Abstract
Coimbatore district is facing problems of groundwater depletion, and the quality of groundwater deteriorates to a greater extent. This study aims to determine the contaminants in groundwater and the sources of contamination in Coimbatore district. Multivariate statistical analysis, cluster analysis (CA) and principal component analysis (PCA) were performed on water quality data of twenty-seven samples collected within Coimbatore district (India), during the pre-monsoon period of the years 2012 and 2013 to study the hydrogeochemical regimes. The correlation between major ions was obtained from PCA, and the factor scores from PCA were used to classify the samples by CA. The possible factors which cause contamination were identified. The electrical conductivity distribution shows that the northern taluks of the district are much more polluted than the southern taluks due to leaching of ions. In the year 2012, the ions dominance was in the order of Mg2+ > Na+ > Ca2+ > K+ = Cl− > HCO3 − > SO4 2− > NO3 − and in the year 2013 the ions dominance was in the order of Na+ > Ca2+ > Mg2+ > K+ = Cl− > HCO3 − > SO4 2− > NO3 −. The statistical results reveal that carbonate dissolution, silicate weathering, ion exchange, leaching of industrial effluents and leaching of fertilizers from agricultural lands alter the groundwater chemistry. CA classified the sampling sites into three groups based on the nature of pollution. Eastern side sampling sites are clustered together, which has more ion concentration due to the flow of groundwater from west to east and more residence time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater contributes to the major source of drinking water in most of the urban and rural areas. It is also an alternative source for irrigation, industrial and commercial purposes (Mishra and Bhatt 2008). Nowadays, the quality of the groundwater deteriorates due to natural and human activities. Therefore, groundwater quality monitoring is given higher priority in environmental protection policies (Mahmood et al. 2011). Geostatistics methods such as inverse distance weighted, Kriging and co-Kriging were used to predict the spatial distribution of groundwater characteristics and to interpolate the groundwater quality (Mehrjardi et al. 2008). The multivariate techniques can be effectively used to demarcate regional and geological factors controlling water chemistry (Vasanthavigar et al. 2009). Multivariate mapping techniques results are useful for the hydrogeochemical spatial variability studies (Arabi et al. 2013). Statistical hydrochemical analysis shows groundwater contamination is closely associated with land use classes and activities (Matiatos and Evelpidou 2013). Multivariate statistical techniques are useful for complex data interpretation, to identify pollution source and water quality variations within a lake base (Kazi et al. 2008). Factor analysis can be used to study anthropogenic effect and intrusion of salt water into the groundwater (SenthilKumar et al. 2008). Principal component analysis, factor analysis and cluster analysis (CA) can be used to evaluate temperate and spatial variations to interpret large water quality data sets (Yang et al. 2009). The possible sources that influence water systems can be identified by these techniques (Reghunath et al. 2002; Lambrakis et al. 2004; Nosrati and Van Den Eeckhaut 2012).
Davraz (2015) studied the groundwater contamination in southwestern Anatolia, Turkey, and concluded that Mn, Al, Fe, As and F contents of groundwater were above critical values. Edet (2009) used factor analysis to determine the source of ions and total hydrocarbon content in the groundwater of Niger delta, Nigeria. Five factors which cause contamination in the groundwater were determined.
In Coimbatore district, surface water sources including river, reservoirs and groundwater contribute to drinking water. The groundwater along Noyyal is not suitable for drinking and irrigation purposes (Santhosh and Revathi 2014). In most of the places along Noyyal River in Coimbatore district, the groundwater is not suitable for irrigation and industrial purpose because of high range of electrical conductivity and total hardness (Sundar and Saseetharan 2008). The Noyyal River of Cauvery basin, which is feeding the different tanks, is polluted by domestic and industrial sewage (Priya et al. 2011). The groundwater quality was unfit for drinking in some areas within Singanallur sub-basin of Coimbatore district scoring a water quality index greater than 100 (Priya et al. 2012). Seasonal variations in the groundwater quality of Coimbatore city were analyzed, and the changes in quality based on water quality index are represented using ArcGIS (Jebastina and Prince Arulraj 2015). Evaporation, silicate weathering, ion exchange and dissolution of carbonate minerals are the major geochemical processes in the district (Sajil Kumar and James 2016).
The objective of the present study is to determine the contaminants in groundwater and the source of contaminants using statistical methods.
Study area
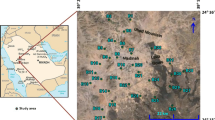
Coimbatore district is situated in the western part of Tamil Nadu, India, which lies between 10°10′N and 11°30′N latitude and 76°40′E and 77°30′E longitude. The total area of the district is 4850 km2. The average annual rainfall ranges between 550 and 900 mm. The main rivers in the district are Bhavani, Noyyal, Amaravathi and Ponnani. The temperature of the district ranges from 19.8 to 35.9 °C. The geological formations of the district are Archean crystallines, alluvial and colluvial formations (CGWB 2008). The district is divided into eight taluks, namely Annur, Coimbatore North, Coimbatore South, Kinathukadavu, Mettupalayam, Pollachi, Sulur and Valparai. The study area with the sampling points is shown in Fig. 1.
Sample collection and analysis
Twenty-seven borewell samples were collected from the study area during pre-monsoon period of 2012 and 2013. The collected samples were analyzed for pH using pH meter, EC using conductivity meter and TDS by gravimetric method. The samples were analyzed for major ions by adopting standard methods (APHA 1998). The amount of chlorides (Cl−) was determined by standard silver nitrate titration. Sodium (Na+) and potassium (K+) were determined by flame photometry, whereas calcium (Ca2+) and magnesium (Mg2+) were determined by titration with standard EDTA. Nitrate (NO3 −) and fluoride (F−) were determined by ion-sensitive electrode. The amount of sulfate (SO4 2−) was determined by spectrophotometer and bicarbonate (HCO3 −) by titration.
Data analysis
Statistical computations were done using the software EXCEL 2003 and SPSS 20 (Statistical Package for the Social Sciences). Deterministic interpolation techniques create surfaces from measured points, based on the extent of similarity (inverse distance weighted) or based on the degree of smoothing. Inverse distance weighted method was used to create the spatial distribution maps of electrical conductivity. Spatial distribution of factor scores was done using the software ArcGIS 10.1.
Results
The descriptive statistics of groundwater quality based on the twenty-seven water samples during 2012 and 2013 are summarized in Table 1. It gives the minimum, maximum, mean and standard deviation of the results for each of the twelve parameters.
Box plot is a statistical tool used to represent the parameters influencing water chemistry in the study area. The variations of the major groundwater ion concentrations are shown in box and whisker plot (Fig. 2a, b). Mg2+, Na+, Ca2+, K+, Cl−, HCO3 −, SO4 2−, NO3 − and F− ions influence in the year 2012. Na+, Ca2+, Mg2+, K+, Cl−, HCO3 −, SO4 2−, NO3 − and F− ions influence in the year 2013.
Electrical conductivity is a good indicator to assess the quality of groundwater. Electrical conductivity concentration depends on basic rock type, soil and the amount of rainfall received (Balachandar et al. 2010). Spatial distribution of electrical conductivity is shown in Fig. 3a, b during the years 2012 and 2013.
Statistical analysis including correlation analysis, principal component analysis (PCA) and CA was performed on the groundwater quality data to ascertain the relationship between variables, to determine the source of ions in groundwater and to group the sampling sites. A correlation analysis was performed to describe the degree of relation between two hydrochemical parameters. The interrelationships between the variables are determined using the correlation matrix. The correlation matrices for the years 2012 and 2013 are represented in Tables 2 and 3, respectively.
The number of principal components considered to understand the underlying data structure is given by the scree plot (Liu et al. 2003). From the scree plots (Fig. 4a, b), a drop in slope is noted after three components in the year 2012 and two components in the year 2013. Therefore, two principal components from both the years are taken during the present study which explains about 77% of the total variance for both the years.
The variability among observed variables is described by factor analysis in terms of factors. A factor is said to be significant if its eigenvalue is greater than unity. The factor analysis score is given in Table 4.
During the year 2012, factor 1 contributes to 61% of the total variance with high loadings of Ca2+, Mg2+, Cl−, SO4 2−, EC and TDS. Factor 2 contributes to 16% of the total variance. During the year 2013, factor 1 contributes to 66% of total variance with high loadings of Na2+, Mg2+, Cl−, HCO3 −, SO4 2−, NO3 −, K+, F−, EC and TDS. Factor 2 contributes to 12% of total variance with high loadings of pH, which indicates biogenic control of pH value. To determine the spatial variation of factor representation and to identify zone of representation, factor scores were calculated. Factor scores are computed for each sample by a matrix multiplication of the factor score coefficient with the standardized data. The value of each factor score represents the importance of a given factor at the sample site. A factor score >+1 indicates intense influence by the process. Very negative values <−1 reflect areas virtually unaffected by the process, while near-zero scores reflect areas with only moderate effect of the process (Prasanna et al. 2010). Regression method was used to determine factor score in the study area. Spatial variation of factor score is done using ArcGIS 10.1. The factor score values of each sampling point are given in Figs. 5a, b and 6a, b.
A CA classifies a set of samples into classified groups called clusters. CA methods are hierarchical clustering, two-way joining (block clustering) and k-means clustering. Hierarchical Q mode CA was performed to group the sampling sites. The data were represented by a horizontal hierarchical tree plot or dendrogram (Fig. 7) with three individual groups in both the years.
Discussion
Hydrogeochemical parameters
The values of the hydrogeochemical parameters presented in Table 1 show large fluctuations during both the years. The pH value shows that the water is slightly alkaline in both the years. Maximum pH values are recorded in Pollachi and Kinathukadavu taluks which are predominated by Archean crystalline rock. Electrical conductivity ranges from 120 to 5200 and 170 to 5740 µs/cm, respectively, during the years 2012 and 2013. Higher EC values were noted in the northern taluks during both the years. The concentration of Ca2+ ranges from 8 to 140 and 2 to 152 mg/L during the years 2012 and 2013, respectively. The average values of Mg2+ are 50.09 and 32.67 mg/L during the years 2012 and 2013. Higher concentrations of Ca2+ and Mg2+ are due to dissolution of carbonate minerals (Nosrati and Van Den Eeckhaut 2012). High amount of Na+ ion was noted in northeast and southeast region. This is due to the direction of flow of groundwater and residence time (Sajil Kumar and James 2016). Concentration of K+ averages 20.07 mg/L in the year 2012 and 18.89 mg/L in the year 2013. Higher concentration is observed in Pollachi, Kinathukadavu and Mettupalayam taluks, which is due to leaching from agricultural areas (Juned and Arjun 2011).
Natural geological processes contribute to the concentration of bicarbonates. Eastern side taluks have low concentration of bicarbonate as the movement of groundwater in the district is from western to eastern side (Sajil Kumar and James 2016). Mettupalayam taluk is found to have higher Cl− concentration during both the years, which is predominated by peninsular gneiss rock type. Higher concentration of SO4 2− (76.58 mg/L) was observed during the year 2012, which can be due to oxidation of industrial sulfur gases entering the ground during precipitation (Saxena 2004). Higher concentration of NO3 − was observed during the year 2013, which is due to leaching from agricultural areas (Nolan et al. 1998). Disposal of human and domestic wastes into the environment also contributes to the NO3 − concentration in groundwater (Edet 2009). The increase of F− content in groundwater originates from the solution of apatite and more commonly from the solution of fluoride-bearing micas (Davraz 2015).
Box and whisker plot in Fig. 2a shows that Mg2+, Na+, Ca2+, K+, Cl−, HCO3 −, SO4 2−, NO3 − and F− ions influence in the year 2012. The source of magnesium may be from dissolution of gneiss. The origin of Cl− is usually from dissolution of halite in groundwater (Mullaney et al. 2009). Figure 2b shows that Na+, Ca2+, Mg2+, K+, Cl−, HCO3 −, SO4 2−, NO3 − and F− ions influence in the year 2013. Gneiss is the major geological formation in the study area which contributes to the higher concentration of Na+, Ca2+, K+ and HCO3 − (Sajil Kumar and James 2016). High concentration of fluoride in groundwater is associated with rock types, especially granite and gneiss (Kim et al. 2011).
The spatial distribution of electrical conductivity in Fig. 3a, b shows that higher values of EC (>5000 µS/cm) are observed in the northern taluks, indicating polluted nature of groundwater due to leaching of ions (Thilagavathi et al. 2012). Industrial disposal and domestic sewage also contribute to this (Uwidia and Ademoroti 2011). The southern taluks have lower concentration of EC, indicating good quality of groundwater.
Statistical analysis
Computation of correlation matrix is the foremost step in multivariate statistical analysis. The correlation matrix for the years 2012 and 2013 is shown in Tables 2 and 3, respectively. Correlation coefficient gives the extent of statistical relationship between the parameters. Correlation coefficient >0.7 represents excellent correlation, and correlation coefficient <0.3 represents poor correlation. In the study area, during both the years there exists a good correlation between EC and TDS due to linear correlation between dissolved ions and conductivity. In 2012, there is an excellent correlation between Mg2+ and Cl−. Good correlation exists between Ca2+–Mg2+, Na+–K+, Na+–HCO3 −, K+–HCO3 −, Ca2+–Cl−. Poor correlation exists between F− and other ions. Correlation of Cl− with Mg2+ and Ca2+ indicates dissolution and leaching of salts (Prasanna et al. 2010). Correlation of HCO3 − with Na+ and K+ indicates chemical weathering (Prasanna et al. 2010). Significant correlation of SO4 2− with other ions and NO3 − with K+ indicates influence of human activities on the system (Srinivasamoorthy et al. 2012). In 2013, there exists an excellent correlation between Na+ and Cl− indicating silicate weathering and anthropogenic sources (Srinivasamoorthy et al. 2012). Good correlation exists between Mg2+–Na+, HCO3 −–Mg2+, HCO3 −–Na+. There exists a good correlation of Cl− with Ca2+, Mg2+, Na+ indicating dissolution of salts (Prasanna et al. 2010). There exists a good correlation of SO4 2− with other ions indicating anthropogenic impact (Odat 2015). Poor correlation exists between F− and other ions.
PCA is used to identify patterns by compressing the data without much loss of information (Smith 2002). PCA converts the original variables into uncorrelated new variables called principal components, which are linear combinations of original variables (Sarbu and Pop 2005). From the scree plots (Fig. 4a, b), two principal components from both the years are taken in the present study. R mode factor analysis gives useful information concerning relationships between elements and is more widely used for the interpretation of geochemical data (Odokuma-Alonge and Adekoya 2013). The factor analysis score is given in Table 4. During the year 2012, factor 1 contributes to 61% of the total variance with high loadings of Ca2+, Mg2+, Cl−, SO4 2−, EC and TDS. The influence of Ca2+, Mg2+ may be due to carbonate dissolution (Prasanna et al. 2010). The influence of Cl− may be from secondary salt precipitation due to agricultural return flow (Kumar et al. 2009). The influence of SO4 2− is due to weathering action of dissolution of gypsum. Factor 2 contributes to 16% of the total variance. During the year 2013, factor 1 contributes to 66% of total variance with high loadings of Na2+, Mg2+, Cl−, HCO3 −, SO4 2−, NO3 −, K+, F−, EC and TDS. High loading of Na2+ indicates natural weathering and ion exchange process (Drever 1997). Influence of Cl− indicates leaching of industrial effluents (Kumar and Alappat 2005) Mg2+ and HCO3 − from silicate weathering of rocks; SO4 2− is due to weathering action of dissolution of gypsum; K+, NO3 − might be from leaching of fertilizers from agriculture land (Mahlknecht et al. 2004); and F− indicates leaching from fluoride-rich minerals. Factor 2 contributes to 12% of total variance with high loadings of pH, which indicates biogenic control of pH value. The factor score values are shown in Figs. 5a, b and 6a, b. Factor I and factor II show that the northern taluks and the eastern sides have high factor score than the western side. Figure 7 shows the dendrogram of hierarchical Q mode CA. “Distance” in CA is used to represent how close each pair of objects is. Euclidean distance is the most common distance measurement in CA. The Euclidean distance between any two objects i and k (d ik) is given by Eq. 1.
In CA, it is desirable that the distances between objects within a cluster are small and the distances between different clusters are large (Yang 2004). The distance between clusters is determined by the methods which determine the relationship between clusters called linkage. There are several linkage methods such as complete linkage, single linkage, average linkage and Ward’s linkage. Ward (1963) proposed a clustering procedure seeking to form the partitions P n, P n−1,…, P 1 in a manner that minimizes the loss associated with each grouping and to quantify that loss in a form that is readily interpretable. From Fig. 7, group I sites (1, 3, 7, 8, 9, 11, 13, 14, 15, 16, 18, 20, 26) are from western side of the study area which is less polluted. Group II sites (2, 4, 5, 10, 12, 17, 19, 21, 22, 23, 24, 25, 27) are from eastern side of the study area which has more ions concentration due to flow of groundwater toward east (Sajil Kumar and James 2016). Group III site (6) has more EC (>5000 µs/cm).
Conclusions
Carbonate dissolution, silicate weathering, ion exchange, leaching of industrial effluents and leaching of fertilizers from agricultural lands alter the groundwater chemistry. Mg2+, Na2+, Cl−, HCO3 − ions dominate the quality of groundwater during the year 2012, and Na2+, Ca2+, Cl− and HCO3 − ions dominate the quality of groundwater during the year 2013. Ion exchange process and dissolution of gypsum have taken place. Spatial distribution of EC shows that northern taluks of the district have higher concentration of EC. EC and TDS are highly correlated. Two factors were derived from factor analysis with 66% of the total variance which shows carbonate and gypsum dissolution, anthropogenic activities and industrial discharge in the study area. Three clusters were formed by Q mode CA from which the eastern side sampling sites of the study area are having more ion concentration due to the groundwater flow direction and residence time.
References
American Public Health Association (APHA) (1998) Standard methods for the examination of water and wastewater, 19th edn. APHA, Washington, pp 3–180
Arabi NE, Idris Y, Fekry A (2013) Temporal and spatial change detection of variations in the groundwater composition by multivariate statistical techniques. NY Sci J 6:38–48
Balachandar D, Sundararaj P, Murthy RK, Kumaraswamy K (2010) An investigation of groundwater quality and its suitability to irrigated agriculture in Coimbatore district, Tamil Nadu, India—a GIS approach. Int J Environ Sci 1(2):176–190
Central Ground Water Board (CGWB) (2008) District groundwater brochure Coimbatore district, Tamilnadu. CGWB technical report series, pp 1–15
Davraz A (2015) Studies of geogenic groundwater contamination in southwestern Anatolia, Turkey. Procedia Earth Planet Sci 15:435–441
Drever JI (1997) The geochemistry of natural waters, 3rd edn. Prentice Hall, Englewood, p 436
Edet A (2009) Characterization of groundwater contamination using factor analysis in the Niger Delta (Nigeria). J Hydrol Eng 14(11):1255–1261
Jebastina N, Prince Arulraj G (2015) Seasonal variation of groundwater quality in Coimbatore district using GIS. Int J Environ Sci 10(6):1949–1956
Juned SA, Arjun BB (2011) Analysis of chloride, sodium and potassium in groundwater samples of Nanded city in Mahabharata, India. Eur J Exp Biol 1(1):74–82
Kazi TG, Arain MB, Jamali MK, Jalbani N, Afridi HI, Sarfraz RA, Baig JA, Shah AQ (2008) Assessment of water quality of polluted lake using multivariate statistical techniques: a case study. Ecotoxicol Environ Saf 72:301–309
Kim Y, Kim JY, Kim K (2011) Geochemical characteristics of fluoride in groundwater of Gimcheon, Korea: lithogenic and agricultural origins. Environ Earth Sci 63:1139–1148. doi:10.1007/s12665-010-0789-7
Kumar D, Alappat BJ (2005) Analysis of leachate pollution index and formulation of sub-leachate pollution indices. Waste Manag Res 23(3):230–239
Kumar M, Ramanathan AI, Keshari AK (2009) Understanding the extent of interactions between groundwater and surface water through major ion chemistry and multivariate statistical techniques. Hydrol Process 23:297–310. doi:10.1002/hyp.7149
Lambrakis N, Antonakos A, Panagopoulos G (2004) The use of multicomponent statistical analysis in hydrogeological environmental research. Water Res 38:1862–1872
Liu CW, Lin KH, Kuo YM (2003) Application of factor analysis in the assessment of groundwater quality in a Blackfoot disease area in Taiwan. Sci Total Environ 313:77–89. doi:10.1016/S0048-9697(02)00683-6
Mahlknecht J, Steinich B, de Navarro LL (2004) Groundwater chemistry and mass transfers in the independence aquifer, central Mexico by using multivariate statistics and mass balance models. Environ Geol 45:781–795
Mahmood A, Muqbool W, Mumtaz MW, Ahmad F (2011) Application of multivariate statistical techniques for the characterization of groundwater quality of Lahore, Gujranwala and Sialkot (Pakistan). Pak J Anal Environ Chem 12:102–112
Matiatos I, Evelpidou N (2013) Assessment of groundwater quality contamination by nitrate leaching using multivariate statistics and Geographic Information Systems. In: Understanding freshwater quality problems in a changing world—proceedings of H04, IAHS-IAPSO-IASPEI Assembly 361:183–190
Mehrjardi RT, Jahromi MZ, Mahmodi Sh, Heidari A (2008) Spatial distribution of groundwater quality with geostatistics (case study: Yazd-Ardakan Plain). World Appl Sci J 4:09–17
Mishra A, Bhatt V (2008) Physico-chemical and microbiological analysis of under ground water in V.V Nagar and nearby places of Anand district, Gujarat, India. E-J Chem 5:487–492
Mullaney JR, Lorenz DL, Arntson AD (2009) Chloride in groundwater and surface water in areas underlain by the glacial aquifer system, Northern United States. Sci Investig Rep 2009–5086:1–41
Nolan BT, Ruddy BC, Hitt KJ, Helsel DR (1998) A national look at nitrate contamination of ground water. Natl Water-Qual Assess Progr 39(12):76–79
Nosrati K, Van Den Eeckhaut M (2012) Assessment of groundwater quality using multivariate statistical techniques in Hashtgerd Plain, Iran. Environ Earth Sci 65:331–344
Odat SA (2015) Cluster and factor analysis of groundwater in Mafraq Area, Jordan. Curr World Environ 10(2):422–431
Odokuma-Alonge O, Adekoya JA (2013) Factor analysis of stream sediment geochemical data from Onyami drainage system, Southwestern Nigeria. Int J Geosci 4:656–661
Prasanna MV, Chidambaram S, Srinivasamoorthy K (2010) Statistical analysis of the hydrogeochemical evolution of groundwater in hard and sedimentary aquifers system of Gadilam river basin, South India. J King Saud Univ (Sci) 22(3):133–145
Priya KL, Jebastina N, Prince Arulraj G (2011) Groundwater quality in the Singanallur sub-basin of Coimbatore city. J Ind Pollut Control 27(1):15–18
Priya KL, Jebastina N, Prince Arulraj G (2012) Assessment of water quality indices for groundwater in the Singanallur sub-basin, Coimbatore, India. Sci Rep 1:186. doi:10.4172/scientificreports.186
Reghunath R, Murthy TRS, Raghavan BR (2002) The utility of multivariate statistical techniques in hydrogeochemical studies: an example from Karnataka, India. Water Res 36:2437–2442
Sajil Kumar PJ, James EJ (2016) Identification of hydrogeochemical processes in the Coimbatore district, Tamil Nadu, India. Hydrol Sci J. doi:10.1080/02626667.2015.1022551
Santhosh P, Revathi D (2014) Hydrogeochemical analysis of ground water parameters in Coimbatore District, Tamilnadu. India Res J Chem Environ Sci 2:89–93
Sarbu C, Pop HF (2005) Principal component analysis versus fuzzy principal component analysis. A case study: the quality of Danube water (1985–1996). Talanta 65:1215–1220
Saxena VK (2004) Geothermal resources of India. Allied Publishers, Chennai, pp 48–70
Senthilkumar G, Ramanathan AL, Nainwal HC, Chidambaram S (2008) Evaluation of the hydro geochemistry of groundwater using factor analysis in the Cuddalore coastal region, TamilNadu, India. Indian J Mar Sci 37(2):181–185
Smith LI (2002) A tutorial on principal component analysis. http:www.cs.montana.edu
Srinivasamoorthy K, Vasanthavigar M, Chidambaram S, Anandhan P, Manivannan R, Rajivgandhi R (2012) Hydrochemistry of groundwater from Sarabanga minor basin, Tamilnadu, India. Proc Int Acad Ecol Environ Sci 2(3):193–203
Sundar LM, Saseetharan MK (2008) Ground water quality in Coimbatore, Tamilnadu along Noyyal river. J Environ Sci Eng 50(3):187–190
Thilagavathi R, Chidambaram S, Prasanna MV, Thivya C, Singaraja C (2012) A study on groundwater geochemistry and water quality in layered aquifers system of Pondicherry region, southeast India. Appl Water Sci 2(4):253–269
Uwidia IE, Ademoroti CMA (2011) Characterisation of domestic sewage from an estate in Warri, Nigeria. Int J Chem 3(3):81–86
Vasanthavigar M, Srinivasamoorthy K, Vijayaragavan K, Rajiv Ganthi R, Chidambaram S, Sarama VS, Anandhan P, Manivannan R, Vasudevan S (2009) Hydro geochemistry of Thirumanimuttar basin: an indication of weathering and anthropogenic impact. Int J Environ Res 3(4):617–628
Ward JH (1963) Hierarchical grouping to optimize an objective function. J Am Stat Assoc 58(301):236–244
Yang K (ed) (2004) Multivariate statistical methods and quality, Downloaded from Digital Engineering Library @ McGraw-Hill. http://www.digitalengineeringlibrary.com
Yang LI, Linyu XU, Shun L (2009) Water quality analysis of the Songhua river basin using multivariate techniques. J Water Resour Prot 2:110–121
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jebastina, N., Prince Arulraj, G. Contamination analysis of groundwater in Coimbatore district, India: a statistical approach. Environ Earth Sci 75, 1447 (2016). https://doi.org/10.1007/s12665-016-6253-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-016-6253-6