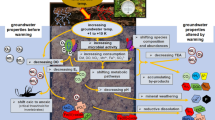
Abstract
Geothermal energy plays an increasingly important role as a renewable energy source. However, it induces temperature changes in natural thermally static groundwater ecosystems. Temperature impacts can considerably alter the groundwater chemical composition and quality, the metabolism of organisms, and, consequently, biogeochemical processes and ecosystem functions. Combining original data from current studies with a compact review of recent findings, we show that a moderate increase in groundwater/aquifer temperature [+5 to 10 Kelvin (K)] generally causes only minor changes in water chemistry, microbial biodiversity, and ecosystem function in non-contaminated and energy-poor (oligotrophic) groundwater systems. In aquifers that are contaminated with organics, nutrients, and heavy metals—typical in urban areas and at sites with intensive land use (e.g., agriculture)—and particularly at temperatures ≥30 °C as regularly reached when heat is actively stored in aquifers, significant changes in water quality and ecological patterns can result. Here most critical are the heat-related mobilization of organic matter and contaminants (e.g., arsenic), the reduction and depletion of dissolved oxygen, and consequently the consecutive shift to anaerobic redox processes that may produce toxic and corrosive products (e.g., hydrogen sulfide) and greenhouse gases (e.g., methane and carbon dioxide). Severe temperature alterations lead to a reduced biodiversity of the aquifer’s microbial community with the establishment of atypical thermophilic assemblages. Groundwater fauna, which is specifically adapted to the cold groundwater habitat, may be sensitive to thermal changes at temperature increases of only 5 K with long-term emigration or direct lethal effects. From an ecological point of view, long-lasting or reoccurring temperature alterations need to be carefully evaluated and regulated in the future. We suggest developing local and regional vulnerability concepts for the sustainable and ecologically sound use of subterranean heat and cold.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The use of geothermal energy plays an increasing role as a regenerative energy source (Lund and Boyd 2016). Extraction of heat and cold from shallow aquifers induces temperature alterations in the typically temperature-constant subsurface (e.g., Pannike et al. 2006; Hecht-Méndez et al. 2010; Hähnlein et al. 2010; Molina-Giraldo et al. 2011). Geothermal systems can be distinguished into closed-loop systems, such as vertical borehole heat exchangers and horizontal heat collectors, and open-loop systems, such as aquifer heat and cold storage systems and wells for the extraction and recharge of groundwater for cooling purposes (Malin and Wilson 2000). Dependent on the type of system, temperature changes range from a few Kelvin (K) in the direct vicinity of borehole heat exchangers and horizontal heat collectors (Griebler et al. 2015) to large heat plumes downgradient of process water recharge wells with water temperatures ≥20 °C (Brielmann et al. 2009). Aquifer heat storage systems may show groundwater temperature extremes of 60–80 °C or more (Würdemann et al. 2014).
Several hundred thousand closed-loop systems and thousands of shallow open-loop systems are operative in Germany (GtV BUNDESVERBAND GEOTHERMIE 2014). In contrast, while aquifer heat and cold storage systems are already abundant in some European countries, i.e., The Netherlands with >2000 systems (Bonte 2013), these types of geothermal energy use systems are still rare in Germany.
In addition to the positive aspects of the sustainable use of a natural infinite source of energy from the shallow subsurface, a critical discussion has recently gained momentum on how anthropogenically induced temperature changes in the subsurface impact the groundwater quality, organismic communities, and ecosystem function (Brielmann et al. 2008, 2011; Bonte 2013; Jesußek et al. 2013a, b; Griebler et al. 2015). Moreover, the reconcilability of geothermal energy use and drinking water production have been controversially discussed with respect to the possible risk of hygienic and chemical (e.g., heavy metals) impact on water quality. While individual guidelines and scientific publications recommend to keep geothermal facilities away from drinking water production plants (DVGW 2013; Bonte et al. 2011, 2013a; Hähnlein et al. 2013), other studies did not find evidence for an increased risk (Köber et al. 2015).
Since there is a clear lack of knowledge about the fast technological and economic development of geothermal energy use from the shallow subsurface, we provide a compilation of recent findings in terms of moderate temperature changes, groundwater quality, and ecosystem status. We briefly introduce original data on the effects of temperature alterations on aquifers to sediment-porewater composition obtained from a model exercise and batch experiments considering different geological settings and degrees of anthropogenic contamination. Moreover, results are introduced from sediment column experiments that evaluate temperature effects on microbiological patterns under oligotrophic conditions and mimic contamination with labile organic carbon and nutrients as well as with petroleum hydrocarbons. Further, recent studies are reviewed and critically discussed. Finally, we provide recommendations for the future management of heat and cold impact in the shallow subsurface.
Materials and methods
Modeling of sediment–porewater physicochemical equilibria
Changes in porewater chemical conditions with a consecutive shift in temperature were modeled for different hydrogeological units, including (i) sands and gravels of the Lower Rhine area, (ii) gravels and moraines of the alpine foothills, (iii) limestones of the alpine region, and (iv) sandstone layers of the early Triassic, to provide a range of environmental settings. Description of the individual hydrogeological units was provided by Kunkel et al. (2004). For modeling, the program environment Phreeqc (Parkhurst and Appelo 1999) version 2 was applied using the Lawrence Livermore National Laboratory (LLNL) aqueous model database (llnl.dat, Daveler and Wolery 1992). The model assumes equilibrium conditions in closed systems and neglects microbial activities (i.e., respiration and biomass growth).
Sediment–porewater batch experiments
Temperature-related changes in the porewater concentrations of dissolved organic carbon (DOC), nutrients, and major ions were assessed in batch tests conducted with aquifer sediments freshly collected from a shallow quaternary aquifer outside (referred to as non-contaminated) and from the central downtown area of (referred to as ‘contaminated’) the city of Munich, Germany. The shallow aquifers in the area of Munich belong to the hydrogeological unit gravels and moraines of the alpine foothills as characterized by Kunkel et al. (2004). Fifty microliters of sediment was incubated together with 150 mL of fresh 0.2 µm filtered groundwater from the respective sites in batch tests (triplicates) first overnight at 10 °C for acclimatization followed by incubation at 4, 10, 15, 20, 30, 45, 70, and 90 °C. Groundwater from the non-contaminated site incubated without sediment served as a sediment-free control. Small volumes (5 mL) of water from the batch tests were collected repeatedly over a time period of 5 and 12 days, respectively, for the analysis of DOC and selected ions/nutrients (K+, Na+, Ca2+, Mg2+, Cl−, NO3 −, NO2 −, NH4 +, and SO4 2−). DOC was analyzed as non-purgeable organic carbon in acidified samples using high-temperature combustion with infrared detection of CO2 on a TOC analyzer (Shimadzu TOC-5050). Ions were analyzed by ion chromatography (Dionex Model DX 100). Data acquisition was performed with the PEAKNET software (Dionex). For quantification, commercial standards were used (Fig. 1).
Experiments in mini-sediment columns
Column setup
Fresh sediments were obtained by drilling from an oligotrophic shallow quaternary aquifer (hydrogeological unit gravels and moraines of the alpine foothills) near Freising, Germany (Brielmann et al. 2009). Prior to the experiments, the screened medium sand fraction (0.2–0.63 mm) of the sediment was autoclaved 3 times for 60 min at 121 °C. Subsequently, the sediment was kept at 12 °C and continuously infiltrated with natural groundwater to establish a stable ‘natural’ microbial community. After 3 months of sediment conditioning, glass columns (100 mm length, 16 mm inner diameter) were packed with sediment and fully saturated. The average saturated pore volume was 5 cm3. Glass columns were closed with a stainless steel mesh and viton stopper (16 mm Ø, 12 mm length; Ochs) and protected from light with an aluminum foil cover. Stainless steel needles served as inlet and outlet ports. Viton tubing (Ismatec) and stainless steel capillaries of the same inner diameter (1 mm) were used to connect the columns to peristaltic pumps (Ismatec), the reservoir, and waste outlets (see Fig. 2 in Brielmann et al. 2011). The installation of copper T-brass connections close to the column inlets connected by stainless steel capillaries and viton tubing to either syringe or peristaltic pumps allowed us to control the experimental conditions as described below. A more detailed description of the mini-sediment-column setup is provided by Hofmann et al. (2016). Columns were operated in up-flow mode at an average pump rate of ~0.54 mL min−1 with values ranging from 0.45 to 0.65 mL min−1. Based on an average porosity of 0.31 determined by the appropriate volumetric and gravimetric measurements, the mean residence time of water in the columns was approximately 9 min. Thus, the average pore water flow velocity (12.4 m day−1) was within the range of natural groundwater flow velocities at the site of sampling.
Before specific treatments, the columns were kept for preadaptation at six distinct incubation temperatures at 4, 10 °C (ambient groundwater temperature), 15, 20, 30, and 45 °C for two (toluene addition) or three (substrate addition in form of R2A medium) months. The incubated aquifer sediments then faced two different treatments besides the temperature change, i.e., first the addition of modified R2A medium to overcome the possible limitation in DOC and major nutrients, and second the addition of toluene to simulate organic contamination with petroleum hydrocarbons. For each treatment, three replicates and two control columns were incubated at each temperature. The sediment sampling was preceded by a sampling of groundwater at the column outlets.
Supply of extra DOC and nutrients (R2A)
For a period of 1 week, a subset of the pre-incubated sediment columns (triplicates) were continuously supplied at each of the 6 temperatures with a modified R2A medium (Reasoner and Geldreich 1985) consisting of 0.5 g L−1 yeast extract, 0.5 g L−1 peptone, 0.5 g L−1 casamino acids, 0.5 g L−1 sodium acetate, 0.5 g L−1 soluble starch, 0.3 g L−1 sodium pyruvate, 0.3 g L−1 K2HPO4, and 0.024 g L−1 MgSO4 × 7H2O at pH 7.2. Via the T-brass connections, the R2A medium was mixed at the column inlets into the continuous groundwater supply. Syringe pump rates were adjusted to achieve an average DOC concentration of 2.8 ± 1.9 mg L−1 entering the columns. To avoid back growth of bacteria into the medium reservoir, the respective supply capillaries were equipped with sterile filters at both ends. Duplicate columns at each temperature receiving no medium addition (natural ingoing DOC concentration of 0.6 mg L−1) served as controls.
Contamination with a petroleum hydrocarbon (toluene)
For a period of three weeks, a subset of the sediment columns at each of the 6 temperatures received a continuous supply of toluene as a model petroleum hydrocarbon compound. Duplicate columns at each temperature received no toluene addition and served as controls. Oxygen-free groundwater containing toluene (200 mg L−1) was stored without headspace and protected from light in inert 5-L Tedlar bags (SKC, PA, USA). A glass syringe was used as a drip feed from which injection needles, Fluran® tubing (Ismatec), and stainless steel capillaries distributed the toluene mixture to the peristaltic pumps and further to the T-brass connections downstream the column inlets. Peristaltic pump rates were adjusted to achieve a toluene column influent concentration of about 1.5 mg L−1. A more detailed description of the mini-column setup is provided in Hofmann et al. (2016). The ratio of oxic groundwater to anoxic toluene medium was kept as high as possible to ensure well-oxygenated conditions and to avoid the dilution of nutrients. Toluene degradation efficiency was estimated from concentration differences between the respective column inlets and outlets. Toluene concentrations were obtained by GC–MS analysis as described in Bauer et al. (2008).
Porewater characteristics
The temperatures in the incubation chambers were independently measured with a thermometer. Porewater analysis further included pH measurements in the column influents and effluents using a pH electrode (WTW). DOC, nutrients, and major ions were analyzed as described above and in Brielmann et al. (2009). For each experimental setup at each temperature, planar oxygen sensor spots (PreSens GmbH, Regensburg, Germany) were attached to the inner wall at the upper and lower end of one column. Accordingly, porewater oxygen concentration/saturation was determined regularly as described elsewhere (Warkentin et al. 2007). Special care was taken to individually calibrate each spot according to the manufacturer’s specifications. Occasionally, the sediment-porewater microbial communities were analyzed for total cell counts (TCC) (data not shown).
Sediment microbiological characterization
At the end of the experiments, all columns were subjected to sediment microbiological analysis. The abundance of sediment prokaryotic cells was determined as TCC by means of flow cytometry (FCM) following the protocol described in Bayer et al. (2016). FCM measurements were performed using a BD LSR II flow cytometer equipped with a blue 488-nm laser. Following the method of Hammes and Egli (2005), green (B530) and red (B610) fluorescence intensities were collected at 550 and 650 nm, respectively, and combined to separate the bacterial populations from background particles and to minimize background noise. All analyses were performed at a minimum flow rate (about 10 µL min−1). The standard instrument error on FCM measurements was always below 5 %.
Bacterial production in the column sediments was estimated via leucine incorporation into bacterial protein (Kirchman 1993) following a modified protocol of Buesing and Gessner (2003). In short, three replicate samples and one blank sample (0.5 cm3 sediment each) were amended with non-labeled and [3H]-labeled leucine (final concentration 100 nM; ARC Research Products) and 750 µL of autoclaved, 0.22 µm filtered porewater. Blanks were stopped immediately with trichloroacetic acid [final concentration 5 % (v/v)], and replicates were stopped after incubation for 6 h at each respective incubation temperature. Samples were centrifuged at 15,000 g for 10 min, and the supernatant discarded. Following a washing step with Milli-Q water, the samples underwent an alkaline extraction (0.6 M NaOH, 25 mM EDTA, 0.1 % SDS) of the proteins on a thermomixer for 1 h at 99 °C and 1000 rpm. After cooling, the samples were centrifuged (15,000g; 10 min), and an aliquot (100 µL) of the supernatant was submitted to liquid scintillation counting.
Amplicon pyrosequencing of bacterial 16S rRNA genes was performed on a 454 GS FLX Titanium system (Roche, Penzberg, Germany) as reported by Pilloni et al. (2012). Briefly, bar-coded amplicons for multiplexing were prepared using the primers Ba27f and Ba519r and extended with the respective adapters, key sequences, and multiplex identifiers (MIDs) as recommended by Roche. Emulsion PCR was performed in a Mastercycler ep gradient (Eppendorf, Hamburg, Germany), and the amplicons were subsequently purified and sequenced following the manufacturer protocols (Pilloni et al. 2012). Quality filtering of the pyrosequencing reads was performed using the automatic amplicon pipeline of the GS Run Processor (Roche). Reads were further trimmed and de-noised as described previously (Pilloni et al. 2012; Karwautz and Lueders 2014). Read affiliation was done using the RDP classifier (Wang et al. 2007) with a confidence threshold set to 80 % (default).
Statistics
One-way ANOVA was used to test the hypothesis that there were no significant differences between several measurements of an individual variable within the selected temperature incubations. Here, the level of significance was set at P ≤ 0.001. If significant differences were obtained, in a second step, a multiple comparison test (Holm–Sidak) was run to identify the ones significantly different (significance level P ≤ 0.05) from the ‘natural condition’ (10 °C). Both tests were run in SigmaStat version 12.0.
Results and discussion
Groundwater quality is related to temperature changes
The chemical composition and quality of groundwater are determined by a multitude of factors including its origin, the properties of the aquifer matrix, the water residence time, as well as physicochemical and biological processes. A central parameter for water chemical composition in sedimentary systems is temperature, which is directly related to the density and viscosity of water, the solubility of gases, geochemical processes such as dissolution or precipitation of minerals, and biological processes. Changes in temperature consequently go along with abiotically and biotically induced compositional changes of the groundwater (e.g., Bonte et al. 2013a; Jesußek et al. 2013a).
Modeling of the porewater chemical composition with changing thermal conditions in four hydrogeological settings revealed considerable changes in concentration for some of the nutrients and major ions tested, particularly at temperatures ≥40 °C. In general, we observed a negative relationship between temperature and porewater pH (Fig. 2), which was not only caused by an increase in CO2. In fact, modeled CO2 did not show any pronounced change in the sandstone formation, but had a positive correlation with temperature in all other settings (Fig. 2). It is important to note that although CO2 at higher temperatures partitions from water to the atmosphere due to reduced gas solubility, the model assumes the system to be closed, leading to a pronounced CO2 increase in sediment-porewater. Similarly, porewater calcium and magnesium concentrations declined with increasing temperature for all hydrogeological settings tested except for the sandstone aquifer (Fig. 2). Sulfate, for example, showed a clear decreasing trend with increasing temperature for the sands and gravels of the Lower Rhine area only (Fig. 2). Nitrite increased in all porewater samples. However, the concentrations were below the routine analytical limit of detection (Fig. 2). For sodium, only a slight increase over time was indicated by the model. The model indicated no significant change with temperature for nitrate, chloride, and potassium, independent from the geological setting tested (Fig. 2).
Temperature-related changes in the porewater concentration of selected hydrochemical species as modeled for four different hydrogeochemical aquifer units using Preeqc. Note that the blue triangles (gravels and moraines of the alpine foothills) are hidden behind the green symbols (sandstones of the early Triassic) for nitrite, sodium, and sulfate
The model described above assumed equilibrium conditions in closed systems and ignored any microbial activities (i.e., respiration and biomass growth). Therefore, as an extension further open batch experiments were performed with groundwater and respective sediments from the hydrogeological unit gravels and moraines of the alpine foothills (Kunkel et al. 2004), mimicking processes in the shallow aquifer close to the groundwater table. Material was collected at a rural (referred to as non-contaminated) and at an urban (referred to as contaminated) location.
As depicted in Figs. 3, 4, and 5, contaminated and non-contaminated groundwater was considerably different in chemical composition. While groundwater from the non-contaminated aquifer outside Munich contained about 1 mg L−1 DOC, the contaminated groundwater from downtown Munich exhibited a fivefold higher concentration (Fig. 3). A striking difference was found with sulfate, which was below 10 mg L−1 in the non-contaminated groundwater and above 100 mg L−1 in groundwater from the urban area (Fig. 4). Groundwater from the contaminated aquifer was also higher in sodium, potassium, and calcium, but was lower in chloride (Fig. 5).
The experiments with the ‘non-contaminated’ and the ‘contaminated’ aquifer sediments revealed clear temperature-related dynamics with respect to organic matter. The concentration of DOC in the sediment-free control batch tests slightly increased with increasing temperature in the first 24 h of incubation before leveling off during the additional incubation period (data not shown). In sediment-porewaters, DOC concentration increased with increasing temperature and incubation time. In the non-contaminated set, this trend was not significant at temperatures below 45 °C (Fig. 3a). Here, variations were in the range of the analytical error. A systematic pattern was observed at temperatures between 70 and 90 °C, with a 2.5–12-fold increase in porewater DOC over time (Fig. 3a). Similarly, with the aquifer material from downtown Munich, the temperature increase led within 12 days to a significant mobilization of DOC at higher incubation temperatures (Fig. 3b). At 45 °C, the DOC concentration in the porewater doubled; at 70 °C, it increased by sixfold; and at 90 °C, a 30-fold DOC concentration was found (Fig. 3b).
In all control incubations containing groundwater without sediments, no effect or clear trend was observed with regard to the nutrients and major ions tested (Figs. 4, 5). With the non-contaminated aquifer material, nitrate and sulfate had elevated concentrations in sediment-porewater after 5 days of incubation. SO4 2− concentration increased at temperatures above 30 °C, and NO3 − increased at temperatures >45 °C (Fig. 4). With both species, the original concentrations increased by about 30 %. With the contaminated aquifer material, the effects were more pronounced. Sulfate porewater concentrations showed a striking positive correlation with temperature (Fig. 4). After five days of incubation, the sulfate level was twice as high at 90 °C compared to ambient conditions (10 °C). While nitrate did not show a systematic change with temperature, a positive correlation was detected for nitrite and ammonium. Both were detected in porewater at temperatures ≥30 and 45 °C (Fig. 4).
Sodium (Na+) as well as potassium (K+) showed an increase in concentration already in the control groundwater samples, while magnesium (Mg2+) decreased with temperature (Fig. 5). Calcium (Ca2+) showed a slight decrease at a moderate temperature increase before it decreased again at temperatures >70 °C (Fig. 5). No clear effects were obtained for chloride (Cl−) in the control groundwater. With the sediment incubations, the patterns were more pronounced. In the porewater of the non-contaminated aquifer material, Na+, K+, and Cl− showed a positive relationship with temperature. A negative relationship was observed with Ca2+ and Mg2+ decreasing at temperatures >45 °C (Fig. 5). The porewater of the contaminated aquifer material contained higher concentrations for all cations, but Mg2+. Temperature-related patterns followed similar trends as for the non-contaminated aquifer material, with an increase of Na+, K+, and Cl− and a decrease of Mg2+. However, here, no significant patterns were observed for Ca2+ (Fig. 5).
Sorption/desorption kinetics and dissolution/precipitation equilibria are temperature dependent. It is well documented that a substantial increase in temperature leads to the mobilization of organic matter (Brons et al. 1991; Christ and David 1996; Kaiser et al. 2001; Bonte et al. 2013a). In column experiments with Tertiary lignite sands, Jesußek et al. (2013a) observed a threefold release of DOC at 70 °C when compared to columns at ambient groundwater temperature. Similar results were obtained by Bonte et al. (2013a), who showed an increase in DOC at temperatures ≥60 °C with different sediments tested. The mechanisms behind the mobilization of DOC include desorption of DOC from sediment surfaces including metal oxides (Filius et al. 2000), as well as partial abiotic and biotic transformation of sediment particulate organic matter (Brons et al. 1991). Our data, in particular, emphasize that with sediments from aquifers with considerable organic contamination, DOC mobilization at high temperatures may exceed values reported so far, generating a vast pool of substrates and electron donors for microbial growth and activity (see the section on ‘ecosystem functions’ below).
An increase in water temperature leads to a reduction in the solubility of gases. Considering that dissolved oxygen (DO), which is required for aerobic processes, becomes less available when water gets warmer, and taking into account that microbial activities are at the same time stimulated and more OM becomes available, a switch from aerobic to anaerobic processes and from oxic to reduced conditions is likely at thermally impacted conditions (more details in the section on ‘ecosystem functions’ below). Besides DO, CO2 is also increasingly lost from heated waters (Brons et al. 1991), resulting in new equilibria, especially in carbonate-buffered systems. The decrease in Ca2+ and Mg2+ ions can be attributed partly to the outgassing of CO2 and the subsequent precipitation of Ca2+ and magnesium carbonate (Griffioen and Appelo 1993). While other studies did not reveal pronounced changes in Ca2+ and Mg2+ concentrations in sediment-porewaters at temperatures ≤40 °C (Jesußek et al. 2013a; Brons et al. 1991), our modeling approach showed that this is related to the sediment mineralogical composition. In the carbonate-rich sands and gravels, the effects are already visible at moderate temperature changes (Fig. 2). Similarly, Ca2+ concentrations already decreased in groundwater and sediment-porewater from the non-contaminated aquifer at temperatures ≥15 °C (Fig. 5). Mg2+ decreased at temperatures ≥30 °C. Only at high temperatures (70 °C), a decrease of Ca2+ and Mg2+ as well as an increase of K+ was observed in the column experiments of Jesußek et al. (2013a). With respect to Na+ and K+, our modeling indicated only a slight increase for Na+ but no change for K+ with temperature in the sediments tested (Fig. 2). However, in the batch tests with carbonaceous non-contaminated groundwater and aquifer material, a clear increase in both Na+ and K+ was observed, at 90 °C in the groundwater and at ≥70 °C in the sediment incubations (Fig. 5). No significant effects were observed in the sediment column experiments with a similar sediment material for a temperature range between 4 and 45 °C (Brielmann et al. 2011). Batch tests with the contaminated aquifer material showed a clear positive relationship between the two cations and temperature (Fig. 5). Differences between the in silico and batch study may be partly related to differences in the sediment mineral composition. The hydrogeological unit gravels and moraines of the alpine foothills are defined by a mean mineral composition that may not necessarily reflect the situation in the periphery of the city of Munich. At temperatures >50 °C, NaHCO3 precipitates to Na2CO3 while releasing CO2. Here, the carbonate partially binds to Ca2+ and Mg2+, reducing their amounts in solution by a concomitant increase in free Na+. Moreover, the modeling did not consider the contribution of any biological processes. Interrelated with the dissolution and precipitation of cations is the negative relationship between temperature and pH, which has been repeatedly reported (Balke 1978; Brielmann et al. 2011; Jesußek et al. 2013a; Bonte et al. 2013a; this study).
For the anions tested, the model did not indicate changes in the concentrations of nitrate and chloride, but with nitrite and sulfate, the latter, however, only in the sands and gravels of the Lower Rhine area (Fig. 2). The batch experiments underline these findings and extend them to some degree. Measureable changes in nitrite concentration were seen in the contaminated aquifer material. Here, ammonium also appeared (Fig. 4). The batch tests also revealed changes in porewater nitrate with higher temperatures, independent of contamination (Fig. 4). Most surprisingly were the findings for sulfate dynamics. While the model experiments revealed a decrease in sulfate with increasing temperature due to precipitation with Ca2+ to CaSO4, our batch experiments revealed the opposite pattern. One reason for the elevated sulfate concentrations in groundwater from the city sediments is sulfate contained and leached from construction and artificial fillings with construction waste (Jang and Townsend 2001). Sulfate is contained in bricks, gypsum wallboards, and gypsum and anhydrite [CaSO4] which are basic ingredients of cement. An increase in sulfate in solution with elevated temperature may be related to a different dissolution behavior of individual sulfate minerals not considered in the model. Changes in nitrate and sulfate concentrations have also been reported in other studies, however, here in combination with microbial activity, i.e., nitrate and sulfate reduction, which consequently led to a decrease and/or complete depletion of these anaerobic electron acceptors (Jesußek et al. 2013a, b; Bonte et al. 2013a).
Most studies available conclude that a moderate increase in temperature has little impact on the quality of groundwater and drinking water (Brielmann et al. 2009, 2011; Jesußek et al. 2013a; Possemiers et al. 2014; this study); in particular, this is the case when the groundwater is of good quality as characterized by low concentrations of DOC and nutrients as well as the absence of contaminants. However, it has been shown that in anoxic aquifers, a moderate increase in aquifer temperature from 10 to 25 °C can mobilize sediment-bound arsenic (Bonte et al. 2013a). Additionally, at temperatures of 60 °C, increased concentrations of molybdenum, boron, vanadium, and fluorine were detected in sediment-porewater (Bonte et al. 2013a). The authors suggest the mobilization of these trace elements to be related to the temperature-related increased solubility of silicates, a process reported by numerous authors (Carroll and Walther 1990; Köhler et al. 2003; Arning et al. 2006; Jesußek et al. 2013a, b).
Groundwater temperature alterations and effects on biological patterns
Since groundwater ecosystems are generally poor in biodegradable carbon, nutrients, and energy, a weak resistance and resilience against disturbances such as temperature alterations are assumed (Griebler et al. 2014). Temperature, in particular, is a key factor influencing the physiological activity, growth, and consequently the presence/absence of individual groups of organisms and species (Lengeler et al. 1999). Organisms in groundwater are physiologically adapted to constant environmental conditions, such as the constant groundwater temperature, with an often narrow range of high performance and tolerance, specifically for Metazoans. Groundwater temperatures resemble the local mean annual air temperature, with values in Germany typically ranging between 9 and 14 °C (Tiehm et al. 2012) and an annual fluctuation of ±1 °C. As known from other ecosystems, changes in the temperature regime (e.g., climate change) cause dynamic changes in community composition and ecosystem processes (Zogg et al. 1997; Bradford et al. 2008; Castro et al. 2010; Yergeau et al. 2012). A similar effect is thus true for groundwater ecosystems.
Ecosystem functions impacted by changes in thermal conditions and OM availability
Although still hardly considered, organisms in aquifers—in particular microorganisms—are related to important ecosystem services, including the natural purification of the infiltrating water, the biodegradation of pollutants, the elimination of pathogens, and carbon and nutrient cycling (Griebler and Avramov 2015). An increase in temperature generally leads to the enhancement of metabolic activities and a decrease in temperature to an activity reduction. It is, however, important to consider that every (eco) species has its individual temperature range with an optimum and a lower and upper limit for individual activities (e.g., growth). Thus, the moment the tolerated temperature range of an individual species is exceeded, activities will cease, and then, organisms will switch to dormancy or die (Price and Sowers 2004). It is expected that with alterations of groundwater and aquifer temperature, biologically mediated processes will potentially be stimulated or impaired. Consequently, results from laboratory cultures need to be interpreted with caution when dealing with complex communities in the environment.
A field study targeting an oligotrophic shallow porous aquifer impacted by the recharge of warm process water revealed no significant changes in groundwater microbial activities, such as bacterial carbon production (carbon turnover) and extracellular phosphatase activity (P acquisition), in the temperature range of 8–18 °C (Brielmann et al. 2009). Similarly, the microbial biomass (total prokaryotic cell counts), a result of bacterial growth, did not show any significant differences in groundwater samples from temperature-impacted and non-impacted areas. This early study was later evaluated in laboratory sediment column experiments. Using the same aquifer material, the temperature impact was experimentally extended to 45 °C, and the analysis included not only groundwater but the sediment fraction, which carries the majority of the microbial biomass (Alfreider et al. 1997; Griebler et al. 2002). More recently, microbial-driven carbon turnover and nutrient acquisition were found to be significantly changed at temperatures ≥20 °C (Brielmann et al. 2011). These observations are quoted as evidence for changed ecosystem functions. The authors of both studies, the field and sediment column study, speculated that the energetic limitations (lack of sufficient labile DOC and essential nutrients) present in the studied aquifer caused the small changes observed at temperatures ≤20 °C (Brielmann et al. 2009, 2011). Consequently, in anthropogenic impacted aquifers, e.g., at urban sites and in areas of intense land use, significant changes in microbial activities and biodiversity may be expected at small temperature changes (±5 K) (Brielmann et al. 2011).
Building on the experiments of Brielmann et al. (2011), the sediment columns were run simulating two types of contamination, i.e., (i) a simultaneous increase in labile dissolved organic carbon and nutrients and (ii) a moderate petroleum hydrocarbon contamination (in this study, toluene). Both treatments were compared to control columns that were run under close to in situ conditions with respect to groundwater and sediment characteristics, but were incubated as well at different temperatures, i.e., at lower (4 °C) and higher (15–45 °C) temperatures when compared to the ambient aquifer temperature (10 °C, referred to as the reference columns). The results depicted in Fig. 6 strikingly underline the effects when overcoming the energetic limitations for microbes in oligotrophic aquifers. At all temperatures, the number of bacteria (as measured by TCC) attached to the sediment as well as their growth performance (as measured by BCP) was significantly higher (P ≤ 0.001) in the two treatments with carbon addition (labile DOC and toluene) than in the uncontaminated columns. TCCs in general were two orders of magnitude higher in the substrate-fed columns and more than one order of magnitude higher in the toluene-fed columns. With respect to BCP, the substrate-fed columns exhibited one order of magnitude higher rates than the controls. In the toluene-fed columns, BCP increased on average by a factor of 2.5. However, while both parameters, TCC and BCP, sensitively reacted to the addition of substrate and toluene at all temperatures tested, they did not reveal significant changes within the different temperatures in the contaminated sediments when compared to the control (10 °C). The only exceptions were the toluene-contaminated sediments incubated at 45 °C (Fig. 6). We also observed that treatment with organic carbon was followed by its aerobic degradation, resulting in a significant lowering of porewater oxygen (DO) concentrations. Where additional DOC and nutrients were continuously fed to the columns, the DO concentration decreased by about 4 mg L−1 compared to the non-fed columns (Fig. 6), which was in line with the amount of substrate converted at the different temperatures. In contrast, microbial toluene degradation efficiency varied with the different temperatures, being significantly lower at 4 °C and highest at 20 °C. The degradation efficiency for toluene was significantly stimulated at 20 °C. Again, toluene degradation patterns were mirrored by the deviation of DO values from the untreated controls (Fig. 6).
Temperature dependence of porewater-dissolved oxygen and selected microbial parameters, i.e., organic carbon turnover efficiency estimated from the inflow and outflow measurements of DOC and toluene in sediment-porewater, total cell counts (TCC), bacterial carbon production (BCP), and bacterial Shannon diversity of microbial communities attached to sediments. Values are means of duplicates or triplicates (±SD). Values (symbols and bars) marked with an asterisk are significantly different (P ≤ 0.05) from the reference (value for the 10 °C incubation, highlighted by the gray background) within similar treatments
The hypothesis of Brielmann et al. (2009, 2011) mentioned above was not supported by our current results. Unexpectedly, we did not find striking temperature-related effects to microbial activities at conditions with a considerable background contamination (elevated DOC, nutrients, and/or organic pollutants). Differences between the amended and non-amended columns were mainly driven by overcoming the organic carbon and nutrient limitations, with temperature playing a minor role in additionally steering carbon turnover, bacterial growth, and biomass. Note that there was also considerable variability between control columns and treatment, most likely a time effect caused by different times of preadaptation and experiments. However, more importantly, the organic contamination immediately led to a decrease in porewater oxygen concentrations, reaching critically low or even hypoxic conditions at temperatures ≥30 °C in our experiments. The switch from oxic to anoxic conditions in an aquifer is without doubt linked to severe changes in water quality, microbially catalyzed processes, and community patterns. However, since our sediment column study simulated only moderate contamination (addition of 1.3–3 mg carbon L−1) and covered a rather short time scale (8–19 days), no switch to reduced conditions was observed. Patterns from the short-term experiments await midterm and long-term evaluation and need to be interpreted with caution when transferred to field conditions.
Convincing evidence that a temperature increase in aquifers can readily lead to a switch of biogeochemical processes and consequently to active ecosystem functions comes from other studies. Using aquifer sediment (tertiary lignite sand) and tap water, Jesußek et al. (2013a, b) showed in column experiments that with increasing temperature, redox processes changed. At ambient temperature (10 °C), oxic conditions and aerobic respiration prevailed. At 25 and 40 °C, conditions changed to nitrate- and iron reducing, respectively. At 70 °C, sulfate reduction dominated (Jesußek et al. 2013a). In a follow-up experiment, the continuous addition of acetate led to the establishment of sulfate-reducing conditions at all four temperatures tested. At 40 °C, the highest sulfate reduction kinetics were found, followed by 25, 10, and 70 °C. At 25 °C, methane was found in solution, but not at other temperatures (Jesußek et al. 2013b). A similar study, conducted by Bonte et al. (2013a), showed that by performing column experiments with sediments from an unconsolidated anoxic sandy aquifer that a temperature increase from 11 °C (ambient) to 25 °C could cause a shift from iron-reducing to sulfate-reducing and methanogenic conditions. A further temperature increase (>45 °C) resulted in the emergence of a thermophilic microbial community specialized in fermentation and sulfate reduction (Bonte et al. 2013a). These studies underline the potential impact of temperature alterations to ecosystem processes.
As already mentioned, an increase in temperature generally leads to an increase in the metabolic activity of organisms. This can also be seen as a stimulation of ecosystem functions and thus a beneficial effect, in particular at sites with organic background contamination (see below). In fact, with regard to the natural cycling of carbon, an increased turnover may not necessarily be desired, because at the same time, dissolved oxygen is depleted, as discussed earlier. Another aspect is the long-term depletion of natural organic carbon pools. As a consequence, the initially stimulated turnover rates will cease at a later time point (Bradford et al. 2008; Frey et al. 2008). Moreover, temperature shifts influence interactions within food webs. For ciliates (protozoa), it has been shown that for a constant production more energy (food) is needed when the ambient temperature shifts to lower or higher values. At elevated temperatures, negative growth rates were observed (Weisse et al. 2002). The consequent reduction or even loss of a predator to bacteria will also affect the overall carbon turnover. Numerous feedback mechanisms such as these are known from different environments, but are not yet well understood and/or considered for groundwater ecosystems.
Currently, a frequent topic of discussion is whether thermally enhanced bioremediation, i.e., the application of geothermal energy use at sites where the subsurface is already organically contaminated, is a promising and beneficial treatment approach (Jesußek et al. 2013b; Zuurbier et al. 2013; Köber et al. 2015). The potentially enhanced microbial activity at increased temperatures is assumed to accelerate contaminant biodegradation. Zeman et al. (2014) showed in laboratory microcosms that at 22 and 30 °C a higher biogas (methane) production, a significant degradation of monoaromatic hydrocarbons, and an overall greater loss of hydrocarbons was achieved in comparison with lower (4 and 9 °C) and higher (35 and 40 °C) thermal conditions. A comparable study by Friis et al. (2007) revealed complete dechlorination of TCE to ethane between 10 and 30 °C and ceasing TCE transformation only at temperatures ≥50 °C. Our toluene experiments exhibited the highest biodegradation rates at 20 °C, 5–10 K above a typical aquifer temperature (Fig. 6). Thus, further investigations for specific groups of chemicals and environmental conditions are highly recommended.
Temperature-related effects on groundwater ecosystem biodiversity
Microbial communities
The direct effect of temperature changes on microbial community composition is indisputable. Microbial communities at ambient groundwater temperatures as found in Germany (8–14 °C) are mainly composed of psychrophilic and psychrotolerant prokaryotes. An increase in temperature to 20 °C may already cause a shift to a dominance of mesophiles and temperatures above 40 °C to thermophilic microbes. This will not only affect biodiversity but likely also microbial processes. As repeatedly shown for soil systems, changes in microbial community composition occur already with a moderate but chronic temperature increase of only 2–3 K (Zogg et al. 1997; Bradford et al. 2008; Castro et al. 2010; Yergeau et al. 2012). Similar selective forces act on groundwater fauna. To date, studies for groundwater ecosystems are scarce.
In a field study by Brielmann et al. (2009), in an oligotrophic shallow aquifer dynamically impacted by a heat plume from the recharge of warm (≥20 °C) process water, a significant positive correlation was shown between groundwater bacterial diversity and temperature. A significant decrease in sediment bacterial diversity at 4 and 45 °C compared to ambient conditions (10 °C) was subsequently reported by Brielmann et al. (2011) for sediment column experiments packed with aquifer material from the same oligotrophic aquifer. In the present study, sediments amended with R2A-medium (labile DOC and nutrients) or toluene showed a significant loss in bacterial diversity compared to non-amended controls (Fig. 6). In the toluene treatments, the diversity loss was highly related to temperature, with a significantly higher diversity at 4 °C and a significantly lower diversity at 30 and 45 °C. No significant differences in Shannon diversity between the temperature treatments were found for the R2A-amended columns. It is clear that the results from the simulation of elevated nutrient and DOM concentrations with the treatment of easy degradable compounds can only be translated into more complex environmental conditions with caution.
Pyrosequencing libraries of sediment-attached microbiota revealed marked distinctions in dominating taxonomic lineages for the different treatments (Fig. 7). Generally, substrate treatment was connected to a decreased abundance of Alphaproteobacteria and a stimulation of Betaproteobacteria (or also Gammaproteobacteria). Under R2A-treatment, a stimulation of Duganella spp. was especially observed at 4 and 15 °C, which was replaced by an increased frequency of Lysobacter spp. at 30 and 45 °C. Both appeared to be competing in a temperature-dependent manner for resources provided by the treatment. Under toluene addition, microbes related to Methyloversatilis spp. were highly stimulated, especially with increasing temperature (Fig. 7). Methylibium spp. also appeared to be stimulated under toluene treatment, albeit only below 45 °C. Both are well-known methylotrophs which have been described to harbor toluene catabolism (Kane et al. 2007; Smalley et al. 2015). Intriguingly, Methyloversatilis was consistently stimulated at 45 °C, albeit to a lesser extent, irrespective of toluene treatment.
Phylogenetic affiliation of bacterial 16S rRNA gene amplicon libraries generated from sediment columns. Total communities are resolved to phylum/class level (a), while selected dominating and treatment-responsive lineages are highlighted at the genus or family level (b). Taxa with a maximal abundance <2.5 % in any given library are not shown in b
These results highlight just some of the pronounced temperature impacts observed for defined lineages in our column experiment and also for those which may be readily linked to defined functions such as toluene degradation. An in-depth discussion of our sequencing libraries would be beyond the scope of this manuscript. It is not impossible but generally difficult to link environmental microbes observed in sequencing libraries to putative functions and also to infer more general ecological traits (Langille et al. 2013). We also have to caution such interpretations since amplicon libraries were non-replicated.
Still, several other laboratory (batch and column sediment microcosms) and aquifer mesocosm studies are partly consistent with our present findings. At aquifer temperatures above 30 °C, the microbial diversity repeatedly declined as a consequence of considerable community reorganization, with the disappearance of psychro- and mesophiles and a shift toward thermophilic representatives (Aragno 1983; Adinolfi et al. 1994; Schippers and Reichling 2006). Detailed information on community composition changes comes from a sediment column study applying sediments from an unconsolidated anoxic sandy aquifer testing temperature variations of 5–80 °C (Bonte et al. 2013b). DNA fingerprinting analysis and pyrosequencing of bacterial 16S rDNA from porewater (column effluent) and sediments revealed differences with changing temperature. While the bacterial communities were quite similar in the column inflow and outflow at temperatures between 5 and 25 °C, at 60 °C a clear change in community composition was observed, especially for the sediment communities, which shifted from Proteobacteria dominated to Firmicutes and Chloroflexi. Temperature-related shifts in the occurrence of bacterial taxa that could be linked to specific redox processes were evident. At moderate temperatures, a high diversity of putative sulfate reducers from Proteobacteria and Firmicutes was abundant, while sulfate-reducing Proteobacteria disappeared at 60 °C. Likewise, proteobacterial genera known for their iron-reducing capabilities were no longer detected. Already at 25 °C, Desulfuromonas and Geobacter were present at lower abundance in the sediments than at 5 and 11 °C, in line with the observed lower iron reduction inferred from hydrochemical data. Finally, Archaea were found to be more abundant at moderate temperatures (Bonte et al. 2013b).
Research on the microbiology and biogeochemistry of (deep) aquifer thermal energy storage plants revealed dynamic changes in microbial community composition in the fluids related to periods of heat recharge, discharge, or plant switch-off times (Würdemann et al. 2014; Westphal et al. 2015). The microbes and their mediated processes that bear a serious risk for corrosion and clogging of pipelines were always less in abundance in the hot (68–73 °C) than in the cold (46 °C) fluids and considerably differed in composition (Lerm et al. 2013). These studies, although not directly comparable to geothermal heat impacts in shallow groundwater ecosystems, again emphasize the pronounced effects of temperature on microbial community composition and dominating processes.
Groundwater fauna
With higher organisms, i.e., metazoans, different terminology is used with respect to temperature adaptation. Animals with a narrow temperature tolerance are called stenothermic, and those with a broad temperature range are eurythermic. Representatives of the true groundwater fauna (stygobites) are cold-stenothermic with only a few exceptions (e.g., Issartel et al. 2005). Effects of short-term on long-term temperature changes to stygobites have been little studied. The few studies available show that an increase in temperature at the species- and specimen level goes along with an increase in metabolic activity, typically causing stress and mortality (Glatzel 1990; Issartel et al. 2005; Colson-Proch et al. 2010; Brielmann et al. 2011; Avramov et al. 2013). At the level of groundwater communities, evidence showed a negative correlation between temperature in aquifers and faunal diversity (Brielmann et al. 2011). A study on groundwater microbes and fauna in local aquifers below basins collecting surface runoff during extreme rain events found that groundwater fauna was almost absent at spots that were impacted by significant temperature dynamics, with maximum temperatures of up to 22 °C, although these sites were characterized by the highest microbial biomass (Foulquier et al. 2011), i.e., a potential food source for these invertebrates. In fact, due to the enhanced recharge of DOC and the elevated temperatures, temporary hypoxic and even anoxic conditions were established. The key role of temperature deviations versus the availability of sufficient dissolved oxygen awaits further clarification. Without a doubt, the fluctuating conditions, causing increased microbial activities but the absence of the fauna, led to a temporal disconnection of the energy flow between the trophic levels from microbes to invertebrates at that site and consequently impaired carbon cycling (Foulquier et al. 2009).
A recent survey on the relationship between aquifer thermal regimes and groundwater fauna composition and diversity in southwest Germany was recently conducted by Spengler and Hahn (in prep.). In this survey, temperature was identified as the main driver shaping groundwater faunal communities at the regional scale in addition to organic matter. Increasing temperatures revealed a reverse trend for groundwater faunal diversity. In particular, typical groundwater amphipods and cyclopoids species seem to be specifically sensitive to elevated groundwater temperature, while other groups and species (e.g., representatives of the Bathynellidae) turned out to be more tolerant.
Conclusions and outlook
The use of geothermal energy as renewable energy source will increase in the future, as well as the storage of heat and cold in the subsurface. When planning the intensified use of the subsurface, aspects related to the protection of groundwater as a drinking water resource as well as related to groundwater ecosystem properties such as biodiversity and ecosystem functions need to be considered. Temperature is a key factor influencing abiotic and biotic processes. The data summarized in this paper underline that temperature changes in aquifers go along with changes in groundwater chemical composition, biodiversity, community composition, as well as microbial processes and consequently ecosystem functions. The effects include the mobilization of heavy metals and organic matter, a decrease in gas solubility, as well as increased dissolution and precipitation of major elements. Importantly, the depletion of oxygen, as a concerted effect of elevated temperature, lower gas solubility, and stimulated microbial activities, may turn the groundwater ecosystem anoxic, with a serious change in carbon and nutrient cycling, meaning a significant change in ecosystem functions. A change in temperature is always accompanied by a change in microbial community composition. It is noted that at sites highly impacted by organic pollution and characterized by a biocenosis way off from a natural reference status, an increase in temperature might be beneficial in stimulating microbial biodegradation processes. For the generally cold-stenotherm groundwater fauna, even temperatures exceeding 14–20 °C may be lethal in the long run. The long-term consequences of such changes on the ecosystem level have been little studied, and more experiments and field studies are needed to gain a fundamental understanding that will allow predictive prognoses.
We are aware that there are initiatives and working groups that claim an intensified use of the subsurface in terms of geothermal energy use and heat/cold storage. The comparativeness between a loss in biodiversity and changes in ecosystem functions and the more comprehensive use of the subsurface is a point that deserves intensive scientific evaluation and discussion. From an ecological point of view, we highly recommend restricting temperature changes to a minimum, especially in systems where freely available energy, i.e., considerable amounts of degradable organic matter and a surplus in nutrients, is present to microbes. Here, special attention should be on avoiding the transition from oxic to anoxic conditions in aquifers. In the future, we recommend systematic planning and management of the subsurface to not only optimize its economic usage for energy production but also to clearly prevent ecological impacts and foster the sustainable protection of essential resources.
References
Adinolfi M, Koch M, Ruck W (1994) Ökologische und mikrobielle Folgen der Wärmespeicherung im Aquifer. Stuttgarter Berichte zur Siedlungswasserwirtschaft, Oldenbourg Industrieverlag GmbH, München
Alfreider A, Krössbacher M, Psenner R (1997) Groundwater samples do not reflect bacterial densities and activity in subsurface systems. Water Res 31:832–840
Aragno M (1983) Impacts Microbiologiques. Premier cycle d’exploitation de l’installation pilote SPEOS. Annex 10
Arning E, Kölling M, Schulz HD, Panteleit B, Reichling J (2006) Einfluss oberflächennaher Wärmegewinnung auf geochemische Prozesse im Grundwasserleiter. Grundwasser 11:27–39
Avramov M, Rock TM, Pfister G, Schramm KW, Schmidt SI, Griebler C (2013) Catecholamine levels in groundwater and stream amphipods and their response to temperature stress. Gen Compar Endocrinol 194:110–117
Balke KD (1978) Problematik der Kühlwassereinleitung in den Untergrund. S.1978371-1978389
Bauer R, Zhang Y, Maloszewski P, Meckenstock RU, Griebler C (2008) Mixing controlled biodegradation in a toluene plume–results from two-dimensional laboratory experiments. J Contam Hydrol 96:150–168
Bayer A, Drexel R, Weber N, Griebler C (2016) Quantification of aquatic sediment prokaryotes–a multiple-steps optimization testing sands from pristine and contaminated aquifers. Limnologica 56:6–13
Bonte M (2013) Impacts of shallow geothermal energy on groundwater quality–A hydrochemical and geomicrobial study of the effects of ground source heat pumps and aquifer thermal energy storage. Dissertation, Free University of Amsterdam
Bonte M, Stuyfzand PJ, van den Berg GA, Hijnen WAM (2011) Effects of aquifer thermal energy storage on groundwater quality and the consequences for drinking water production: a case study from the Netherlands. Water Sci Technol 63:1922–1931
Bonte M, Röling WFM, Zaura E, van der Wielen PWJJ, Stuyfzand PJ, van Breukelen BM (2013a) Impacts of shallow geothermal energy production on redox processes and microbial communities. Environ Sci Technol 47:14476–14484
Bonte M, van Breukelen BM, Stuyfzand PJ (2013b) Temperature-induced impacts on groundwater quality and arsenic mobility in anoxic aquifer sediments used for both drinking water and shallow geothermal energy production. Wat Res 47:5088–5100
Bradford MA, Davies CA, Frey SD, Maddox TR, Melillo JM, Mohan JE, Reynolds JF, Treseder KK, Wallenstein MD (2008) Thermal adaptation of soil microbial respiration to elevated temperature. Ecol Lett 11:1316–1327
Brielmann H, Griebler C, Schmidt SI, Michel R, Lueders T (2009) Effects of thermal energy discharge on shallow groundwater ecosystems. FEMS Microbiol Ecol 68:273–286
Brielmann H, Lueders T, Schregelmann K, Ferraro F, Avramov M, Hammerl V, Blum P, Bayer P, Griebler C (2011) Oberflächennahe Geothermie und ihre potentiellen Auswirkungen auf Grundwasserökologie. Grundwasser 16:77–91
Brons HE, Griffioen J, Appelo CAJ, Zehnder AJB (1991) (Bio)geochemical reactions in aquifer material from a thermal energy storage site. Water Res 25:729–736
Buesing N, Gessner MO (2003) Incorporation of radiolabeled leucine into protein to estimate bacterial production in plant litter, sediment, epiphytic biofilms, and water samples. Microb Ecol 45:291–301
Carroll SA, Walther JV (1990) Kaolinite dissolution at 25°, 60° and 80 °C. Am J Sci 290:797–810
Castro HF, Classen AT, Austin EE, Norby RJ, Schadt CW (2010) Soil microbial community responses to multiple experimental climate change drivers. Appl Environ Microbiol 76:999–1007
Christ MJ, David MB (1996) Temperature and moisture effects on the production of dissolved organic carbon in a Spodosol. Soil Biol Biochem 28:1191–1199
Colson-Proch C, Morales A, Hervant F, Konecny L, Moulin C, Douady CJ (2010) First cellular approach of the effects of global warming on groundwater organisms: a study of the HSP70 gene expression. Cell Stress Chaperones 15:259–270
Daveler SA, Wolery TJ (1992) EQPT, A data file preprocessor for the EQ3/6 software package–User’s guide and related documentation (Version 7.0): Lawrence Livermore National Laboratory, UCRL-MA-110662 PT II. http://www.wipp.energy.gov/library/CRA/2009_CRA/
DVGW (2013) Erdwärmenutzung in Einzugsgebieten von Trinkwassergewinnungsanlagen. DVGW-Inf 23(07):2013
Filius JD, Lumsdon DG, Meeussen JCL, Hiemstra T, Van Riemsdijk WH (2000) Adsorption of fulvic acid on goethite. Geochim Cosmochim Acta 64:51–60
Foulquier A, Malard F, Barraud S, Gibert J (2009) Thermal influence of urban groundwater recharge from stormwater infiltration basins. Hydrol Process 23:1701–1713
Foulquier A, Malard F, Mermillod-Blondin F, Montuelle B, Doledec S, Volat B, Gibert J (2011) Surface water linkages regulate trophic interactions in a groundwater food web. Ecosystems 14:1339–1353
Frey SD, Drijber R, Smith H, Melillo J (2008) Microbial biomass, functional capacity, and community structure after 12 years of soil warming. Soil Biol Biochem 40:2904–2907
Friis AK, Heimann AC, Jakobsen R, Albrechtsen H-J, Cox E, Bjerg PL (2007) Temperature dependence of anaerobic TCE-dechlorination in a highly enriched Dehalococcoides-containing culture. Water Res 41:355–364
Glatzel T (1990) On the biology of Parastenocaris phyllura Kiefer (Copepoda, Harpacticoida). Stygologia 5:131–136
Griebler C, Avramov M (2015) Groundwater ecosystem services–a review. Freshw Sci 34:355–367
Griebler C, Mindl B, Slezak D, Geiger-Kaiser M (2002) Distribution patterns of attached and suspended bacteria in pristine and contaminated shallow aquifers studied with an in situ sediment exposure microcosm. Aquat Microb Ecol 28:117–129
Griebler C, Malard F, Lefébure T (2014) Current developments in groundwater ecology—from biodiversity to ecosystem function and services. Curr Opin Biotech 27:159–167
Griebler C, Kellermann C, Kuntz D, Walker-Hertkorn S, Stumpp C, Hegler F (2015) Auswirkungen thermischer Veränderungen infolge der Nutzung oberflächennaher Geothermie auf die Beschaffenheit des Grundwassers und seiner Lebensgemeinschaften–Empfehlungen für eine umweltverträgliche Nutzung. UFOPLAN, FKZ 3710 23 204, 154 pp
Griffioen J, Appelo CAJ (1993) Nature and extent of carbonate precipitation during aquifer thermal energy storage. Appl Geochem 8:161–176
GtV Bundesverband Geothermie (2014) http://www.geothermie.de/
Hähnlein S, Molina-Giraldo N, Blum P, Bayer P, Grathwohl P (2010) Ausbreitung von Kältefahnen im Grundwasser bei Erdwärmesonden. Grundwasser 15(2):123–133
Hähnlein S, Bayer P, Ferguson G, Blum P (2013) Sustainability and policy for the thermal use of shallow geothermal energy. Energy Policy 59:914–925
Hammes FH, Egli T (2005) New method for assimilable organic carbon determination using flow-cytometric enumeration and a natural microbial consortium as inoculum. Environ Sci Technol 39:3289–3294
Hecht-Méndez J, Molina-Giraldo N, Blum P, Bayer P (2010) Evaluating MT3DMS for Heat Transport Simulation of Closed Geothermal Systems. Ground Water 48(5):741–756
Hofmann R, Größbacher M, Griebler C (2016) Mini sediment columns and two-dimensional sediment flow-through microcosms–versatile model systems for studying biodegradation of organic contaminants in groundwater ecosystems. In: McGenity TJ, Timmis KN, Nogales B (eds) Protocols for Hydrocarbon and Lipid Microbiology. Springer-Verlag, Berlin-Heidelberg. doi:10.1007/8623_2016_210
Issartel J, Hervant F, Voituron Y, Renault D, Vernon P (2005) Behavioural, ventilatory and respiratory responses of epigean and hypogean crustaceans to different temperatures. Comp Biochem Physiol Part A 141:1411–1417
Jang Y-C, Townsend TG (2001) Sulfate leaching from recovered construction and demolition debris fines. Adv Environ Res 5:203–217
Jesußek A, Grandel S, Dahmke A (2013a) Impacts of subsurface heat storage on aquifer hydrogeochemistry. Environ Earth Sci 69:1999–2012
Jesußek A, Koeber R, Grandel S, Dahmke A (2013b) Aquifer heat storage: sulfate reduction with acetate at increased temperatures. Environ Earth Sci 69:1763–1771
Kaiser K, Kaupenjohann M, Zech W (2001) Sorption of dissolved organic carbon in soils: effects of soil sample storage, soil-to-solution ratio, and temperature. Geoderma 99:317–328
Kane SR, Chakicherla AY, Chain PSG, Schmidt R, Shin MW, Legler TC, Scow KM, Larimer FW, Lucas SM, Richardson PM et al (2007) Whole-genome analysis of the methyl tert-butyl ether-degrading beta-Proteobacterium Methylibium petroleiphilum PM1. J Bacteriol 189:1931–1945
Karwautz C, Lueders T (2014) Impact of hydraulic well restoration on native bacterial communities in drinking water wells. Microb Environ 29:363–369
Kirchman DL (1993) Leucine incorporation as a measure of biomass production by heterotrophic bacteria. In: Kemp PF, Sherr BF, Sher EB, Cole JJ (eds) Handbook of methods in aquatic microbial ecology. Lewis Publishers, Boca Raton, pp 359–367
Köber R, Dörr C, Lüders K, Koprach N, Schäfer D, Dahmke A (2015) Geochemische Beeinflussungen des Grundwassers durch Wärmespeicherung. Geothermische Energie, Heft 82:20–21
Köhler SJ, Dufaud F, Oelkers EH (2003) An experimental study of illite dissolution kinetics as a function of pH from 1.4 to 12.4 and temperature from 5 to 50°C. Geochim Cosmochim Acta 67:3583–3594
Kunkel R, Wendland F, Voigt H-J, Hannappel S (2004) Die natürliche, ubiquitär überprägte Grundwasserbeschaffenheit in Deutschland. Schriften des Forschungszentrums Jülich Reihe Umwelt/Environment Band/Volume 47
Langille MGI, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA et al (2013) Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotech 31:814–821
Lengeler JW, Drews G, Schlegel HG (1999) Biology of Prokaryotes. Thieme Verlag, Stuttgart-New York
Lerm S, Westphal A, Miethling-Graff R, Alawi M, Seibt A, Wolfgramm M, Würdemann H (2013) Thermal effects on microbial composition and microbiologically induced corrosion and mineral precipitation affecting operation of a geothermal plant in a deep saline aquifer. Extremophiles 17:311–327
Lund JW, Boyd TL (2016) Direct utilization of geothermal energy 2015 worldwide review. Geothermics 60:66–93
Malin N, Wilson A (2000) Ground-source heat pumps: are they green? Environ Build News 9:1–22
Molina-Giraldo N, Bayer P, Blum P (2011) Evaluating the influence of thermal dispersion on temperature plumes from geothermal systems using analytical solutions. Int J Therm Sci 50(7):1223–1231
Pannike S, Kölling M, Panteleit B, Reichling J, Scheps V, Schulz HD (2006) Auswirkung hydrogeologischer Kenngrößen auf die Kältefahnen von Erdwärmesondenanlagen in Lockersedimenten. Grundwasser 11(1):6–18
Parkhurst DL, Appelo CAJ (1999) User's guide to PHREEQC (Version 2) : a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. Water-Resources Investigations Report 99–4259. U.S. Department of the Interior, U.S. Geological Survey, Denver, Colarado, p 312
Pilloni G, Granitsiotis MS, Engel M, Lueders T (2012) Testing the limits of 454 pyrotag sequencing: reproducibility, quantitative assessment and comparison to T-RFLP fingerprinting of aquifer microbes. PLoS One 7:e40467
Possemiers M, Huysmans M, Batelaan O (2014) Influence of aquifer thermal energy storage on groundwater quality: a review illustrated by seven case studies from Belgium. J Hydrol Reg Stud 2:20–34
Price PB, Sowers T (2004) Temperature dependence of metabolic rates for microbial growth, maintenance, and survival. PNAS 101:4631–4636
Reasoner DJ, Geldreich EE (1985) A new medium for the enumeration and subculture of bacteria from potable water. Appl Environ Microbiol 49:1–7
Schippers A, Reichling J (2006) Laboruntersuchungen zum Einfluss von Temperaturveränderungen auf die Mikrobiologie des Untergrundes. Grundwasser 11:40–45
Smalley NE, Taipale S, De Marco P, Doronina NV, Kyrpides N, Shapiro N, Woyke T, Kalyuzhnaya MG (2015) Functional and genomic diversity of methylotrophic Rhodocyclaceae: description of Methyloversatilis discipulorum sp. nov. Int J Syst Evol Microbiol 65:2227–2233
Tiehm A, Schmidt K, Augenstein T, Betting D (2012) Wärmeträgerfluide in der Geothermie: Exemplarische Gefährdungsabschätzung anhand von Strukturaufklärung, Abbaubarkeit und Toxizität. DVGW-Forschungsvorhaben W 1/01/09/Badenova Innovationsfonds-Vorhaben 2010-3
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naive bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267
Warkentin M, Freese HM, Karsten U, Schumann R (2007) New and fast method to quantify respiration rates of bacterial and plankton communities in freshwater ecosystems by using optical oxygen sensor spots. Appl Environ Microbiol 73:6722–6729
Weisse T, Stadler P, Lindstrom ES, Kimmance SA, Montagnes DJS (2002) Interactive effect of temperature and food concentration on growth rate: a test case using the small freshwater ciliate Urotricha farcta. Limnol Oceanogr 47:1447–1455
Westphal A, Lerm S, Miethling-Graff R, Seibt A, Wolfgramm M, Würdemann H (2015) Effects of plant downtime on the microbial community composition in the highly saline brine of a geothermal plant in the North German Basin. Appl Microbiol Biotechnol. doi:10.1007/s00253-015-7181-1
Würdemann H, Westphal A, Lerm S, Kleyböcker A, Teitz S, Kasina M, Miethling-Graff R, Seibt A, Wolfgramm M (2014) Influence of microbial processes on the operational reliability in a geothermal heat store—results of long-term monitoring at a full scale plant and first studies in a bypass system. Energy Proc 59:412–417
Yergeau E, Bokhorst S, Kang S, Zhou J, Greer CW, Aerts R, Kowalchuk GA (2012) Shifts in soil microorganisms in response to warming are consistent across a range of Antarctic environments. ISME J 6:692–702
Zeman NR, Renno MI, Olson MR, Wilson LP, Sale TC, De Long SK (2014) Temperature impacts on anaerobic biotransformation of LNAPL and concurrent shifts in microbial community structure. Biodegradation 25:569–585
Zogg GP, Zak DR, Ringelberg DB, MacDonald NW, Pregitzer KS, White DC (1997) Compositional and functional shifts in microbial communities due to soil warming. Soil Sci Soc Am J 61:475–481
Zuurbier KG, Hartog N, Valstar J, Post VEA, van Breukelen BM (2013) The impact of low-temperature seasonal aquifer thermal energy storage (SATES) systems on chlorinated solvent contaminated groundwater: modeling of spreading and degradation. J Contam Hydrol 147:1–13
Acknowledgments
This research was funded by the German Environment Agency (UFO-PLAN; Forschungskennzahl 3710 23 204) and the Life Science Foundation (http://www.life-science-stiftung.org/). We are grateful to B. Kirschbaum (UBA, Dessau-Berlin), W. Adam (Wasserwirtschaftsamt Freising), H. König and F. Meyfarth (Texas Instruments Germany, Freising), E. Schrade, V. Hammerl, R. Schaupp, K. Groißmeier and A. Balmert (all TUM) as well as G. Hinreiner, G. Teichmann, S. Schaefer, and M. Stoeckl (Helmholtz Zentrum München, IGÖ) for project assistance, support, and valuable discussion. We further thank the members of the scientific board of the UBA project for critical comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of a Topical Collection in Environmental Earth Sciences on ‘Water in Germany’, guest edited by Daniel Karthe, Peter Chifflard, Bernd Cyffka, Lucas Menzel, Heribert Nacken, Uta Raeder, Mario Sommerhäuser, and Markus Weiler.
Rights and permissions
About this article
Cite this article
Griebler, C., Brielmann, H., Haberer, C.M. et al. Potential impacts of geothermal energy use and storage of heat on groundwater quality, biodiversity, and ecosystem processes. Environ Earth Sci 75, 1391 (2016). https://doi.org/10.1007/s12665-016-6207-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-016-6207-z