−1. The Kd values suggested the adsorption of Alachlor in nine soils in the following sequence:
∆G, a thermodynamic parameter, is the Gibbs free energy, its values in present work stretched between − 14 and − 22 kJ mol−1. The Freundlich kinetics was analyzed for the Alachlor adsorption on all nine soils. The Freundlich adsorption distribution coefficient (Kf) ranged from 2.07 to 11.09 µg mL−1. The highest Kf value was observed in soil sample S2 while the lowest in S1. The R2 values for Freundlich isotherm ranged from 0.72 to 0.95 giving an indication of a good fit with Freundlich model. na was calculated from the regression equation which lied between 0.33 and 1.46.
Alachlor removal
Juglans regia-based activated carbon was utilized for Alachlor removal from soils. FTIR characterization of activated carbon revealed several new peaks formed after the activation (Fig. 4). Abundant functional groups were present on the carbon surface, however, most of the peaks centered between 1000 and 1800 cm−1. Functional groups can act as an attachment site for pesticide thus facilitating in removal. The results showed the removal of Alachlor by Juglans regia shell-based activated carbon after 3 and 6 h duration in 5 ppm concentration was highest in S7 (84%) and least in S1 (53%). Highest removal in 7.5 ppm concentration was in S9 (73%) and least removal was from S5 (43%) (Fig. 5).
Discussion
Soil properties
Pakistani soils displayed a slight alkaline trend. This alkaline range of pH in all soil specimens was due to the fact that Pakistani soils have generally been found to be moderately alkaline in nature. The abundance of Na content in soil tends to make it sodic as well as alkaline rendering these soils to accumulate a higher percentage of salts. A higher pH adversely affects the soil complex imparting instability and causing disintegration of clay fragments which later clog the soil pores (Naqushband et al. 2017). The soil organic matter (OM) substantially influences other soil properties (Powlson et al. 2013). The soils of the current experiment were found to possess very low OM. Generally, soils in a higher range of pH contain less amount of soil organic matter (Kuramae et al. 2012; Peralta et al. 2013). Elevated pH values of soils curtail water uptake which increases the sodium content consequentially impeding the biochemical phenomena imperative for the sustenance of OM (Uzoma et al. 2011). The increased salinity is also responsible for the reduced microbial activity required for the maintenance of OM (Mavi et al. 2012). All soil samples exhibited high EC values. The higher values of EC indicated more saline nature of soils, thus making it difficult for plants to uptake water even if the soils are wet (Bauder et al. 2011). According to soil samples, S8 was most probable to the risk of salinity hazard.
Alachlor adsorption
The adsorption of Alachlor on soils was found to decrease with the increase in concentration of herbicide applied. More molecules of herbicide with increased concentration occupied greater number of sorption sites on soils (Doretto et al. 2014). Alachlor adsorption studies in three different soils from India with pH ranging from 4.93 to 7.97 with moderate Kd ranges, also revealed increase in the phenomena of adsorption with increasing Alachlor concentration. Highest adsorption in Indian soils was observed in soils with highest OM. However, the isotherm obtained in Indian soils was an “L” type instead of the “C” type obtained in the current research (Janaki et al. 2012). Dal Bosco et al. (2012) also presented the isotherm of their Alachlor adsorption experiment to be “L” type. These isotherms show an initial slope which is not further increased with increasing the concentration of the pesticide. However, the slope of the isotherm of the current research increased with increasing Alachlor concentration. Hence, the type C isotherm indicates a constant availability for adsorption sites for the complete range of Alachlor concentration. Experiments on Alachlor adsorption by Jaya et al. (2009) also exhibited an “L”-type isotherm in Indian soils. Pesticides have been categorized into three classes on the basis of their mobility in soil. These classes are characterized by the Koc values. First, a very high mobility group with Koc values less than 50; second, high mobility group with Koc values between 150 and 500; and finally, low mobility group with Koc values higher than 500 (Ahmad 2017d). A research conducted on adsorption of herbicide on Chinese soils indicated that maximum values of Koc lied in the range close to 500 µg mL−1. The preeminent variance in those soil samples was the low pH which interred mostly in acidic range. The only exception was one black soil with an alkaline pH (8.06) that showed the highest value of Koc indicating least mobility (Liu et al. 2010a, b). The soils of current research also lied in an alkaline range and thus exhibited low mobility of Alachlor. The Alachlor adsorption results by Schwab et al. (2006) displayed very high Koc values in American soils mainly because of the presence of a very high amount of organic carbon in them. The negative ∆G values represented the type of interaction between the pesticide and soil. Its value equal or less than − 40 kJ mol−1 indicates physisorption while above it indicates chemiosorption. Its values depicted the phenomena of physisorption between Alachlor and soil particles. It also indicated that the interaction occurred through weak Van der Waal’s forces and hydrogen bonding, and implied the reaction to be spontaneous and exothermic. Dal Bosco et al. (2012) confirmed that herbicides of this group adsorb through Van der Waal’s forces that exist between the nuclei of aromatic carbon and the aromatic ring occurring on the OM surface. While the hydrogen bonding exists between the carbonyl oxygen of amide part of the herbicide and the hydrogen of hydroxyl and carboxyl groups on OM surface.
The investigated soils were also assessed by the Freundlich isotherm model. Loamy and clay loam soils depicted enhanced adsorption on the basis of Kf. The Kf values in Chinese soils have been found to be in a closer range with that of Pakistani soils (Liu et al. 2010a, b) while another study on Indian soils revealed Kf values to be less than 10 in all samples because of high pH (Shivaramaiah 2014). Kf values in soils from Tamil Nadu, India, were found to be very low as well (< 2 µg mL−1) (Janaki et al. 2012) as the soil pH was high. On comparing the Kf values of current Alachlor adsorption experiments with different other researches, it was found that Kf values of Pakistani soils were in closest proximity with Chinese soils. The na values indicate the extent of irreversibility of adsorption among all soil samples. Mainly, the highest values of na were seen in loamy soils. The na values in all Brazilian soils was found around 1 (Dal Bosco et al. 2012). According to Kodešová et al. (2011), a low range of Kf value are obtained for low range of n values.
On the basis of the adsorption results obtained, it was deduced that soils belonging to loamy class showed higher rate of adsorption compared to others. The texture of soils greatly influences the rate of adsorption. Kodešová et al. (2011) depicted that least adsorption occurred in sandy soils. OM and clay content in soils greatly facilitated the rate of Alachlor adsorption in soils. The lowest amount of OM (0.6%) rendered a very low value of adsorption in soil sample S3 (6.15 µg mL−1). Soil sample S7 contained the highest percentage of clay (50%) thus categorized as clay loam. As a consequence of more clay content, S7 was found to comprise lowest pH value and a high adsorption rate. The soil sample S6 possessed highest sand content (82%) thus categorized as sandy loam soil. It showed a reduced rate of adsorption (28 µg mL−1). pH values showed an inverse relation with the rate of adsorption. In soils with elevated pH values, the availability of sites for pesticide adsorption are reduced while in lower pH soils there are more protonated surfaces on the adsorbent. Although S6 possessed a low pH yet a reduced adsorption was observed. This was due to the fact that this soil contained the lowest amount of organic matter providing less sites for Alachlor adsorption. High OM percentage soils also show more tendency for buffer capacity against changing pH (Liu et al. 2010a, b; Ahmad and Rashid 2015). Schwab et al. (2006) also deduced from their research that an increase in adsorption with increasing OM and clay content results in a decrease in bioavailability. More Alachlor molecules will bind to soil particles with higher OM consequentially resulting in impeding the movement of pesticide towards groundwater. Another reason for increased Alachlor adsorption to soils with greater OM is that OM acts as the most significant sorbent surface for pesticides because of the phase partitioning that is induced by hydrophobic interactions (Wang et al. 2010). The increasing rate of pesticide adsorption in soils with increasing OM and decreasing pH has also been confirmed in various previous researches (Dal Bosco et al. 2012; Janaki et al. 2012).
The physiochemical properties of soils and their Kd values were assessed statistically. To analyze their relationship, regression analysis was performed which showed that the pH was indirectly proportional to the adsorption rate (R2 = − 0.70) while OM (R2 = 0.80) and TOC (R2 = 0.79) are directly proportional to it (Table 4). Thus, it is quite evident that an increase in OM and TOC will consequently enhance the adsorptive interactions and a decrease in pH will also increase adsorption. According to the results, the soil sample which had the highest value of Kd also had the highest amount of organic matter present in it. This justifies the statement that there is a direct relationship among organic matter and the soil’s adsorption capacity. Univariate ANOVA analysis was done on the soil samples along with the Kd values. In this analysis, the P value was found lower than the alpha value (α) (0.05). P value < 0.05 indicates the significance of the experimental results. Residual plots have been obtained from the statistical software package Minitab 17. Four types of plots were obtained from the one-way ANOVA analysis: normal probability plots, versus fit plots, histogram and versus order plots. These plots determined the accuracy and goodness of fit of the experimental results. Normal probability plots depicted the normal data distribution while residual plots displayed a constant variance of data (Fig. 6).
Removal by activated carbon
Each functional group can generate several peaks in the spectrum. The fingerprint region of the FTIR spectra ranges from 500 to 1500 cm−1 while after it lies in the functional group region. The number of peaks before activation in the fingerprint region was six while after activation it increased to ten. Overall, the increase in functional groups was to 14. Several peaks were obtained at 561 cm−1 (C–Br stretch), 665 cm−1 (–C=H:CH bend), 896 cm−1 (=C–H bend), 1028 cm−1 (C–N stretch), 1157 cm−1 (C–O stretch), 1425.51 cm−1 (C–C stretch), 1510.31 cm−1 (N–H bend), 2018.4 cm−1 (–C=C stretch) and 3406.4 cm−1 (=C–H stretch).
It was observed that Alachlor removal was fast during the initial phase observed after 3 h contact time. However, a slower decrease in Alachlor absorbance was seen after 6 h of contact time. This is because sufficient free sites are available for pesticide attachment to the activated carbon in the initial phase. These results depicted that Alachlor removal by activated carbon had an indirect relation with concentration of pesticide, i.e. greater the concentration lower will be removal. Since the amount of AC is constant throughout the experiment (for each concentration), the variation in Alachlor concentration revealed higher removal in lower concentration of Alachlor (Liu et al. 2010a, b). In the present study, all soils followed this trend, and removal was greater in percentage in 5 ppm rather than in 7.5 ppm. Also the individual percentage removal was more after 3 h contact time than after 6 h as the pesticide removal phenomena tends to reach closer to its equilibrium.
Conclusion
UV–Vis spectrophotometry revealed the physical adsorption of Alachlor on selected soils. Adsorption of Alachlor to soil is directly related to organic matter, total organic carbon content and clay content, as in soil S2, S9 and S5. High organic matter resulted in elevated Kd and Kf values, thus proposing greater ability for adsorption. S4 possessed lower Kd value, but high organic matter, which was due to textural influence, lower clay content and higher sand. The results depicted low probability of Alachlor leaching and reduced mobility in soils. The research also led environmental friendly method for the removal of Alachlor from soils using activated carbon prepared from biomass. It can be used as an effective adsorbate for number of organic pollutants. So activated carbon prepared from biomass waste such as Juglans regia can be effectively used rather than commercially prepared activated carbon from non-biomass sources.
References
Afolabi TJ, Alade AO, Jimoh MO, Fashola IO (2016) Heavy metal ions adsorption from dairy industrial wastewater using activated carbon from milk bush kernel shell. Desalin Water Treat 57(31):14565–14577
Agrawal A, Pandey RS, Sharma B (2010) Water pollution with special reference to pesticide contamination in India. J Water Res Prot 2(05):432
Ahmad KS (2017a) Green electrokinetic remediation of Thiabendazole adsorbed soils via mineralization. Agrochimica 61(3):190–205
Ahmad KS (2017b) Investigating the impact of soils’ physicochemical composition on chlorsulfuron pedospheric sorption. Stud Univ Babes Bolyai Chem 62(1):165–174
Ahmad KS (2017c) Pedospheric sorption investigation of sulfonyl urea herbicide Triasulfuron via regression correlation analysis in selected soils. S Afr J Chem 70(1):163–170
Ahmad KS (2017d) Determination of Rimsulfuron sorption parameters in eight Pakistani soils. Agrochimica 61(2):140–153
Ahmad KS, Rashid N (2015) Sorption-desorption behavior of newly synthesized N-(1H-benzimidazole-2 ylmethyl) acetamide (ABNZ) on selected soils and its antifungal activity. J Chem Soc Pak 37(4):841–849
Ahmad KS, Rashid N, Tazaiyen S, Zakria M (2014) Sorption-Desorption characteristics of benzimidazole based fungicide 2-(4-fluorophenyl)-1H-benzimidazole on physicochemical properties of selected Pakistani soils. J Chem Soc Pak 36(6):841–849
Ahmad KS, Rashid N, Zakria M (2015) Adsorption and desorption characteristic of metsulfuron-methyl in Pakistani soils. J Chem Soc Pak 37(2):380–389
Bauder TA, Waskom RM, Sutherland PL, Davis JG, Follett RH, Soltanpour PN (2011) Irrigation water quality criteria. Service in action; no. 0.506
Bouhamed F, Elouear Z, Bouzid J, Ouddane B (2016) Multi-component adsorption of copper, nickel and zinc from aqueous solutions onto activated carbon prepared from date stones. Environ Sci Pollut Res 23(16):15801–15806
Caldas SS, Demoliner A, Costa FP, D’Oca MG, Primel EG (2010) Pesticide residue determination in groundwater using solid-phase extraction and high-performance liquid chromatography with diode array detector and liquid chromatography-tandem mass spectrometry. J Braz Chem Soc 21(4):642–650
Dal Bosco TC, Sampaio SC, Coelho SR, Cosmann NJ, Smanhotto A (2012) Effects of the organic matter from swine wastewater on the adsorption and desorption of alachlor in soil. J Environ Sci Health B 47(6):485–494
Dehghani M, Nasseri S, Zamanian Z (2013) Biodegradation of alachlor in liquid and soil cultures under variable carbon and nitrogen sources by bacterial consortium isolated from corn field soil. Iran J Environ Health Sci Eng 10(1):21
Doretto KM, Peruchi LM, Rath S (2014) Sorption and desorption of sulfadimethoxine, sulfaquinoxaline and sulfamethazine antimicrobials in Brazilian soils. Sci Total Environ 476:406–414
El-Nahhal Y, Hamdona N (2017) Adsorption, leaching and phytotoxicity of some herbicides as single and mixtures to some crops. J Assoc Arab Univ Basic Appl Sci 22:17–25
El-Nahhal Y, Nir S, Polubesova T, Margulies L, Rubin B (1998) Leaching, phytotoxicity, and weed control of new formulations of alachlor. J Agric Food Chem 46(8):3305–3313
El-Nahhal Y, Nir S, Margulies L, Rubin B (1999) Reduction of photodegradation and volatilization of herbicides in organo-clay formulations. Appl Clay Sci 14(1–3):105–119
Germany PAN (2016) PAN international list of highly hazardous pesticides. Via http://www.pan-germany.org
Hall KE, Ray C, Ki SJ, Spokas KA, Koskinen WC (2015) Pesticide sorption and leaching potential on three Hawaiian soils. J Environ Manag 59:1–8
Janaki P, Chinnusamy C, Nalini K (2012) Adsorption and desorption behaviour of alachlor in different soils of Tamil Nadu. J Indian Soc Soil Sci 60(3):230–232
Jaya M, Singh SB, Kulshrestha G, Arya S (2009) Adsorption behaviour of alachlor on soil, FYM and charcoal. Pest Res J 21(1):101–104
Kodešová R, Kočárek M, Kodeš V, Drábek O, Kozák J, Hejtmánková K (2011) Pesticide adsorption in relation to soil properties and soil type distribution in regional scale. J Hazard Mater 186(1):540–550
Kuramae EE, Yergeau E, Wong LC, Pijl AS, van Veen JA, Kowalchuk GA (2012) Soil characteristics more strongly influence soil bacterial communities than land-use type. FEMS Microbiol Ecol 79(1):12–24
Liu W, Zhang J, Zhang C, Wang Y, Li Y (2010a) Adsorptive removal of Cr (VI) by Fe-modified activated carbon prepared from Trapa natans husk. Chem Eng J 162(2):677–684
Liu Y, Xu Z, Wu X, Gui W, Zhu G (2010b) Adsorption and desorption behavior of herbicide diuron on various Chinese cultivated soils. J Hazard Mater 178(1):462–468
Manocha S, Manocha LM, Joshi P, Patel B, Dangi G, Verma N (2013) Activated carbon from biomass. In: AIP conference proceedings, vol 1538, no 1, pp 120–123. AIP
Martin N, MacDonald F (2009) Evaluating the impact of insecticides on Scaptomyza flava and its parasitoid, Asobara persimilis. NZ J Crop Hortic Sci 37(3):243–252
Mavi MS, Marschner P, Chittleborough DJ, Cox JW, Sanderman J (2012) Salinity and sodicity affect soil respiration and dissolved organic matter dynamics differentially in soils varying in texture. Soil Biol Biochem 45:8–13
Nabiyouni G, Ghanbari D (2012) Thermal, magnetic, and optical characteristics of ABS-Fe2O3 nanocomposites. J Appl Polym Sci 125(4):3268–3274
Naqushband M, Niazi MT, Mehmood K, Dogar A (2017) Tackling of problematic soil and brackish water in Pakistan and improvement in the efficiency of urea and can fertilizers. NFC IEFR J Eng Sci Res 2:58–66
Nazari G, Abolghasemi H, Esmaieli M (2016) Batch adsorption of cephalexin antibiotic from aqueous solution by walnut shell-based activated carbon. J Taiwan Inst Chem Eng 58:357–365
OECD (2005) Guideline for the testing of chemicals. Adsorption–desorption using a batch equilibrium method
Peralta RM, Ahn C, Gillevet PM (2013) Characterization of soil bacterial community structure and physicochemical properties in created and natural wetlands. Sci Total Environ 443:725–732
Powlson DS, Smith P, Smith JU (eds) (2013) Evaluation of soil organic matter models: using existing long-term datasets, vol 38. Springer Science and Business Media, Berlin
Reddy KR, Chirakkara RA (2013) Green and sustainable remedial strategy for contaminated site: case study. Geotech Geo Eng 31(6):1653–1661
Rowell DL (2014) Soil science: methods and applications. Routledge, London
Schwab AP, Splichal PA, Banks MK (2006) Adsorption of atrazine and alachlor to aquifer material and soil. Water Air Soil Pollut 177(1):119–134
Shivaramaiah HM (2014) Adsorption, desorption and movement of endosulfan in agricultural soil. Int J Food Vet Sci 4(1):53–61
Sizmur T, Quilliam R, Puga AP, Moreno-Jiménez E, Beesley L, Gomez-Eyles JL (2016) Application of biochar for soil remediation. Agric Environ Appl Biochar Adv Barriers 63:295–324
Słaba M, Szewczyk R, Piątek MA, Długoński J (2013) Alachlor oxidation by the filamentous fungus Paecilomyces marquandii. J Hazard Mater 261:443–450
Sondhia S, Khare RR (2014) Soil adsorption studies of a rice herbicide, cyhalofop-butyl, in two texturally different soils of India. Environ Monit Assess 186:5969–5976
Szewczyk R, Soboń A, Słaba M, Długoński J (2015) Mechanism study of alachlor biodegradation by Paecilomyces marquandii with proteomic and metabolomic methods. J Hazard Mater 291:52–64
Tao HC, Zhang HR, Li JB, Ding WY (2015) Biomass based activated carbon obtained from sludge and sugarcane bagasse for removing lead ion from wastewater. Biores Technol 192:611–617
Tiryaki O, Temur C (2010) The fate of pesticide in the environment. J Biol Environ Sci 4(10):29–38
Uzoma KC, Inoue M, Andry H, Fujimaki H, Zahoor A, Nishihara E (2011) Effect of cow manure biochar on maize productivity under sandy soil condition. Soil Use Manag 27(2):205–212
Wang H, Lin K, Hou Z, Richardson B, Gan J (2010) Sorption of the herbicide terbuthylazine in two New Zealand forest soils amended with biosolids and biochars. J Soils Sed 10(2):283–289
World Health Organization (2010) The WHO recommended classification of pesticides by hazard and guidelines to classification 2009
Yang J, Qiu K (2010) Preparation of activated carbons from walnut shells via vacuum chemical activation and their application for methylene blue removal. Chem Eng J 165(1):209–217
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahmad, K.S. Sorption and Juglans regia-derived activated carbon-mediated removal of aniline-based herbicide Alachlor from contaminated soils. Environ Earth Sci 77, 437 (2018). https://doi.org/10.1007/s12665-018-7633-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-018-7633-x