Abstract
The study area Faridabad District is situated in the south-eastern part of state Haryana in the National Capital Region Delhi, India. From past few decades, change in land use pattern has affected water resources in the area both in terms of quantity and quality. To avoid further degradation of groundwater, the present study aims to identify the sources and the processes contributing to groundwater salinity and pollution, based on hydrogeochemistry in integration with GIS. The groundwater vulnerability has been assessed by rock–water interaction, geochemical processes, river/canal seepage, pollution and intermixing, variation in land use activities. The study suggests that the region-specific factors such as unplanned land use pattern and waste disposal, drainage as well as intermixing of groundwater play significant role in groundwater pollution besides geochemical processes. Salinity in shallow aquifers is usually as a consequence of leaching of evaporates in waterlogged areas along canals during rain or irrigation; mineral weathering; evaporation induced concentration of dissolved salts; saline groundwater movement from deeper to shallower aquifers due to continued indiscriminate groundwater over-abstraction; and expanding lateral extent of pollution in the overexploited aquifers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The concept National Capital Region (NCR), Delhi, originated to relocate the commercial activities to keep pace with increasing migration of population, dynamic changes in land use and land cover; and to achieve environmentally sustainable development. Over the past decades, the NCR, India’s largest and the world’s second largest agglomeration of people has faced rapid growth in population, urbanization, industrialization and agricultural intensification, which caused scarcity of water. Faridabad District in Haryana State—a part of the NCR is one of the largest industrial estates of Asia, housing a number of small, medium and large industries, such as agro-based, chemicals, electroplating, fertilizers, dyeing, textile, weaving, pharmaceuticals, machinery parts, and wooden products. Along with industrialization, the area has also experienced high agricultural development. The consumption of fertilizer increased from 130.77 kg/Ha in 2000–2001 to 329.55 kg/Ha in 2010–2011 (Agricultural Department of Haryana 2013). Increasing urbanization and industrial activities also lead to increase in solid waste generation. Presently, 200 MLD of sewage is generated, and about 85 MLD is being discharged to the River Yamuna without any treatment. The present industrial water demand is about 20MLD and the same is expected to reach 100MLD in 20 years. In 2001, sewer network covered 65 per cent of urban population in the district which has increased to cover only 70 per cent of the population in 2011 (NCRPB 2013).
Although Agra canal and Gurgaon canal were constructed to meet the irrigation water demand, yet, about one-third of the treated sewage in the area is utilized for irrigation. The rest of sewage is discharged into Budhiya Nala and Gaunchi drains meant for flood control ultimately adds to the Yamuna River. Thus, the quality of water in the Yamuna River, Agra canal, Gurgaon canal, Budhiya Nala and Gaunchi drain gets degraded. Inadequate good quality surface water supply has forced the agricultural and industrial activities to depend to a large extent on groundwater. The area is thus faced with the challenge to control indiscriminate withdrawal of groundwater and its contamination, resulting in continuous depletion of this vital resource. Water table is declining at a rate of 0.2 m/year, and water table is recorded 10–15 m deep in the region.
However, assessment of the recent status of vulnerability of groundwater to pollution in this highly industrialized area has yet to be made especially for the development of secured economic zone. In this context, an attempt has been made to assess the factors influencing the groundwater quality and its vulnerability to pollution, based on hydrogeochemistry in integration with GIS; and strategies have been suggested for the NCR concept to achieve desired success.
Study area climate, geology and geohydrology
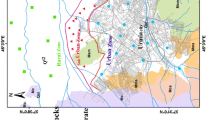
The Faridabad District (Fig. 1a, 27°39′, 28° 31′ N and 76°40′, 77°32′ E) is located in the south-eastern part of Haryana state in the NCR. The area experiences semi-arid climate with extreme dry air during summer months April to July; receives cold weather from January to March; and receives about 542 mm average annual rainfall from Southwest monsoon during last week of June to September. Along with cash crop, study area is under dense rice–wheat cropping system, which requires more water. As a consequence, groundwater development in the study area is more than replenishable groundwater recharge and facing the problem of falling water table.
The Quaternary alluvium comprising sand, clay, silt, kankar and gravel, occupying the major part of the area has two units: Khadar—the low lying flood plain in the eastern part with ~ 10–15 m thick sandy newer alluvium, and Banger—an upland plain of older alluvium in west. The north-western and western parts are occupied by the Delhi supergroup Proterozoic rocks as isolated hills, discontinuous parallel ridges and inselberg, mainly comprising quartzite with intercalation of mica-schist, amphibole-quartzite, minor Fe–Mg amphibole-schist, kyanite-schist, garnet-schist, chlorite-schist, sillimanite-schist, porphyroblastic K-feldspar schist, amphibolite, and impure marble. Saltpetre (Nitre) occurs around Ujina, Hattin, Hodel and Palwal.
The potential groundwater aquifer zones occur in the semi-consolidated sand beds with negligible thickness and in the underlying weathered or fractured quartzite outcrops in the northwest to > 350-m-thick sandy alluvium in the eastern parts near Yamuna River (CGWB 2011). Beyond this depth, the stratum is not a potential aquifer. In the quartzite, the ground water flow occurs through secondary permeability imparted by joints and fractures. The tube wells are 65–110 m deep, and the depth to water level remains 3–18 mbgl during pre-monsoon and 4–21 mbgl during post-monsoon. In some parts of the Palwal and Hatin block, 2–5 m water level was observed. In eastern parts of Faridabad and Ballabhgarh blocks, significant decline in water table was observed and the water level occurs at 10–25 mbgl. In Ballabhgarh, average water level declined from 23.31 mbgl in 2007 to 26.22 mbgl in 2011, and seepage from the Yamuna River, Agra canal and Budhiya Nala drain occurs in this part. The groundwater sampling locations as well as geomorphology of the investigated area is shown in the Fig. 1.
Groundwater sampling and analytical procedure
Based on topographic sheet, satellite images, extensive field survey was done in post-monsoon period February 2013 and twenty two groundwater samples were collected. Samling location were geocoded with Garmin GPS and brought into GIS environment. Samples were collected from tube wells and first few minutes, water was left running to reduce the impact of iron pipes and to achieve constant conductivity values. At each site, water samples were collected in two separate clean polypropylene bottles (Tarsons; 250 and 125 ml), rinsed 2–3 times with groundwater to be sampled. One of the bottles (125 ml) containing sample was acidized adding several drops of ultra-pure nitric acid to stabilize trace metals and for determination of major cations. The other bottle (250 ml) containing un-acidified sample was used for anions analysis. The water physico-chemical parameters were analysed by following the standard procedure. The physical parameters pH, electrical conductivity (EC), and total dissolved solids (TDS) were measured onsite using respective electrodes. The samples were stored in ice-containing styrofoam boxes and brought to laboratory and stored at 4 °C for further analysis. A total of 14 water quality parameters have been analysed by following the standard procedure of APHA (American Public Health Association 2005). Carbonate and bicarbonate were also analysed on field using titration method. Samples were immediately filtered through 0.22 µm Millipore cellulose membrane filters for analysis. Anions (F−, Cl−, SO42−, and NO3−) were analysed using ion chromatography (ICS-90, Dionex). Major cations (Ca2+, Mg2+, Na+, K+) were analysed using atomic absorption spectrophotometer (Thermo Fisher). Dissolved silica was analysed by the molybdosilicate method (APHA 2005). The quality of the chemical analyses was carefully inspected by taking and analysing blanks and duplicate samples, and by checking ion balances. The ion balance errors for the analyses were generally within ± 5%.
The spatial variation of different water quality parameters was studied using spatial analyst of Arc GIS 10. Inverse distance weighing technique (IDW) is used to generate spatial distribution of various water parameters. IDW assigns weights by taking the inverse of the distance from an observation’s location to the location of the point being estimated (Burrough and McDonnell 1998). The saturation index (SI) values of minerals were calculated using the PHREEQC software. Details have been discussed in results and discussion.
Results and discussion
The groundwater quality parameters of the area show a wide range (Table 1), suggesting contributions from multiple sources and processes. The pH of groundwater is alkaline. Very high values of EC (384–11,108 µS/cm; mean 3462 µS/cm); TDS (192–5554 mg/L; mean 1776.2 mg/L); Cl (6.3–2406.6 mg/L; mean 659.0 mg/L); and 60% samples of Na–Cl type clearly indicate occurrence of saline groundwater and insufficient flushing. The Piper (1944) plot (Fig. 2) suggests that rest samples are of Na–HCO3, Mg–Cl, and Mg–HCO3 type, and groundwater is dominated with Na+, Mg2+, Ca2+, Cl−, and HCO3−. Na+, Mg2+ and Ca2+ constitute 95.02% of the total cations in the order Na+ > Ca2+ > Mg2+> K+ and Cl−, SO42− and HCO3− constitute about 87.69% of the total anions, in the order Cl− > SO42− > HCO3− > NO3. A comparison of the quality parameters in Table 1 with the World Health Organization (WHO 2008) drinking water standards indicates that the most of groundwater is not suitable for drinking purposes.
Mechanisms controlling groundwater quality
In order to identify the processes controlling groundwater quality in the area, the hydrochemical data have been subjected to various graphical plots. In the scatter diagram of groundwater Ca versus HCO3 as represented in Fig. 3a, the samples above and below the equiline 1:1 suggest contribution from both weathering of carbonate and silicate minerals present in the geological formation, as observed earlier in the region (Datta and Tyagi 1996; Garrels and Mackenzie 1967; Holland 1978). The scatter diagram of Ca2+ + Mg2+ vs HCO3 + SO4 (Fig. 3b) shows that the points fall along the equiline (Ca2+ + Mg2+ = HCO3 + SO4), suggesting weathering of carbonates and sulphate minerals also (Datta et al. 1996a, b). In the (Ca + Mg) versus (SO4 + HCO3) plot (Fig. 3b), the samples which fall below the equiline indicate origin from silicate weathering and suggest an excess of (SO4 + HCO3) in the water is balanced by alkalis (Na+ + K+), and the samples above the equiline indicate origin from carbonate weathering (Datta et al. 1996a, b; Rajmohan and Elango 2004).
The study area is a part of Indo-Gangetic alluvial plain, so the aquifers are rich in calcite minerals (e.g. detrital carbonate grains and kankar concretions), interaction of groundwater with these minerals produces high Ca2+ content during carbonate weathering, which is evident in Fig.3a. Weathering of schist, quartzite rocks, and alumino-silicates minerals including feldspar and mica release Ca and Mg into water. The study area being rich in kankar, dolomite and lime, the Ca2+/Mg2+ molar ratio of one suggests dissolution of dolomite (Maya and Loucks 1995), and ratio > 1 indicates more calcite contribution; and Ca2+ ions are released to the groundwater during recharge from rainfall, irrigation or leaching. In the denudated hills with scarce soil cover, CO2 pressure during recharge being low the HCO3 uptake and the potential to dissolve calcite are also low. Due to root and microbial respiration and organic matter oxidation partial pressure of CO2 (Pco2) in the soil zone being is generally greater than the atmospheric PCO2 of 10−3.5 bar (Mitrov 1961) which influences soil water interaction with sediments carbonate minerals. Hence, anthropogenic high CO2 level and PCO2 (10−1.4–10−3.03 bar) in the area, polluted organic rich infiltration water from municipal wastes, landfill sites and leaky sewage sources also seem to be source of HCO3 in groundwater.
The iso-contours of groundwater HCO3, Cl and SO4 (Figs. 3c, 4a, b) show relatively low levels in the north and western part of the area and represent mostly fresh groundwater recharged by rainfall infiltrated further south of the Aravalli hills. Presuming 3–10 mg/L Cl of fresh rain water, considerable recharge from rainfall seems to be taking place in these parts representing the recharge zone of the aquifer. The eastern, south-eastern and southernmost parts of the area having higher HCO3 levels (Fig. 3c) and Cl and SO4 levels represented in Fig. 4a, b indicate the discharge zone of the aquifer. Very high levels of Cl− and SO42− have been observed in parts adjacent to the Yamuna River, Budhiya Nala and Gaunchi drain, carrying the sewage and anthropogenic wastes of the area to Badshahpur STP, Mirzapur STP, Pratapgarh STP. Groundwater over-abstraction-induced decline in water table in these areas and change in hydraulic gradient-induced seepage from these water bodies may contribute to high SO42− and Cl− levels in the groundwater. Groundwater with high SO42− and Cl− may be also related to semi-arid climate-induced prolonged evaporative enrichment of water, leading to high salinity (Subba Rao 2006).
A good correlation between Cl− and SO42− as in Fig. 5a indicates common source of these ions. Leaching of halite, chloride schist, fertilizers and irrigation return flow can also be the possible sources of high chloride levels in the groundwater, as observed by studies in other areas (Andreasen and Fleck 1997; Lowrance et al. 1997). The groundwater in the area has wide range of Na/Cl molar ratio 0.47–6.29. The molar ratio = 1 indicates dissolution of halite, while ratio > 1 indicates release of Na from silicate weathering or due to salts present in the soil (Stallard and Edmond 1983). High Cl levels in groundwater may be attributed to leaching of surface/sub-surface salts, anthropogenic untreated domestic and industrial wastes, and irrigation water return flow, as observed earlier in the adjacent Delhi region (Datta et al. 1997; Datta 2013a) in the north of the Faridabad District. The Na and Cl straight line relationship (Fig. 5b) suggests intermixing of multiple-sourced highly saline groundwater with freshwater along specific flow paths, induced by overexploitation, increasing the lateral extension of pollution, as observed earlier (Datta et al. 1996a, b).
The (Na + K) versus Cl scatter plot represented in Fig. 5c also suggests availability of abundant alkalis (Na+ over K+) to form alkali carbonates/sulphate, and greater resistance of K+ to chemical weathering and its adsorption on clay minerals (Kumari et al. 2011). The positive values of the water chloroalkaline indices CAI1 and CAI2 expressed below (ion concentrations in meq/l) (Schoeller 1965) also confirm exchange of water Na+ or K+ ion with Mg or Ca in aquifer material. The samples with CAI1 positive and CAI2 negative indicate both ion exchange and reverse ion exchange.
The Ca2+ being common in CaCO3 and CaSO4 (gypsum or anhydrite), both salts compete for Ca2+ in dissolution–precipitation equilibriums and affect each other substantially. In the study area, most of the samples have excess of Ca2+ over SO42−. Samples which plot around the equiline with Ca2+/SO42− molar ratio ~ 1:1 (Fig. 5c) represent the groundwater in which Ca2+ levels are mainly controlled by the dissolution of CaSO4. A few samples lying above the equiline indicate excess of SO42− over Ca2+ suggesting precipitation of calcite. Higher SO42− can be also from anthropogenic sources such as agrichemicals, industrial discharge, and burning fossil fuels deposition, as observed earlier in other semi-arid areas (Kumari et al. 2011).
Fluoride level in groundwater ranges from 0.6 to 6.9 mg/L with mean value 1.5 mg/L. 45.4% of the samples exceed the maximum desirable limit (1 mg/L) set by the Bureau of Indian Standards (BIS 1991) and WHO standards (2009) (1.5 mg/L). Iso-contours of groundwater fluoride (Fig. 6) indicate that most of the places have high fluoride level except in Aravalli foothill parts in the north, eastern parts adjacent to Yamuna River and south-western parts. Preferential dissolution of the Delhi supergroup rocks having mineralogical assemblages of quartz, K-feldspar, plagioclase, pegmatites, biotite, fluorite, apatite and amphiboles containing high fluoride, and the sediments derived from these make the region naturally prone to high F-levels in groundwater. Seepage from canal and drain passing through the industrial parts of Faridabad, Ballabhgarh and Palwal area, carrying industrial effluents, also could be source of very high F-level in groundwater. Na+/Ca2+ and HCO3−/Ca2+ ratios ≫ 1 in all the samples also favour fluoride dissolution.
NO3 iso-contours in groundwater show a very wide range 4.0–1431.1 mg/L (Fig. 6), suggesting contributions from both point and non-point sources. In most places, NO3 concentration is higher than maximum permissible level (10 mg/L) of NO3–N by the US Environmental Protection Agency (US EPA 2000); and 36.3% samples show higher than the BIS (1991) and WHO (2009) recommended limit of 45 mg/L for drinking water. The highest NO3 level is observed in the north-western part adjacent to the Gurgaon canal. Since the study area has intense agricultural and industrial activities, groundwater irrigation and water logging induced leaching of fertilizers, animal waste, crop residue; and presence of saltpetre, septic tanks and effluents from industries can be also attributed to high Cl and NO3 levels in groundwater in the study area. It is a matter of great concern, because high NO3 level > 45 mg/L in drinking water causes methaemoglobinaemia in infants (WHO 2009).
Geochemical modelling
The geochemical reactions controlling groundwater chemistry depend on the solubility of minerals in the flow pathways, and their saturation state, i.e. degree of equilibrium of respective mineral with water (Koetsiers and Walraevens 2006). Geochemical modelling has been done using PHREEQC Interactive 2.13.2 software. Saturation index for anhydride, aragonite, calcite, chalcedony, chrysolite, CO2, dolomite, gypsum, halite and sepiolite has been calculated using following equation:
where IAP = ion activity product of the dissociated mineral; K t = equilibrium solubility at mineral temperature.
Figure 7 shows the saturation index variation of different minerals in the geological formation of the area. SI < 0 indicates that the groundwater is under-saturated with respect to a particular mineral, and reflects insufficient amount of the mineral in the formation for dissolution by water or short residence time of water. SI > 0 specifies that the water is oversaturated with respect to a particular mineral and therefore incapable of dissolving more of the mineral; and reflects formation containing ample amount of the mineral with sufficient groundwater resident time to reach equilibrium. Figure 7 clearly indicates that in most of the places groundwater is under-saturated with respect to dissolution of anhydrite, chrysolite, CO2, gypsum, halite and sepiolite, suggesting significant contribution of Ca2+, Mg2+ and other ions to the groundwater by dissolution of these minerals. SI values also indicate oversaturation of groundwater with aragonite, calcite, chalcedony, and dolomite. Besides halite-albite minerals and ion exchange, Na is contributed by anthropogenic sources also as clear from the plot of (Na + K) versus Cl (Fig. 5c).
Gypsum and anhydrite having SI < 0 are responsible for occurrence of higher SO4 levels in groundwater, besides various anthropogenic activities (Kumari et al. 2011). Out of twenty-two samples, only two samples are under-saturated with respect to calcite and three samples under-saturated with aragonite. The rest of the samples are oversaturated with calcite and dolomite. Dolomite dissolution of calcite being a relatively rapid reaction water rapidly acquires Ca2+ (Palmer and Cherry 1984; Busenberg and Plummer 1982; Drever 1982), which on reacting with dolomite acquires Mg2+ during infiltration and also increases Ca2+, CO32− and HCO3− content in the groundwater. This process can lead to oversaturation of calcite and precipitation in order to maintain equilibrium with loss of Ca2+ and CO32− ions in water, which then demands more dissolution of dolomite, leading to increase in Mg2+ in water. In addition, dedolomitization with simultaneous dissolution of anhydrite and dolomite, together with calcite precipitation, may be another important process as expressed below controlling hydrochemical characteristics in the area:
As dedolomitization progresses, dissolution of gypsum releases Ca2+ ions into solution. The elevated PCO2 for soil water has a major impact on the interaction with carbonate minerals present in reservoir. The saturation indices for different minerals of the study area suggest that evaporite dissolution, ion exchange along with anthropogenic activities are controlling the hydrogeochemistry of groundwater.
Salting of soil
The suitability of water for irrigation depends on its quantity and quality, cropping practices, soil texture and climate (Todd 1980; Domenico and Schwartz 1990). High Na causes poor permeability and drainage of soil, soil mineral particles dispersal, and less infiltration of water (Jalali 2007). The Na % (Wilcox 1955) has been calculated by the formula given below where ion concentrations are in meq/l
The Na % (Table 2) shows that none of the sample is excellent; 27% samples are good, 23% are in permissible class, 45% are in doubtful class, and groundwater at one location is unsuitable for irrigation. High concentration of sodium causes soil mineral particles to disperse and less water to infiltrate. This results in poor permeability and drainage of soil, leading to restricted air and water circulation in it and thus makes it hard.
The water samples are grouped in three classes on Doneen’s chart represented in Fig. 8 (Domenico and Schwartz1990) of the water total ion content and the permeability index (PI) expressed by the following equation given below (Doneen 1964), where concentration of ions is in meq/L:
The Doneen’s chart classified the water into three classes:
-
Class I Low PI; the water is of good quality for irrigation;
-
Class II Higher PI value with respect to class I; the water is generally acceptable;
-
Class III Very high PI; water is completely unsuitable for irrigation.
Doneen’s diagram (Fig. 8) shows that only groundwater from six locations is good for irrigation, one sample is acceptable and the rest samples are unsuitable.
Based on EC, the suitability of groundwater for irrigation has been also been assessed by salinity hazard index (Table 3). It was observed that groundwater from only three locations is good for irrigation; from six locations, it is doubtful; and water from thirteen sampling locations is unsuitable.
The USSL plot (1955) of groundwater EC and SAR (Fig. 8) shows three samples of C2S1 type; five samples of C3S1 type, i.e. relatively good and moderate quality water suitable for irrigation, and one sample of the C3S2 type, i.e. high salinity and medium sodium hazard, and tolerable for irrigation. One sample is of C4S3 type, i.e. very high salinity and high Na hazard, and unsuitable for irrigation. Water samples from twelve locations are of high salinity (C3, C4, and C5 type) and Na hazard, and unfit for irrigation on soils with restricted drainage. It is clear from all approaches that most of the groundwater in the area are unsuitable for irrigation also.
At most of the sampling location value of EC and SAR and other constituent are very high so in both Doneen’s and USSL diagram (Fig. 8a, b), only few samples are coming within the diagram limit. Rest of the samples can not be used for irrigation purposes.
Groundwater vulnerability assessment
The present study intends to assess vulnerability of groundwater to pollution for general planning and decision-making, and not for precise future prediction of contamination. An earlier study in the region indicated that during Pleistocene Period, a drier interpluvial phase of alluvial and lake formation along with interstitial saline water and intermixing of fresh water with older saline water contributed to high salinity in groundwater (Kulkarni et al. 1989). Other studies in the Delhi region indicated that limited groundwater recharge and flushing; evaporite dissolution; irrigation water return flow; pollutant sources; as well as intermixing of fresh water with saline/polluted water as the main causes for groundwater contamination in aquifers (Datta et al. 1996a, b, 1997). Since assessment of vulnerability to pollution by probabilistic models and statistical or overlay/index methods has many limitations, in the present study, the groundwater vulnerability has been assessed by extensive field studies based on spatiotemporal variability of land use, river/canal seepage; pollution and intermixing; relative abundances of minerals in the geological formations; soil/rock–water interactions; geochemical processes; correlation matrix of water quality parameters; and anthropogenic activity, as assessed in other areas (Kumari et al. 2011).
The correlation matrix (Table 4) of the degree of dependency of water quality parameters standardizing the data with the criterion presented by Davis (2002) shows that EC has strong correlation (coefficient > 0.7) with salinity, TDS, Na+, Ca2+, Cl− and SO42− and moderate correlation (coefficient 0.5–0.7) with Mg and HCO3− indicating their origin from the same source, and their involvement in physiochemical reactions such as oxidation–reduction and ion exchange. Strong positive correlation of Cl−–SO42−, Na–Cl, Na–SO4, and Na–Ca also indicates their release from common source and anthropogenic activities. A high positive correlation of Na+ and Cl− indicates presence of highly saline groundwater and groundwater intermixing in the study area. Correlation of Mg–NO3, Ca–SO4, and Mg–Cl indicates their origin from agricultural chemical sources. Since high pH, HCO3− and Na+ in groundwater favour release of fluoride from minerals into groundwater (Singh et al. 2012), F− shows positive correlation with HCO3− (Corr. Coeff. 0.50).
The study indicates that relatively low surface gradients and fine-grained poorly drained sediments, and over-irrigation by canal water and groundwater cause stagnation of water which along with warm dry semi-arid climate favours salts accumulation in soil. Canal/river seepage and slow infiltration of agricultural and urban surface runoff accumulated in stagnant water pools contribute to groundwater recharge significantly. The groundwater over-abstraction-induced changes in the geohydrological conditions have triggered the mobilization of salts and salinity in groundwater. It is reported that the water quality of Yamuna River, Budhiya Nala and Gaunchi drain (all with pH 7.2–8.7; EC 1110–4220 µS/cm); and Agra canal and Gurgaon canal (both with pH 7.1–8.2; EC 910–1820 µS/cm) does not meet the standards prescribed under Water and Environmental Protection Acts (Haryana State Pollution Control Board 2010). Thus, the canal/river seepage has caused significant deterioration in the groundwater quality from the 2009–2010 reported values of EC (872–6710 µS/cm); HCO3 (176–1280 mg/L); Cl (69–1956 mg/L); SO4 (27–840 mg/L); NO3 (2.4–245 mg/L); and F (0.0–3.85 mg/L) monitored by the Central Ground Water Board in Faridabad District (Haryana State Pollution Control Board 2010).
The present-day rainwater δ18O (−4 to −10‰) and 3H (5–7 TU), shallow groundwater δ18O (−6 to −10‰) and 3H (6–12 TU), and river water δ18O (−6 to −10‰) and 3H (6–15 TU) also suggest limited modern groundwater recharge (Av. < 5–15%) from rainfall over the past decades, as reported earlier (Goel et al. 1977; Datta 2013b) to flush the groundwater system from salinity and pollution. Therefore, it is quite evident that even after taking engineering measures of flood control and effluent treatment, due to not so effective infrastructures to check water pollution; the shallow groundwater in the area is vulnerable to degradation, specifically in the eastern, central and southern parts. Deeper groundwater is less vulnerable because of longer travel time required by the pollutants to reach the groundwater. Therefore, land use decisions and land management have to be made keeping in mind that all anthropogenic cannot be carried out in isolation of groundwater.
Concluding remarks
In the study area, salinity and pollution of groundwater in shallow aquifers is not homogeneous in its lateral extent and caused by various sources, multiple processes, and dynamics. Different processes that may have caused the groundwater pollution include alterations in water and salt balance; unplanned land use; canals and drains network; and the influence of excessive flood irrigation during the last decades. The indiscriminate anthropogenic influences in the natural conditions during groundwater abstraction have favoured multiple-sourced pollution intermixing and lateral movement of saline/polluted water in the shallow groundwater active circulation zone making it vulnerable. With growing population, the state is facing increasing pressure to resolve crisis involved in distributing its water among citizens, farmers and industry, and under an anticipated warming environment from climate. While building drainage systems to prevent water logging creates artificial discharge areas and thereby augments the risk of mobilizing saline/polluted waters, flood irrigation and water logging should be minimized and prevented.
Resource distribution differs from region to region and is mainly geographically bound entities, which is characterized by specificity of occurrence. Vulnerability assessment of these resources especially groundwater resources is very important in arid/semi-arid region; it plays a vital role in the utilization and protection of groundwater resources. Human influence like induced indiscriminate withdrawal of groundwater is leading to serious consequences on environment and economy of the region.
Water resource management is multidimensional, which depends on reliable assessment of available water, its supply and distribution, its existing depletion, pollution, and its protection from depletion and degradation. For complete and sustainable utilization of this precious resource requires adequate knowledge of spatial and temporal changes in groundwater due to rapid change in land use/land cover activity. For spatial continuity assessment geographic information system (GIS) can play a significant role in planning, risk assessment and decision-making in groundwater resources management and protection of environmental degradation, especially in semi-arid region of developing country. Also for effective implementation of government policy community participation is necessary; still a large gap of knowledge exists in developing country for effective water use. Without bridging this gap, no sustainability can be achieved. More attention should be given to administrative management, improved water supply, capacity building among the local population to operate and self-sustain the system through awareness generation and constant motivation. Regular groundwater monitoring and strict enforcement of regulatory and counteractive measures are very important to minimize and prevent water logging and to develop management strategies to protect groundwater vulnerability to pollution/depletion.
References
Agricultural Department of Haryana (2013). www.agriharyana.nic.in
American Public Health Association (APHA) (2005) Standard methods for the examination of water and waste water, 21st edn. American Public Health Association, Washington, DC
Andreasen DC, Fleck WB (1997) Use of bromide chloride ratios to differentiate potential sources of chloride in a shallow, unconfined aquifer affected by brackish-water intrusion. Hydrogeol J 5(2):17–26
Bureau of Indian Standards (BIS) (1991) Drinking water specification. IS:10500:1991
Burrough PA, McDonnell RA (1998) Principles of geographical information systems for land resources assessment. Oxford University Press, New York
Busenberg E, Plummer LN (1982) The kinetics of dissolution of dolomite in CO2–H2O systems at 1.5 to 65 °C and 0 to 1 atm PCO2. Am J Sci 282:45–78
Central Ground Water Board (2011) Annual report Central Ground Water Board, Ministry of Water Resources, Govt. of India. CGWB, India
Datta PS (2013a) Groundwater vulnerability to changes in land use and society in India. In: Understanding freshwater quality problems in a changing world proceedings of H04, IAHS-IAPSO-IASPEI Assembly, Gothenburg, Sweden 361, pp 345–352
Datta PS (2013b) Ensemble models on palaeoclimate to predict India’s groundwater challenge. Acque Sotterranee Ital J Groundw AS06027:007–018
Datta PS, Tyagi SK (1996) Major ion chemistry of groundwater Delhi area: chemical weathering processes and groundwater flow regime. Geol Soc India 47(2):179–188
Datta PS, Bhattacharya SK, Tyagi SK (1996a) 18O studies on recharge of phreatic aquifers and groundwater flow-paths of mixing in Delhi area. J Hydrol 176:25–36
Datta PS, Deb DL, Tyagi SK (1996b) Stable isotope (18O) investigations on the processes controlling fluoride contamination of groundwater. J Contam Hydrol 24(1):85–96
Datta PS, Deb DL, Tyagi SK (1997) Assessment of groundwater contamination from fertilizers in Delhi area based on 18O, NO3 − and K+ composition. J Contam Hydrol 27(3–4):249–262
Davis JC (2002) Statistics and data analysis in geology. Wiley, Singapore
Domenico PA, Schwartz FW (1990) Physical and chemical hydrogeology. Wiley, New York
Doneen LD (1964) Notes on water quality in agriculture. Published as a Water Science and Engineering Paper 4001
Drever JI (1982) The geochemistry of natural waters, 3rd edn. Prentice-Hall, Englewood Cliffs
Garrels RM, Mackenzie FT (1967) Origin of the chemical composition of some springs and lakes. In: Stumm W (ed) Equilibrium concepts in natural water systems. Advances in Chemistry, vol 67. American Chemical Society, pp 222–242
Goel PS, Datta PS, Tanwar BS (1977) Measurement of vertical recharge to groundwater in Haryana state (India) using tritium tracer. Nordic Hydro 18:211–224
Haryana State Pollution Control Board (2010) Action plan for abatement of pollution in respect of Faridabad Town
Holland HD (1978) The chemistry of the atmosphere and oceans. Wiley, New York
Jalali M (2007) Salinization of groundwater in arid and semi-arid zones: an example from Tajarak, western Iran. Environ Geol 52:133–1149
Koetsiers M, Walraevens K (2006) Chemical characterization of the Neogen aquifer, Belgium. J Hydrogeol 14:1556–1568
Kulkarni KM, Rao SM, Singhal BBS, Parkash B, Navada SV, Nair AR (1989) Origin of saline groundwaters of Haryana State, India. IAHS Publ. no. 182, Regional Characterization of Water Quality. Proceedings of Baltimore symposium, May 1989
Kumari Rina, Datta PS, Singh CK, Mukherjee S (2011) Characterization and evaluation of processes governing the groundwater quality in parts of the Sabarmati basin, Gujarat using hydrochemistry integrated with GIS’. Hydrol Process J. https://doi.org/10.1002/hyp.8284
Lowrance R, Altier LS, Newbold JD, Schnabel RR, Groffman PM, Denver JM, Correll DL, Gilliam JW, Robinson JL, Brinsfield RB, Staver KW, Lucas W, Todd AH (1997) Water quality functions of riparian forest buffers in Chesapeake Bay watersheds. Environ Manag 21:687–712
Maya AL, Loucks MD (1995) Solute and isotopic geochemistry and groundwater flow in the central Wasatch Range Utah. J Hydrol 172:31
Mitrov BA (1961) Gaseous composition of atmosphere and its analysis. Tr. Inst. Prikl. Geofiz., Akad. Nauk S.S.S.R., Moscow (translated by Israel Program For Scientific Translations Published by U.S. Department of Commerce, of Technical Services, Washington, DC 209
National Capital Region Planning Board (NCRPB) (2013) Annual report 2013
Palmer CD, Cherry JA (1984) Geochemical evolution of groundwater in sequences of sedimentary rocks. J Hydrol 75(2):27–65
Piper AM (1944) A graphic procedure in the chemical interpretation of water analysis. Am Geophys Union Trans 25:914–923
Rajmohan N, Elango L (2004) Identification and evolution of hydrogeochemical processes in the groundwater environment in an area of the Palar and Cheyyar River Basins, Southern India. Environ Geol 46:47–61
Schoeller H (1965) Qualitative evaluation of groundwater resource. In: Methods and techniques of ground-water investigation and development, UNESCO, pp 54–83
Singh CK, Kumari R, Singh N, Mallick J, Mukherjee S (2012) Fluoride enrichment in aquifers of the Thar Desert: controlling factors and its geochemical modelling. Hydrol Process. https://doi.org/10.1002/hyp.9247
Stallard RF, Edmond JN (1983) Geochemistry of the Amazon-II. The influence and the geology and weathering environment on the dissolved load. J Geophys Res 88(14):9671–9688
Subba Rao N (2006) Seasonal variation of groundwater quality in a part of Guntur District, Andhra Pradesh, India. Environ Geol 49:413–429
Todd DK (1980) Groundwater hydrology. Wiley, New York, p 535
US Salinity Laboratory Staff (1955) Diagnosis and improvement of saline and alkaline soils. USDA Handbook 60, USDA, Washington
U.S. Environmental Protection Agency (2000) Drinking water standards and health advisories. U.S. Environmental Protection Agency, Office of Water, 822-B-00-001, p 12
World Health Organization (WHO 2008) Guidelines for drinking-water quality, 3rd edn, vol 1
WHO (World health Organization) (2009) Guidelines for drinking water quality. World Health Organization
Wilcox LV (1955) Classification and use of Irrigation waters. USDA, 969: Washington
Acknowledgements
The author (Rina Kumari) thanks SERB, Department of Science and Technology, for providing the Grant (No. SR/FTP/ES-14/2012) for carrying out this research. The author also thank Jawaharlal Nehru University for providing various research facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kumari, R., Datta, P.S., Rao, M.S. et al. Anthropogenic perturbations induced groundwater vulnerability to pollution in the industrial Faridabad District, Haryana, India. Environ Earth Sci 77, 187 (2018). https://doi.org/10.1007/s12665-018-7368-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-018-7368-8