Abstract
Worldwide, groundwater resources have been considered as the main sources of drinking, domestic uses, industrial and agriculture water demands, especially in arid and semiarid regions. Accordingly, the monitoring of the groundwater quality based on different tools and methods becomes a necessity. The aim of this study was to apply several approaches to assess the water quality and to define the main hydrochemical process which affect groundwater of the Maritime Djeffara shallow aquifer. In addition to the hydrochemical approach, two multivariate statistical analyses, hierarchical clusters analysis (HCA) and principal component analysis (PCA), were carried out to identify the natural and the anthropogenic processes affecting groundwater chemistry. Hydrochemical approach, based on 47 analyzed groundwater samples, shows that most of samples present a sulfate to mixed chloride, with sodi-potassic tendency facies. According to their chemically composition, the HCA revealed three different groups (C1, C2 and C3) according to their electrical conductivity (EC) values: C1 (average EC = 4500 µS/cm), C2 (average EC = 7040 µS/cm) and C3 (average EC = 9767 µS/cm). Furthermore, PCA results show two principal factors account 84.05% of the total variance: (1) F1 represents the natural component, and (2) F2 symbolizes the anthropic component. Moreover, the groundwater quality map of the Maritime Djeffara shows three categories: suitable, doubtful and unsuitable water for irrigation. These different results should be taken to protect water resources in arid and semiarid regions, especially at the alluvial coastal regions. Also, they help to make a suitable planning to manage and protect the groundwater resources.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Around the world, groundwater resources were always used to satisfy the human’s activities demands (domestic, industrial and agriculture uses, etc.), particularly in arid and semiarid regions (Voutsis et al. 2015). These regions are characterized by a scarce precipitation and almost negligible surface water supplies with a significant spatiotemporal variability. Moreover, these regions are categorized by their weak fraction of recharge against an intensive exploitation. The latest causes the reduction of freshwater in shallow aquifers and creates a local depression which leads to groundwater quality degradation. Therefore, it is essential to product a robust management plan. For this reason, the groundwater contamination becomes a very seriously environmental problem. The groundwater resources management should be reached by its characterization and determination of its hydrochemistry evolution.
Generally, the groundwater chemistry is controlled by some of natural processes such as aquifer lithology, water and geological formations interactions (dissolution and precipitation), water velocity and its residence time in the aquifer (Appelo and Postma 2005). In addition, the intense anthropogenic activities cause a very important risk to the groundwater quality. This risk was manifested by overexploitation, industries and agriculture fields that can alter the water chemistry through abstraction and irrigation (Huang et al. 2013). For this reason, the identification of the main processes controlling the groundwater hydrochemistry becomes necessary.
Studies in arid and semiarid regions have shown the importance of groundwater assessment for water management and integrated development strategy. Several methods have been developed to assess the groundwater chemistry and to define the main sources of water degradation. Moreover, several approaches are used to track the threat of degradation such as statistical analysis (hierarchical clusters analysis (HCA), principal component analysis (PCA), factorial analysis (FA), etc.) (Kharroubi et al. 2012; Agoubi et al. 2013; Bencer et al. 2016) and geochemical analysis (analytical methods, etc.) (Katz et al. 2011; Zabala et al. 2016; Colombani et al. 2017).
This study is the first attempt which serves to determine the groundwater suitability for irrigation in the Maritime Djeffara shallow aquifer, using the hydrochemical, statistical approaches and the groundwater quality indexes. To estimate the main variations in the groundwater chemistry and to disclose the main hydrochemical process to acquire water mineralization in the Maritime Djeffara shallow aquifer, some hydrochemical approaches and two multivariate statistical analyses were carried out. The first one is the HCA method. It considers an environmental geochemistry to investigate the environmental phenomena affecting the groundwater quality. It has been successfully applied in several hydrogeochemical studies. Principally, it consists of the samples reclassified into distinct hydrochemical groups according to their similarity. This model is a very delicate model because it can lead to an entirely different result if the linkage rule and the similarity measurement weren’t well chosen (Reimann et al. 2008). The second one is the PCA which is a powerful data reduction method. It allows to transform a huge amount of data and water quality variables into a manageable set of uncorrelated factors that represents the most variance observed, allowing to generate hypothesis about the identification of the principal components and their significance (Bayo and López-Castellanos 2016). This model makes possible to distinguish the principal factors (anthropogenic and natural processes) that exert control on the groundwater chemical composition. Generally, the PCA and the HCA are complementary. They group and discover the existing intrinsic structures or underlying behavior of data.
These models are the most used worldwide and almost successfully applied in order to better understand the groundwater evolution and determine the principal processes that control its composition, such as: (1) in Euboea Island (Voutsis et al. 2015), (2) Mzimba aquifer in Northern Malawi (Wanda et al. 2011), (3) Galilee and Eromanga basins, Great Artesian Basin in Australia (Moya et al. 2015), (4) Hajeb ELyoun–Jelma in Central Tunisia (Kolsi et al. 2013), (5) Sidi Bouzid shallow aquifer (Ncibi et al. 2016).
Study area
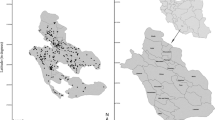
The Maritime Djeffara extending over 3100 Km2 has been selected as the study area for this study. It is situated in the coastal part of southeastern Tunisia (Fig. 1). It represents the Djeffara collapsed part localized in the East of Dhaher Mountain. It is limited by the Mediterranean Sea at the east, the boundary of Gabes governorate at the north and Ben Guerdane region at the south. This region is categorized by Mediterranean climate, which gathers two different types: on the one hand, the coastal semi-humid climate, and on the other hand, the sub-Saharan arid climate. This allows to classify the study area among arid and semiarid regions. The average temperature is about 30.1 °C in summer and 12.4 °C in winter, and the annual average rainfall is of about 149 mm/year, during the period of 1968–2015 (Ayed et al. 2017). Also, the rainfall may reach a maximum of about 376 mm/year (1968–2015) with an average of 27 rainy days per year.
Thus, the climate of the study area favors the evapotranspiration (ETP); hence, the annual average ETP is about 1806.27 mm. In addition, the ETP is characterized by their irregularity (seasonal and monthly). It reaches a rate of 240.66 mm during the summer period. These values decrease sharply during the winter to reach 94.66 mm. Monthly ETP varies between 254 mm in July and 93 mm in December and January.
Generally, in the South of Tunisia, the groundwater of shallow aquifers is lodged in the sandy and sandy clay of the coastal areas. Almost, the aquifer recharge is performed directly from the rainwater infiltration and the deep aquifer groundwater seepage through the faults affecting the area. The wadis underflows have a minor importance which is located mainly at the Zeus and Oum Zessar Wadis, Om Ettamer Wadi, El Fje Wadi and Smar Wadi.
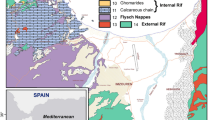
The geological map (Fig. 2) shows a Mio–Plio–Quaternary deposits which consist mainly by alluviums, limestones, gypsum, conglomerates, sands, clays and paralic Sebkhas deposits (Jedoui 2000), while the stratigraphy of the study area is characterized by a series of deposits with ages ranging from the Paleozoic to the Quaternary. The Paleozoic is represented by the Permian deposits (sandstone and dolomitic). The Mesozoic is composed by the Triassic detrital materiel to the Upper Cretaceous carbonate units (Bouaziz 1995). The Jurassic and Cretaceous formations (carbonate facies) are exposed to the eastern part of the Dhaher Mountain. The Mio–Pliocene deposits are composed of red gypsum clays and clay sands intercalated between conglomeratic and sandstone layers (Jedoui 2000; Bouaziz et al. 2003). These layers include the deep and shallow aquifers waters of the Maritime Djeffara. Two distinct hydrogeological entities have been identified in this region: the deep aquifer is characterized by an impermeable substratum dated from the Oligocene (Mamou 1990). This aquifer is overlaid by a semipermeable layer (gypsum clays of the Mio–Plio–Quaternary). The second one is the shallow aquifer, subject of this study (Agoubi 2012). The shallow aquifer is included in the Mio–Plio–Quaternary deposits which are constitutes by two lithological sections (Fig. 3). The Maritime Djeffara aquifer shows a dramatic thickness variation. These deposits are mainly pebbles, gravels, silts, sands and gravel clay. The hydrogeological cross section AA’ with N–S direction correlates eight drilling wells (Fig. 3a). This correlation shows a high subsidence of Jurassic at Drouj drilling well deposits in NE of the study area. It proves a thickness variation of the shallow aquifer from 50 m (at the north) to 90 m (at the south). Moreover, a second hydrogeological section was performed to establish the reservoir geometry (Fig. 3b). It has a NW–SE direction and shows a complex structure of shallow aquifer which is affected by many faults allowing communication between the shallow aquifer and the Triassic deep one. This communication leads to geochemical composition modification in groundwater. At the east of this section, the Jurassic deposits show uprising at Khaoui Elghadir drilling well, where two faults affect the area into the graben and horst structure. Soils in the study area are composed of materials belonging to the Mio–Plio–Quaternary formation. The Maritime Djeffara reservoir geometry is characterized by a high complexity, from a lithologic point of view rather than from a structural point of view. The aquifer is categorized by its significant permeability which ranges between 10−4 and 2.10 × 10−1 m/s.
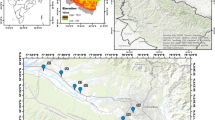
On the other hand, the piezometric map (Fig. 4) shows head values ranging between −10 m especially located at the coastal regions (Boughrara Gulf and Djorf) and 130 m observed at the southwestern part of the study area (Ayed et al. 2017). A general flow direction was detected in the orientation of southwest to northeast.

Reproduced with permission from Ayed et al. (2017)
Piezometric map of the Maritime Djeffara shallow aquifer
Materials and methods
Materials
Groundwater samples were collected from 47 wells in May 2016. A homogeneous distribution was chosen to obtain a representative data concerning the spatial variability of the groundwater quality in the Maritime Djeffara shallow aquifer (Fig. 1). The coordinates of each well sampled were measured using a Trimble Juno ST handheld GPS. The physical parameters (temperature, pH, EC) were measured at the moment of sampling in field using a calibrated pH meters and conductivity meter. The suspended matter in the water samples was separated by filtration through a filter of 0.45 µm pore size. The samples were stored (after filtering) in polyethylene containers without any treatment (acidification and dilution) and refrigerated until analysis within 1–2 days. Polyethylene sample bottles were washed several times firstly with distilled water and secondly by the wells groundwater before taking the samples.
Most of the chemical determinations were carried out in chemical laboratory of the Higher Institute of Water Sciences and Technology of Gabes (Tunisia). The cations and anions of these samples (sodium (Na+), potassium (K+), Lithium (Li+), calcium (Ca2+), magnesium (Mg2+), chloride (Cl−), sulfate (SO4 2−), bicarbonate (HCO3 −), phosphate (PO4 3−), nitrate (NO3 −), bromide (Br−) and fluoride (F−) were analyzed by ion chromatography (Metrohm 850 Professional IC). Concerning the alkalinity as HCO3 − was measured using the titration method, in “Water Sciences laboratory” at the Higher Institute of Water Science and Technology of Gabes. The analytical accuracy of the ion measurement is determined by calculating the absolute error in ionic equilibrium, which does not exceed in all samples the standard limit (± 5%). In addition, the TDS contents were estimated based on the conductivity measurements and the sum of total analyzed dissolved solutes.
To develop the object of this study, several tools were used. The IBM SPSS Statistics version 21 and XLSTAT version 2016 softwares were used to apply the statistical approaches to the groundwater quality data for 47 samples. Also, the “Diagram” and “Excel 2016” softwares were used to the analytical analyses and groundwater hydrochemical data processing.
Statistical analysis methods
In this study, a multivariate statistical analysis (MSA) was applied to determine the patterns of hydrochemical dataset of Maritime Djeffara groundwater. In total, 15 chemical constituents were analyzed in 47 samples collected in May 2016 from the agricultural wells with homogenous repartition. These variables (Na+, K+, Li+, Ca2+, Mg2+, Cl−, SO4 2−, HCO3 −, PO4 3−, NO3 −, Br−, F−, pH and EC) were successfully used in hierarchical cluster analysis and principal component analysis.
Assessing the statement of normality is essential in most statistical measures. For this attempt, the parametric statistical analysis was suggested as the best examples. In this study, the normality was checked by the Kolmogorov–Smirnov (KS) and Shapiro–Wilk (SW) tests.
The KS statistic test belongs to the supremum class of empirical distribution function (EDF). It is based on the largest vertical difference between the empirical distribution and hypothesized (Conover 1999). It gives an “n”-ordered data points (x 1 < x 2 < x 3 < ··· < x n ). Conover (1999) defined this test as following:
where “sup” stands for supremum which means the greatest; F * (x) is the hypothesized distribution function; F n (x) is the EDF estimated based on the random sample.
In KS test of normality, F * (x) is taken to be a normal distribution with known mean (µ) and standard deviation (σ).
The KS statistic test is used for testing:
-
H 0: F(x) = F * (x), for all x from −∞ to ∞ (the data follow a specified distribution);
-
H a: F(x) ≠ F * (x), for at least one value of x (the data do not follow the specified distribution).
Moreover, the popular Shapiro–Wilk test was applied. It is based on the statistic test (W). The comparison with other tests shows that it is the most powerful for small sample size (n ≤ 50). It was the first test that could detect departures from normality due to either skewness or kurtosis (Razali1 and Wah1 2011). The statistic test is defined as:
where y i is the ith order statistic, \( \bar{y}_{i} \) is the sample mean, \( a_{i} = \left( {a_{1} , \ldots ,a_{n} } \right) \, = \frac{{m^{\text{T}} V^{ - 1} }}{{(m^{\text{T}} V^{ - 1} V^{ - 1} m)^{1/2} }}\quad {\text{and}}\quad m = (m_{1} , \ldots m_{n} )^{\text{T}} \) are the expected values of the order statistics of independent and identically distributed random variables sampled from the standard normal distribution, and V is the covariance matrix of those order statistics.
Generally, the W values vary between 0 and 1. Small values of W lead to the rejection of normality, whereas a value equal or close to 1 designates the normality of the data. The higher the W, the greater the compatibility with the credibility of normal law.
Hierarchical cluster analysis
The “hierarchical cluster analysis” (HCA) is a statistical approach. Generally, it is used to classify the groundwater sources into definite hydrochemical groups according to their similarity or dissimilarity (Monjerezi et al. 2011). This statistical analysis was generated using as linkage the Ward’s method (1963) and as similarity measure the squared Euclidian distances (Baig et al. 2010). It is the most used algorithm in MSA to hydrochemical analysis. The result of this method is usually presented as a dendrogram, where the X axis was considered for representing the samples grouped into clusters and the Y axis represents the dissimilarity between different clusters. Each cluster resulting from this analysis group samples is as similar as possible to each other, while their difference is as large as possible.
Principal component analysis
In order to analyze the groundwater data, the principal components analysis (PCA) has been used. This statistical approach is essentially applied to prove the water and sediment interaction (Bošnjak et al. 2012; Kolsi et al. 2013). This method is a mathematical technique and is based on the reduction of a large number to a small number of variables which represent the principal components. This procedure consists on the extracting of related variables and consequently deducing the processes that control the water chemistry (Huang et al. 2013). In addition, it can be used to extract the principal factors presenting the different natural and anthropogenic processes that influence on the groundwater chemical composition (Helena et al. 2000; Cloutier et al. 2008; Güler et al. 2012; Huang et al. 2013).
To apply the PCA, several tests should be used to assess the suitability of the accused data for factor analysis. These tests comprise the Kaiser–Mayer–Olkin (KMO) and Bartlett’s sphericity tests. They are the most used tests in academic and business studies. The KMO test measures the sampling adequacy that is recommended to check the case to variable ratio for the analysis being conducted. It determines if the results given with the sample are adequate or not. Theoretically, the KMO index ranges from 0 to 1, and the world-over accepted index is over 0.5 (Williams et al. 2010).
The Bartlett’s test of sphericity refers to the study significance and thus shows the validity and suitability of the responses collected to the problem being addressed through the study. It indicates the strength of the relationship between variables. This kind of tests determines the null hypothesis that the correlation matrix is an identity matrix. This later is a matrix in which all of the diagonal elements are equal to 1 and all off diagonal elements are close to 0.
For PCA to be recommended suitable, the Bartlett’s test of sphericity must be less than 0.05 (p < 0.05) (Williams et al. 2010).
Analytical methods
To understand the hydrochemical process in the Maritime Djeffara shallow aquifer, the following methods were used:
The groundwater facies were determined based on “Piper diagram” which use the major elements as cations (Na+, K+, Ca2+ et Mg2+) and as anions (Cl−, SO4 2− et HCO3 −) measured in milliequivalents per liter. It represents the hydrochemical evolution of groundwater (Piper 1944). Generally, the groundwater chemistry is modified during this movement through the soil by mineral–water interaction. The determination of the saturation index (SI) can evaluate this phenomenon. It describes the precipitation or dissolution of minerals along flows in the soil–water system (Bhattacharya et al. 2012).
In this study, the saturation index was calculated using the “Phreeqc” extension of the “Diagram” software (Parkhurst and Appelo 2013). It determines the saturation indexes of anhydrite, calcite, dolomite, gypsum and halite minerals.
The groundwater of the Maritime Djeffara shallow aquifer was almost used for irrigation. For this reason, the groundwater suitability to irrigation was determined by assessing the effects of mineral concentrations in water, soils and plants. In the study case, the assessment of groundwater suitability for irrigation was determined through five indexes: electrical conductivity (EC), permeability index (PI), sodium adsorption ratio (SAR), sodium percent (%Na) and magnesium percent (%Mg).
Electrical conductivity assessment
Generally, the determination of salinity content is carried out by EC calculation, in order to identify these affects to crops (Thorne and Peterson 1954). The excess of salinity in water irrigation decreases the osmotic activity of plants in the soil. Also, it affects soil permeability, aeration and soil structure (Costa and Aparicio 2015). Four classes of EC were distinguished: low, moderate, high and very high (Table 1).
Permeability index (PI)
The soil permeability is influenced by the continuos and intensive use of groundwater sources for irrigation. The permeability is affected through the sodium, calcium, magnesium and bicarbonate content in the soil. The permeability index (PI) was determined by Eq. 1 created by Doneen (1964) where all elements are in milliequivalent per liter:
To assess the groundwater suitability for irrigation, Doneen (1964) evolved a water classification based on the PI. Hence, three classes were identified: Class I is characterized by PI < 25% and classifies the water as unsuitable for irrigation, Class II is categorized as good and suitable water for irrigation with a 25% < PI > 75%, and Class III where the PI > 75% is distinguished by an excellent water for irrigation.
Sodium adsorption ratio (SAR)
The excess of sodium concentration in groundwater affects the soil properties and reduces the soil permeability. Hence, the determination of the groundwater suitability for irrigation was performed by calculating the sodium adsorption ratio (SAR). This ratio measures the risk of alkali–sodium for crops. Moreover, it determines the degree from which water tends to enter into cation exchange reactions in soil. Generally, the sodium reaches the aquifer through rain and rocks dissolution. It replaces the absorbed magnesium and calcium and consequently causes the soil structure damage. Because of its effect on soils and plants, it has been considered among the main factors governing irrigation water (Richards 1954). This ratio was determined using Eq. 2:
According to SAR ratio, waters were grouped into three classes. Water with SAR ranging from 0 to 3 is considered as good, while SAR ranging from 3 to 9 is characterized by moderate type and greater than 9 is considered unsuitable for irrigation purpose (Table 1).
Sodium percent (%Na)
In addition to the SAR ratio, the sodium percent (%Na) presents a significant factor to indicate sodium hazard. The Na+ cation has an important effect to deteriorate the subsoil structure and consequently reduces his permeability (Narasimha et al. 2006). Usually, Na+ concentration is stated in terms of sodium percent or soluble-sodium percentage (%Na). A high Na+ concentration in water irrigation leads to its absorption by the clay particles, and it will be substituted by Mg2+ and Ca2+ ions. This substitution reduces the soil permeability. For this aim, the %Na is used for assessing the water quality for agricultural purposes. This parameter is calculated according to Eq. 3 which is expressed as the sodium and potassium percentage against all cationic concentrations.
According to Wilcox diagram (1955), four classes have been distinguished good, permissible, doubtful and unsuitable. Only two classes have established by Eaton (1950): safe and unsafe (Table 1).
Magnesium percent (%Mg)
The magnesium percent or risk of magnesium (%Mg) designates one of the most important qualitative criteria for assessing the water quality for irrigation as long as magnesium and calcium keep the equilibrium state in most waters. The excess of magnesium affects the soil quality (increases the construction of alkalinity) which can decrease the productivity (Ramesh and Elango 2011). The % Mg was calculated as following where the ions concentrations cited are indicated in milliequivalents per liter (Ayers and Westcot 1985):
According to this parameter, two classes have been distinguished: suitable and unsuitable where the %Mg < 50% and %Mg > 50%, respectively (Szabolcs and Darab 1964).
Results and discussion
Water quality
Groundwater samples were analyzed and were tested for charge balance errors (Table 2). The charge balance for all samples was found less than ± 5%. The statistical description summary of the Maritime Djeffara groundwater samples was presented as a minimum, maximum, average and standard deviation (Table 3). The results show that the pH values vary from 6.85 to 7.84 with an average of 7.34, indicating alkaline to neutral conditions. In addition, the TDS values range from 876.61 to 5222.45 mg/L with an average value equal to 3055.64 mg/L. According to Fetter (1990), the TDS values were classified into two classes: TDS < 1000 mg/L corresponding to freshwater and 1000 mg/L < TDS < 10,000 mg/L indicating brackish water. Thus, the study area is characterized by an alkaline and brackish waters type. These waters are located particularly at the north and to the east of the aquifer; zones of study area outlet. Furthermore, the standard deviation values are relatively low compared to the averages for most elements. Sulfate (SO4 2−) shows a very high standard deviation which is equal to 485.5. This last presents more than 50% of the average. Chloride (Cl−) and sodium (Na+) indicate some variability in the groundwater type of the study area. Based on the average values of the chemical parameters, the cations are in following order of abundance Na+> Ca2+> Mg2+> K+> Li+, while the anions reveal an order of abundance as follows SO4 2− > Cl− > HCO3 − > PO4 3− > NO3 − > Br− > F−.
Thus, to determine the chemical trends of Maritime Djeffara groundwater, the chemical analysis results have been plotted on Piper diagram using the “Diagram” software (Simler 2009). Indeed, the diagram analysis (Fig. 5) shows two different facies:
-
Forty-six samples present a SO4–Cl mixed water type with sodi-potassic tendency;
-
One sample presents a Ca–Mg–HCO3 water type.
The groundwater of the study area is characterized by their high concentrations of Na, Cl, Ca and SO4 which may be related to dissolution of calcite (CaCO3), gypsum (CaSO4) and anhydrite (CaSO4), while the Na and Cl high concentrations could be related to halite dissolution (NaCl). These results were also obtained by previous studies which consider the groundwater lodged at the same aquifer (Agoubi et al. 2014).
In the present study, the saturation index (SI) values for the different minerals (anhydrite, calcite, dolomite, gypsum and halite) and their chemical equilibria were calculated using the “Phreeqc” function of the “Diagram” software (Parkhurst and Appelo 2013). The correlation between SI and minerals compositions (Fig. 6) indicates that the carbonate minerals (calcite (CaCO3) and dolomite ((Ca, Mg) (CO3)2) saturation have an over-saturated state. So, they tend to precipitate, indicating that the salt load is not influenced by the water–carbonate mineral interaction. Furthermore, the groundwater is under-saturated with sulfated minerals (gypsum (CaSO4, H2O) and anhydrite (CaSO4)). Consequently, this dissolution contributes to the solutes acquisition and controls the mineralization. According to previous studies (Kharroubi et al. 2012; Agoubi et al. 2013), the halite concentration may be caused by the seawater intrusion and the influences of Sebkhas zones.
Statistical analysis results
Samples characteristics
A Shapiro–Wilk’s test (p > 0.05) and a visual inspection of their normal Q–Q plots showed that the exam scores were approximately normally distributed for all elements (Fig. 7). The same results were obtained by the Kolmogorov–Smirnov test which show that the parameters were approximately normally distributed (Table 4). In the study case, the normal distribution is favored for optimal results and reliable interpretations of the results.
Hierarchical cluster analysis (HCA)
The application of the HCA shows three clusters (C1, C2 and C3) represented in dendrogram (Fig. 8, Table 5). These clusters were regrouped according to their dominant chemical composition as:
-
Cluster 1 includes 24 wells. It is characterized by low values of EC where the median doesn’t exceed 4.5 ms/cm and a low concentration of Na+, Ca2+, Mg2+, SO4 2− and Cl− compared to those of clusters 2 and 3. This cluster is categorized by a SO4–Cl–HCO3 water type;
-
Cluster 2 groups samples with medium concentrations between cluster 1 and cluster 3 and includes 12 wells. The average of EC is equal to 7.04 ms/cm. This cluster is characterized by SO4–Cl–Na–HCO3 water type;
-
Furthermore, cluster 3 groups only 11 wells and is branded by the highest concentrations of SO 2−,4 Cl− and Na+, also, by the highest EC (9767 ms/cm).
Principal component analysis (PCA)
This statistical analysis was performed using 15 variables (pH, EC, HCO3 −, F−, Cl−, Br−, NO3 −, SO4 2−, PO4 3−, Na+, K+, Li+, Ca2+, Mg2+ and TDS) and 47 individuals (wells). These variables were projected on two factors (F1 and F2), which represent 84.05% of the total variance. According to Dagnellie (1992), the significant threshold (Cs) for 47 samples is equal to 0.273. The report between the number of significant relationships (SR) and the number of total relations (TN) in the matrix gives an idea about the distribution nature (Table 6):
TN = 120; SR = 77; then SR/TN = (77/120) = 0.64.
This ratio gives a value greater than the threshold of statistical significance (0.64 > Cs (0.273)) which indicates a good statistical distribution for this correlation matrix.
In this study, the Bartlett sphericity test and KMO were determined for the PCA (Table 7). For the Maritime Djeffara groundwater, the KMO measure is about of 0.799 (Table 7), which can be considered acceptable for the present study. Moreover, the Bartlett’s test of sphericity, close to 0, is significant, while the significance level is small enough to reject the null hypothesis. This means that correlation matrix is not an identity matrix.
The correlation coefficients between the different variables are given by the correlation matrix (Table 6) which was made two by two. This correlation shows that the total dissolved salts (TDS) are strongly correlated with the major elements Cl− (0.973), Br− (0.974), NO3 − (0.974), SO2 − (0.984), Na+ (0.981), K+ (0.974), Ca2+ (0.933) and Mg2+ (0.935) and EC (0.975), with the exception of bicarbonate (HCO3 −) which is poorly correlated with TDS (0.054). Furthermore, the SO4 2− is strongly correlated with all major elements except the HCO3 −, PO4 3− and Li+. Practically, the bicarbonate is weakly correlated with all elements. However, the Ca2+, Mg2+ and K+ ions show a good to moderate correlation with all elements except HCO3 − and Li+. Table 5 shows the following associations: TDS-EC (0.975), TDS-Na (0.981), TDS-Cl (0.973), EC-Na (0.96), EC-Cl (0.97), Na–Cl (0.965). These results reflect the contribution of Na+ and Cl− ions to groundwater mineralization. The PCA shows that these variables are extremely interrelated with a strong correlation especially between EC and Cl−, SO4 2−, Na+, Ca2+ and Mg2+.
In the present study, overall 14 axes have been obtained. Table 8 presents the variance percentages as well as the cumulative variance for each component (factor) axis responsible for this statistical distribution. The adopted axes must have a variance percentage greater than or equal to the value of the ratio between the total cumulative variance and the number of axes obtained. So, the % variance should be ≥ 7.14. Consequently, only the two first axes F1 and F2 are considered. They represent 84.04% of the total variance (Table 8):
-
The first main component F1 explains 65.72% of the total variance; it is positively expressed by EC, Mg2+, SO4 2−, Na+, Ca2+, K+, Cl−, NO3 − and Br−. This confirms that the mineralization is mainly due to the evaporates dissolution (NaCl and CaSO4);
-
HCO3 −, PO4 3−, F+, Li+ and pH are positively carried out by the F2 axis, which expresses 18.325% of the total variance, indicating carbonate dissolution and contamination caused by anthropogenic activities.
The spatial distribution of 47 samples on the factorial plan (F1 × F2) (Fig. 9) shows three different groups:
-
The first group C1 consists of 18 individuals (1, 6, 7, 9, 10, 16, 17, 22, 23, 26, 29, 31, 32, 38, 40, 43, 44 and 45) reflecting the chloride sulfated, sodic and magnesium character. Therefore, F1 carries the samples which have undergone natural effects on mineralization. These samples were located at the downstream part of the aquifer and at the Sebkhas zones where the highest concentrations were observed: TDS, K+ and SO4 2− (with values of 5222.45, 39.4 and 2499.6 mg/L, respectively, for sample 16), Cl− (1292.55 mg/L for sample 7) and Na+ (1050.48 mg/L for sample 1). This result shows the phenomenon related to salts trapped in sediments dissolution processes;
-
The second group C2, formed by 16 wells (3, 4, 11, 13, 14, 18, 19, 20, 21, 24, 28, 34, 36, 37, 39, 41), corresponds to the least mineralized group. The samples corresponding to this group are characterized by the lowest element contents such as TDS, K+ and SO4 2− (with values equal to 876.61, 12.31 and 123.17 mg/L, respectively, for sample 37,), Cl− (303.1 mg/L for sample 36) and Na+ (200.73 mg/L for sample 41);
-
The third group C3 is positioned on the positive part of the factorial axis F2. This axis mainly refers to the effect of anthropogenic activities. This pollution mainly occurs at the wells: 25, 27, 30, 33, 35, 42, 46, 47. C3. It is characterized by high content of PO4 3− and F− (46.8 and 13.78 mg/L, respectively, for sample 25), HCO3 − (726.8 mg/L for well 33) and Li+ (3.25 mg/L for well 42);
The main component analysis (PCA) corroborated the results obtained using the hierarchical cluster analysis (HCA). Indeed, the samples grouped in the same cluster by the HCA are the same grouped by the PCA (Figs. 8, 9).
These two statistical models which are considered the most popular in academic researchers give an approval results for the present study. Generally, they were successfully applied in several studies and were associated with other models such as the factor analysis. Moya et al. (2015) have used the multivariate statistical approaches in order to recognize the hydrochemical patterns and to determine the processes that control the hydrochemical evolution in the Great Artesian Basin of Australia. The authors are satisfied by the obtained results which are significant. Also, Wanda et al. (2011) have chosen the PCA and HCA models to assess the groundwater hydrochemistry patterns in Mzimba aquifer (northern of Malawi).
Groundwater suitability for irrigation
The salt content in irrigation water (EC)
In order to identify the risk on soils, groundwater contaminations and crops, the EC is a good tool to measure salinity. In addition of the waste water uses, the excess salt content is one of the main concerns which affect the water quality. Furthermore, a high salt concentration in water or soil will negatively affect crop yield (decreases osmotic activity of plants) and cause soil degradation (interferes with water absorption and soil nutrients). Table 9 shows that all water samples belong to the very high salinity class. So, it is unsuitable for irrigation in normal conditions (Fig. 10a).
Permeability Index (PI)
In our study, the permeability index (PI) values range from 39.71 to 54.31% with an average of 45.74% (Table 10, Fig. 10b). According to Raghunath (1987), the PI values are included between 25 and 75, showing that the water belongs to the good class and has suitable quality for irrigation;
Sodium adsorption ratio (SAR)
This ratio shows a high value (SAR values > 23) at clusters 2 and 3 which make the soil exposed to an alkalinity phenomenon. Based on the diagram SAR/EC at 25 °C, the groundwater of the Maritime Djeffara is classified as (Figs. 10c, 11):
-
C3-S1 group: characterized by admissible water quality, which can be useful for the tolerant crops to salt. This type of crops should be periodically controlled to assess the salinity evolution. These waters are localized especially at the downstream of the study area (Djorf, Sebkhas el Maidher, Gulf of Bou Ghrara, Zarzis) (some samples of cluster 1);
-
C4-S2 group: these samples are classified among the poor groundwater quality, characterized by a high mineralization. They are suitable for irrigation and categorized by a very good salinity tolerance species and on well-drained and leached soils (some samples of clusters 1 and 2);
-
C5-S3: very poor water quality, highly mineralized and is not suitable for irrigation. That is near the outlets of the shallow aquifer (cluster 3 samples).
According to the SAR ratio, the Maritime Djeffara groundwater is classified between the high alkalization power class (clusters 2 and 3) and the middle class (clusters 1 and 2) where the soil alkalinity risk is appreciable in soils with fine texture and high exchange capacity. The exchangeable Na+ ions can substitute with Ca2+ and Mg2+, which may cause reduce soil permeability (Narasimha et al. 2006).
Sodium percentage (%Na)
The sodium percentage (%Na) was calculated for 47 groundwater samples. %Na values range from 35.2 to 51.07%, with an average equal to 40.54%. According to the Wilcox classification (1955), two classes are represented (Figs. 10d, 12):
-
medium quality: it concerns medium mineralized waters, with an average and low risk of salinization and alkalization, respectively. It characterizes the central groundwater of Maritime Djeffara (sample 37);
-
poor quality: this class characterizes the groundwater localized at the downstream part of the shallow aquifer as well as the Sebkhas zones. These waters have a strong mineralization, presenting an important risk of salinization of the soils, but with medium to strong alkalization.
Magnesium percentage (%Mg)
In Maritime Djeffara groundwater, the magnesium percentage (%Mg) values range from 42.38 to 57.28% (Table 11). According to %Mg, two water classes can be distinguished:
-
Suitable water for irrigation: when the %Mg is less than 50%;
-
Inappropriate and unsuitable water to irrigation: when the %Mg is greater than 50%. These waters adversely affect crop growth.
In the study area, approximately 76.9% of the water samples show %Mg values less than 50%. So, these waters are suitable for irrigation. The remain of the samples (23.4%) have a % Mg > 50%. Therefore, they are unsuitable for irrigation. These wells are located at the north and east parts of the Maritime Djeffara shallow aquifer (Fig. 10e).
Water quality map
The choice of weights to each parameter is based on the statistical analysis of these five parameters. The results are presented as a minimum, maximum, average, standard deviation, skewness and variation coefficient. Table 12 shows that the highest parameter is the %Mg (average = 50.15%). According to the average values, these parameters are classified in abundance order as following: % Mg > PI > % Na > EC > SAR. This indicates that the %Mg parameter represents the great risk to water quality.
On the other hand, the use of the variation coefficient (which allows comparisons when units of measurement are different), calculated for the five parameters (Table 12), shows that the EC parameter contributes immensely to the variability of water quality (38.73%), followed by sodium adsorption ratio (SAR) (31.08%). The most important static parameters which do not significantly contribute to water quality are the %Na (12.09%), the PI (8.65%) and the %Mg (6.15%). By assigning the appropriate weight to each parameter (Table 13), three classes were distinguished (Fig. 13). The intersection of the spatial distribution maps of these parameters gives a final map where water quality varies from the suitable to unsuitable for irrigation successively from the Southwest (recharge zone) toward the Northeast (saline depressions area).
Usually, the use of quality indexes was manifested at numerous studies which are intended to determine the groundwater suitability for irrigation purposes. Ncibi et al. (2016) have used the SAR, %Na, RSC, PI and %Mg to determine the groundwater quality in Sidi Bouzid shallow aquifer (Central Tunisia) showing powerful results that can help in water resources management. These indexes were also used by Aghazadeh and Mogaddam (2010) for assessing the groundwater either for drinking or for irrigation purposes in Oshnavieh Area, Northwest of Iran. Associated with other indexes, Delgado et al. (2010) have determined the groundwater of Yucatán (Mexico) aptitude for irrigation.
Conclusion
The comprehension how groundwater hydrochemistry evolves becomes a necessity to manage the groundwater resources in sedimentary basins. Particularly, in coastal aquifers the groundwater chemistry is influenced by both natural and anthropogenic processes. In this study, results obtained by using five quality indexes show that generally the Maritime Djeffara groundwater is doubtful to suitable for irrigation (localized at the recharge and centrals areas), while the unsuitable waters characterize the Sebkhas zones and the coastal areas. Moreover, we have proved that the use of multivariate statistical methods (PCA and HCA) can be an effective tool for the identification of the different water quality, types and groups. Based on HCA, groundwater was categorized as a SO4–Cl–HCO3 and SO4–Cl–Na–HCO3 water types. Also, the 47 samples were grouped into three clusters according to their chemical similarity. These clusters were categorized by their electrical conductivity (EC): C1 (average = 4450 µS/cm), C2 (average EC = 7040 µS/cm) and C3 (average EC = 9767 µS/cm). Furthermore, according to PCA results, two factors have been defined (F1 et F2) presenting 84.04% of the total variance. F1 was associated with natural processes (rock–water interaction, lithology) and expressed with EC, Mg2+, SO4 2−, Na+, Ca2+, K+, Cl−, NO3 − and Br−. F2 was illustrated by anthropogenic activities (industries, agriculture) and associated with HCO3 −, PO4 3−, F+, Li+ and pH. The factorial plans (F1 × F2) showed three classes. The first category includes water samples with the highest TDS whose ionic acquisition is controlled by the residence time and mineralization of chloride sulfated, sodic and magnesium minerals. The second class contains least mineralized samples. It is most often characterized by the lowest TDS values. The third group categorizes the samples affected by the anthropogenic processes and bicarbonate dissolutions.
The obtained water quality model can determine the groundwater aptitude for irrigation uses in each region. This model can serve as a planning document allowing to take preventive and corrective measures. These methods can be applied in other regions with similar characteristics.
References
Aghazadeh N, Mogaddam AA (2010) Assessment of groundwater quality and its suitability for drinking and agricultural uses in the Oshnavieh area, Northwest of Iran. J Environ Prot 1(01):30
Agoubi B (2012) Caractérisation hydrogéologique et géochimique du système aquifère de la Jeffara maritime: Apport des approches géostatistiques (Hydrogeological and geochemistry characterization of the Maritime Jeffara aquifer system). Faculty of sciences of Sfax
Agoubi B, Kharroubi A, Abichou T, Abida H (2013) Hydrochemical and geoelectrical investigation of Marine Jeffara Aquifer, southeastern Tunisia. Appl Water Sci 3(2):415–429
Agoubi B, Kharroubi A, Abida H (2014) Geochemical assessment of environmental impact on groundwater quality in coastal arid area, south eastern Tunisia. J Environ Sci Eng Technol 2:35–46
Appelo CAJ, Postma D (2005) Geochemistry, groundwater and pollution, 2nd edn. A. A. Balkema, Rotterdam
Ayed B, Jmal I, Sahal S, Bouri S (2017) Assessment of groundwater vulnerability using a specific vulnerability method: case of Maritime Djeffara shallow aquifer (Southeastern Tunisia). Arab J Geosci 10:262. https://doi.org/10.1007/s12517-017-3035-8
Ayers RS, Westcot DW (1985) Water quality for agriculture FAO irrigation and drain, no. 29
Baig JA, Kazi TG, Shah AQ, Kandhro GA, Afridi HI, Arain MB et al (2010) Speciation and evaluation of arsenic in surface water and groundwater samples: a multivariate case study. Ecotoxicol Environ Saf 73:914–923
Bayo J, López-Castellanos J (2016) Principal factor and hierarchical cluster analyses for the performance assessment of an urban wastewater treatment plant in the Southeast of Spain. Chemosphere 155:152–162
Bencer S, Boudoukha A, Mouni L (2016) Multivariate statistical analysis of the groundwater of Ain Djacer area (Eastern of Algeria). Arab J Geosci 9(4):1–10
Bhattacharya P, Sracek O, Eldvall B, Asklund R, Barmen G, Jacks G, Koku J, Gustafsson JE, Singh N, Brokking Balfors B (2012) Hydrogeochemical study on the contamination of water resources in a part of Tarkwa mining area, Western Ghana. J Afr Earth Sci 66:72–84
Bošnjak MU, Capak K, Jazbec A, Casiot C, Sipos L, Poljak V, Dadić Ž (2012) Hydrochemical characterization of arsenic contaminated alluvial aquifers in Eastern Croatia using multivariate statistical techniques and arsenic risk assessment. Sci Total Environ 420:100–110
Bouaziz S (1995) Etude de la tectonique cassante dans la plate-forme et l’Atlas Sahariens (Tunisie méridionale): evolution des paléochamps de contraintes et implications géodynamiques. Unpublished thesis ès-Sciences, Université Tunis II
Bouaziz S, Jedoui Y, Barrier É, Angelier J (2003) Néotectonique affectant les dépôts marins tyrrhéniens du littoral sud-est tunisien: implications pour les variations du niveau marin. Comptes Rendus Geosci 335(2):247–254
Cloutier V, Lefebvre R, Therrien R, Savard MM (2008) Multivariate statistical analysis of geochemical data as indicative of the hydrogeochemical evolution of groundwater in a sedimentary rock aquifer system. J Hydrol 353:294–313
Colombani N, Cuoco E, Mastrocicco M (2017) Origin and pattern of salinization in the Holocene aquifer of the southern Po Delta (NE Italy). J Geochem Explor 175:130–137
Conover WJ (1999) Practical nonparametric statistics, 3rd edn. Wiley, New York, pp 428–433 (6.1)
Costa JL, Aparicio VC (2015) Quality assessment of irrigation water under a combination of rain and irrigation. Agric Water Manag 159:299–306
Dagnellie P (1992) Statistique théorique et appliquée, vol 1. Presses agronomiques de Gembloux, Gembloux
Delgado C, Pacheco J, Cabrera A, Batllori E, Orellana R, Bautista F (2010) Quality of groundwater for irrigation in tropical karst environment: the case of Yucatan, Mexico. Agric Water Manag 97(10):1423–1433
Doneen LD (1964) Notes on water quality in agriculture. Department of Water Science and Engineering, University of California, Davis
Eaton FM (1950) Significance of carbonates in irrigation waters. Soil Sci 69(2):123–134
Fetter CW (1990) Applied hydrogeology. CBS Publishers & Distributors, New Delhi
Güler C, Kurt MA, Alpaslan M, Akbulut C (2012) Assessment of the impact of anthropogenic activities on the groundwater hydrology and chemistry in Tarsus coastal plain (Mersin, SE Turkey) using fuzzy clustering, multivariate statistics and GIS techniques. J Hydrol 414–415:435–451
Helena B, Pardo R, Vega M, Barrado E, Fernández JM, Fernández L (2000) Temporal evolution of groundwater composition in an alluvial aquifer (Pisuerga river, Spain) by principal component analysis. Water Res 34:807–816
Huang G, Sun J, Zhang Y, Chen Z, Liu F (2013) Impact of anthropogenic and natural processes on the evolution of groundwater chemistry in a rapidly urbanized coastal area. South China Sci Total Environ 463–464:209–221
Jedoui Y (2000) Sédimentologie et géochronologie des dépôts littoraux quaternaires: reconstitution des variations des paléoclimats et du niveau marin dans le Sud-Est tunisien. These de doctorat es-sciences géologiques, université de Tunis II
Katz BG, Eberts SM, Kauffman LJ (2011) Using Cl/Br ratios and other indicators to assess potential impacts on groundwater quality from septic systems: a review and examples from principal aquifers in the United States. J Hydrol 397(3):151–166
Kharroubi A, Tlahigue F, Agoubi B, Azri C, Bouri S (2012) Hydrochemical and statistical studies of the groundwater salinization in Mediterranean arid zones: case of the Jerba coastal aquifer in southeast Tunisia. Environ Earth Sci 67(7):2089–2100
Kolsi SH, Bouri S, Hachicha W, Dhia HB (2013) Implementation and evaluation of multivariate analysis for groundwater hydrochemistry assessment in arid environments: a case study of Hajeb Elyoun-Jelma, Central Tunisia. Environ Earth Sci 70(5):2215–2224
Mamou A (1990) Caractéristiques, évaluation et gestion des ressources en eau du Sud-Tunisien. Doctoral dissertation, Paris 11
Monjerezi M, Vogt RD, Aagaard P, Saka JDK (2011) Hydro-geochemical processes in an area with saline groundwater in lower Shire River valley, Malawi: an integrated application of hierarchical cluster and principal component analysis. Appl Geochem 26:1399–1413
Moya CE, Raiber M, Taulis M, Cox ME (2015) Hydrochemical evolution and groundwater flow processes in the Galilee and Eromanga basins, Great Artesian Basin, Australia: a multivariate statistical approach. Sci Total Environ 508:411–426
Narasimha G, Sridevi A, Buddolla V, Subhosh CM, Rajasekhar RB (2006) Nutrient effects on production of cellulolytic enzymes by Aspergillus niger. Afr J Biotechnol 5(5):472–476
Ncibi K, Gaaloul N, Gasmi A (2016) Contribution de l’analyse multivariée et des SIG pour la caractérisation hydrochimique de la nappe phréatique de la plaine de Sidi Bouzid (Tunisie centrale)/[Contribution of the multivariate analysis and the GIS for Hydrochemical characterization of phreatic aquifer to the plain of Sidi Bouzid (Central Tunisia)]. Int J Innov Appl Stud 15(3):667
Parkhurst DL, Appelo CAJ (2013) Description of input and examples for PHREEQC version 3-A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations, U.S. geological survey techniques and methods
Piper AM (1944) A graphic procedure in the geochemical interpretation of water‐analyses. Eos Trans Am Geophys Union 25(6):914–928
Raghunath HM (1987) Ground water. New Age International, Seborga
Ramesh K, Elango L (2011) Groundwater quality and its suitability for domestic and agricultural use in Tondiar river basin, Tamil Nadu, India. J Environ Monit Assess. https://doi.org/10.1007/s10661-011-2231-3
Razali NM, Wah YB (2011) Power comparisons of Shapiro–Wilk, Kolmogorov–Smirnov, Lilliefors and Anderson–Darling tests. J Stat Model Anal 2(1):21–33
Reimann C, Filzmoser P, Garrett R, Dutter R (2008) Statistical data analysis explained: applied environmental statistics with R. Wiley, Chichester
Richards LA (1954) (US Salinity Laboratory) Diagnosis and improvement of saline and alkaline soils, US Department of Agriculture hand book
Simler R (2009) Diagrammes. Laboratoire d’Hydrogéologie d’Avignon, Avignon
Szabolcs I, Darab C (1964) The influence of irrigation water of high sodium carbonate content of soils. In: Proceedings of 8th international congress of ISSS, Trans, II (pp 803–812)
Thorne DW, Peterson HB (1954) Irrigated soils: their fertility and management. Blakiston, New York
Voutsis N, Kelepertzis E, Tziritis E, Kelepertsis A (2015) Assessing the hydrogeochemistry of groundwaters in ophiolite areas of Euboea Island, Greece, using multivariate statistical methods. J Geochem Explor 159:79–92
Wanda E, Monjerezi M, Mwatseteza JF, Kazembe LN (2011) Hydro-geochemical appraisal of groundwater quality from weathered basement aquifers in Northern Malawi. Phys Chem Earth Parts A/B/C 36(14):1197–1207
Ward JH (1963) Hierarchical grouping to optimize an objective function. J Am Stat Assoc 58:236–244
Wilcox LV (1955) Classification and use of irrigation waters, vol 969. USDA Circular, Washington, DC, USA
Williams B, Onsman A, Brown T (2010) Exploratory factor analysis: a five-step guide for novices. J Emerg Primary Health Care (JEPHC) 8(3):990399-1–990399-13
Zabala ME, Martínez S, Manzano M, Vives L (2016) Groundwater chemical baseline values to assess the recovery plan in the Matanza-Riachuelo River basin, Argentina. Sci Total Environ 541:1516–1530
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ayed, B., Jmal, I., Sahal, S. et al. Hydrochemical characterization of groundwater using multivariate statistical analysis: the Maritime Djeffara shallow aquifer (Southeastern Tunisia). Environ Earth Sci 76, 821 (2017). https://doi.org/10.1007/s12665-017-7168-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-017-7168-6