Abstract
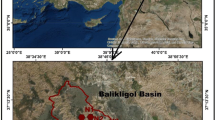
The study area lies between latitudes 6°02′N and 6°24′N, and longitudes 8°00′E and 8°16′E. The area is underlain by Abakaliki shale which belongs to Asu River Group of Albian age. The study determines different water types (facies) via analysis of major ion concentration of water samples using spectrophotometer of HACH DR/2010 series. Twenty (20) water samples were collected and analyzed. The laboratory results were further induced to statistical evaluations using Aqua Chem, and it showed that the groundwater of the area is hard and fresh. The results can be classified into five hydrochemical facies, namely Ca–Mg–Cl–S04, Ca–Mg–HCO3–Cl–SO4, Ca–Mg–HCO3, Na–K–HCO3 and Na–K–Cl–SO4, all of which are dominantly alkaline water. Static water level was measured from hand-dug wells to determine hydraulic heads which delineated local potentiometric surface. Flow directions were deduced, and it showed isolated system of groundwater movement. Such localized pattern of water occurrence can be attributed to the underlying shaley aquiclude across the area. The hydraulic head info was correlated with facie analysis to interpret groundwater recharge zones.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Upper Cross River basin is an extension of Cross River hydrogeological basin to the southeastern Nigeria. It comprises Abakaliki town and some surrounding mineralized villages. The area is groundwater problematic due to the underlying aquiclude-forming shales of Asu River Group of Albian age (Offodile 2002). However, fractures where formed, produce isolated and prolific aquifers with consequent non-regional flow system and thus yielding to different facie zones. According to Okogbue and Ukpai (2013), groundwater terrain of the area has been degrading due to mineralization of major ions, heavy metals and other trace elements. Therefore, the study determined the lithogenic significances (environmental impacts) of chemical elements that quantified to different groundwater types (facies), natural environments of origin according to Kevin (2005) and their physical flow pattern. The importance of this study is that groundwater resource origins were identified. The origin, however, enabled authors to define recharge and discharge zones. These zones will assist groundwater management programs in the future.
Geographical and hydrographical locations
The study area lies within latitudes 6°02′–6°24 north and longitudes 8°00′–8°16′E. The topography of the area is defined by the scattered pyroclastic hills in the Abakaliki town and an upland in the mineralized Ameka village. Generally, the terrain is rolling and undulating. Abakaliki area is located in the hydrographical Cross River basin (Fig. 1) and falls within Niger south hydrological area. The climate is humid and tropical with a marked dry season (November–March) and wet season (April–October). The vegetation is characterized by grasslands, shrubs and scanty trees. The area is drained by Iyokwu River from northcentral, Abia River from northwest, both joining with Ebonyi River. The Ebonyi River then drains other river systems in the area and flows southwards, forming a trellis drainage pattern that stretches beyond the study area to Cross River (Fig. 2).
Geology and mineralization
Offodile (2002) reported that Asu River Group, Albian in age and subdivided into three formations, comprises essentially of over 200 m bluish gray to olive brown shales and sandy shales, fine-grained micaceous and calcareous sandstones and some limestones. There are also some dykes, sills and igneous intrusives associated with the group. The group underlies Abakaliki area, dipping northwestwards and southeastwards in the northern and southern parts, respectively, with strike direction along the axis of Abakaliki anticlinorium-aftermath uplift formed during Santonian tectonic episode of post-Albian that trends northeast–southwest (Fig. 3). According to Reyment (1965), intrusion took place during the post-Albian time which deformed the shale of the area, creating different geologic structures, some of which have mineralizing properties. Thus, the shale of Asu River Group was faulted, fractured and weathered in many places which were identified during field mapping in the course of this study. Many of these structures formed lodes in the study area. Among the lodes are Enyigba main lode, Ameri and Ameka lodes. The mineral lodes/veins are made up of galena (PbS), sphalerites (ZnS), siderite (FeCo3) and the subordinate chalcopyrite (CuFeS2) and marcasite (FeS2).
Materials and methods
Proposed sample locations were visited with the aid of a topographic map of the area which showed the access roads, and information gathered was compiled as regards the names of the locations where the samples would be collected (Table 1). Water sample collection from boreholes commenced the next day after reconnaissance. A Global Positioning System (GPS) of Garmin eTrex model was used to locate each sampled point by measuring latitudes and longitudes of the sampled location. A total of 20 samples were collected from different locations spread across the study area: 16 from boreholes, 3 from hand-dug wells and 1 from the town water supply reservoir. The reservoir sample was collected in order to compare the difference in elemental composition of the pipe-borne water with the groundwater. Each sample was collected using a clean 1-l plastic water bottle and labeled according to the location name. Each plastic bottle was rinsed with the same water to be sampled before the collection to avoid any contamination from the bottle. Physical parameters such as the electrical conductivity (EC) and temperature relevant to the study were measured in situ using a portable WTW LF 90 conductivity meter. Dips and strikes were measured using a Brunton compass, depths to groundwater levels in 21 hand-dug wells were measured using a 100-m tape while the elevation from mean sea level and location of each of the wells was measured using the GPS of Garmin eTrex model. The generated data went through further software analysis using Aqua Chem to determine hydraulic heads. The longitudes and latitudes measured were converted to coordinates in meters on a universal transverse mercator (UTM) using UTM calculator.
Note: The hydraulic head of each well is the difference of elevation and depth to water level in the well. Thus,
The samples were analyzed at Hydrochemistry laboratory of UNICEF assisted water and sanitation project office, Federal secretariat, Ibadan Nigeria. Ions were analyzed using spectrophotometer of HACH DR/2010. The laboratory results were further induced to statistical evaluations to produce a Piper diagram. A resistivity meter was used for vertical electrical sounding (VES) data acquisition. The raw VES data were processed using appropriate constants and were presented as sounding curves which are obtained by plotting graph of apparent resistivity versus half-current electrode spacing (1/2AB) on double logarithmic graph sheets.
Results and discussion
The hydraulic heads as presented in Table 2 are emplaced in Fig. 4. As shown in the figure, the highest static water level is at the central part of the map and the lowest at the northeastern. The hydraulic head data were further used to produce a 3-D surface map (Fig. 5) showing recharge zones indicated by cone-like uplifts, which when correlated with Fig. 4 reflects elevations of higher static water level. Under steady-state condition, the elevation at any point on the water table equals the energy heads (Todd 1980). Therefore, the higher energy heads in the study area are inferred as permeable weathered zones where infiltration flow is more prominent. The flow lines lie perpendicular to water table contours as depicted in Fig. 4. The figure also showed that flow directions are variable, indicating non-regional groundwater flow. According to Kevin (2005), the occurrence of groundwater and the distribution of aquifers, aquicludes and aquitards in a region are determined by lithology (geologic strata in terms of rock composition and texture), stratigraphy (rock sequence and links between various deposits), and the structures (folds, faults, joints and fractures) of the geologic strata present. From analytical results of VES data presented in Fig. 7, the aquifers of the study area are influenced by the structures formed from fractures/weathered zones, while shales of the Asu River Group and the intercalated mudstone layers formed the aquicludes and aquitards, respectively (Table 3). The table showed that underground water contains soil water which correlates with the unsaturated zone and groundwater which correlates with the saturated zone. The latter was used as a criterion for the static water levels measured in the area, which flow system is localized due to intermingled shale and mudstone beds that confined groundwater flow to different restricted environments. This means, therefore, that the hydrogeology of the area is determined by the geology.
Facie analysis and groundwater classifications
Table 4 presents results of the hydrochemical analysis, indicating that 50 % of the water resources are alarming with pollution of total dissolved solids (TDS) when compared with WHO (2004) standard limit, especially around Enyigba area where the concentration falls within brackish range of 1020 mg/l. Similarly, total hardness ranges from 21 to 228 mg/l as CaCO3, with the lowest in representative samples 4 and 18. The mean concentration of the total hardness is 124 mg/l which falls between 75 and 150 mg/l range of water hardness prescription according to Sawyer and McCarty (1967). The table shows the concentrations of major ions from laboratory results in milligram per liter (mg/l) and was converted to milliequivalent per liter (meq/l) (Table 5) purposefully for the groundwater facie evaluation. It was done by dividing the valence (ionic charge) of each major element with its atomic or formula weight; the dividend was used as the converting factor (Table 6) which is multiplied by the concentration in ppm or mg/l to get the meq/l. The concentrations of ions in meq/l were used to plot Piper trilinear diagram according to Ahiarakwem (2004), as developed by Piper (1944). The Piper diagram (Fig. 6) identified five hydrochemical facies, labeled A to E, whereby the facies were presented where projections from the cation and anion triangles intersect on the diamond shape of the Piper trilinear diagram. According to Freeze and Cherry (1979), a hydrochemical facie is a distinct zone that has cations and anions concentrations describable within defined composition category. These were derived from four end zones of the diamond shape. The facies represent water types in the study area and are named as follows:
-
Type (A): Ca–Mg–Cl–SO4 facie.
-
Type (B): Ca–Mg–HCO3–Cl–SO4 facie.
-
Type (C): Ca–Mg–HCO3 facie.
-
Type (D): Na–K–HCO3 facie.
-
Type (E): Na–K–Cl–SO4 facie
From Fig. 6, type A (Ca–Mg–Cl–SO4) constitutes 25 % of the water samples. It includes samples 7,10,14,16 and 19. It has appreciable amounts of chloride ion (Cl−), sulfate ion (SO4 −) and alkaline earth metal ions (Mg2+ and Ca2+). Sulfate is a characteristic of water from atmospheric precipitation (Davis and De Wiest 1966) while chloride-rich water indicates brine origin (Todd 1980). In that case, Offodile (2002) disclosed that brine springs issue out along the axis of the Abakaliki anticlinorium. The brine spring is likely the source of the chloride in this group. Therefore, type A facie is an indication of mixing groundwater environment where water from intermediate zones streamlined from recharge areas that have much influence of precipitation, dilutes salty water harbored in the Asu River sediment. This type of facie falls within the range of normal alkalinity according to Nton et al. (2007). The water type is localized within the Abakaliki town.
Type B (Ca–Mg–HCO3–Cl–SO4) is the dominant water type in the study area and constitutes about 45 % of the water samples. It comprises samples 2, 5, 6, 9, 12, 13, 15, 17 and 20. It is an intermediate facie situated between the two end members of types A and C. Ca2+, Mg2+ and HCO3 − can be attributed to dissolution of carbonate mineral while SO4 − dissolved from sulfide minerals, all of which can be influenced by recharging rainwater. For chloride ion, Cl−, it is indicative of deepwater zone (Freeze and Cherry 1979). So, this group is between recharge zone and deepwater environment, somewhat hard, slightly acidic and weak alkaline water. It is likely an intermediate zone. This group is more prominent from the central to the southern part of the study area.
Type C (Ca–Mg–HCO3) symbolizes water from recharge zone where carbonic acid formed due to reaction of CO2 from organic matters and infiltrating water from rain in the unsaturated zone reacts with earth materials to release Ca2+, Mg2+ and HCO3 −. It constitutes about 15 % of the whole samples and comprises samples 1, 8 and 11. This type of water is characterized by hardness due to carbonate effect, dominated by alkaline earth metals (Ca, Mg) and weak acid of bicarbonate (Nwankwo 1988). The facie is found around the old mine areas (Ameka 1 and Ameri 2) and toward Ishieke village near Ebonyi State University (EBSU) faculty of education campus.
Type D (Na–K–HCO3) constitutes about 5 % of the study area and involves only sample 4. It is alkaline water, which might have evolved through cation exchange process from the weathered zone of the Abakaliki shale, probably dominated by clayey soil (cation exchanger) within the shale of Asu River group. The exchange reaction could be as follows:
where Ca2+ is taken up from the water to the cation exchange material (X) which in exchange releases Na+ to the groundwater, resulting in a Na–HCO3 water type. This reaction causes more dissolution of carbonate minerals as a result of increased affinity of the water to replace the lost Ca2+, thereby releasing more HCO3 − and subsequently raising the pH, and making the groundwater alkaline. The water type is interpreted as groundwater on transit due to the prevalence of the cation exchange process. The water type is found at Enyigba in the southern part of Abakaliki town.
Type E (Na–K–Cl–SO4) constitutes 10 % of the total water types and involves water samples 3 and 18. It can be found in deep subsurface zone where halite or normal salt (NaCl) is harbored. The water type is dominant near the pyroclastic massif which is the site for town water supply reservoir, off Water Works Road in Abakaliki town (sample 18), and at the Ameri village (sample 3) in the located south of Abakaliki town, where salt was mined before the 1967 civil war. Sample 3 which is from hand-dug well (about 250 meters deep) is the particular salt well where the salt was mined. It is therefore observed that the pipe-borne water (sample 18) has the characteristics of deep water type even though its source is apparently from the surface; this could be as a result of reagents applied for treatment before distribution.
Figure 7 depicts the areal distribution of the water types. Type C (recharge zone) is surrounded by type B (intermediate zone) in the northwest and aligns southeastward with type A (mixing groundwater zone). The type A confines type E (deep groundwater zone) at northcentral while type B extends to the center of Abakaliki town where it is overlain by a central refuse dump located beside Iyokwu River, near Tycoon Filling station which is 1/2 km away from Federal Teaching Hospital, Abakaliki, along Enugu–Abakaliki express road. From there, the water type moves to join type A at location 10 (Nkwagu). Toward the south, the dominant type B sandwiches water type C and joins type D; the water type D is inferred as water after a short distance of travel from recharge zone, advancing to the depth regime. It dominates only in location 4.
The sandwiched recharge zone of type C within the serially isolated and wide spread intermediary type B (see Fig. 7) is correlated with the series of isolated recharge points marked by cone-like uplifts as shown in Fig. 5 indicated same trending sources of groundwater recharge. Thus, the recharge areas in hydrogeological studies are obviously signified by levels of higher groundwater heads. A comparison of the facie flow in Fig. 7 with the groundwater flow directions obtained from the water table map (Fig. 4) clearly proved variability of the flow system. The idea of groundwater evolution from recharge zone to the intermediate zone and down to the deep zone according to Chebotarev (1955) was delineated in the facie map which explains flow from water type C, passes through type B to type A and flows downstream to type E, whereas type D may be flowing from type C via cation exchange medium and discharges to the surface, thereby forming a spring or even continues to join the deep water (type E) via anion exchange process. The modeled directions of the facie flow are indicated by the arrows as shown in Fig. 7, which depicts local variable flow system vis-a-vis the groundwater flow pattern exhibited in the water table map (see Fig. 4). The summary characteristics of the water types in the study area are outlined in Table 7.
Conclusions
Five hydrochemical facies, namely Ca–Mg–Cl–SO4 as type A, Ca–Mg–HCO3–Cl–SO4 as type B, Ca–Mg–HCO3 as type C, Na–K–HCO3 as type D and Na–K–Cl–SO4 as type E, are prevalent in the groundwater of the area, each producing a water type that is dominantly alkaline. The water types are interpreted as groundwater from mixing groundwater environment, intermediate groundwater zone, recharge zone, discharge/transit zone and deep groundwater zone, respectively. Their relative abundances as delineated on the hydrofacie map show that Ca–Mg–HCO3–Cl–SO4 which is within an intermediate groundwater zone is the most abundant.
The facie flow was correlated with the groundwater flow, and both confirm the variability of flow system whose pattern is not uniform, rather to various directions indicating non-regional groundwater flow. From the studies of environmental impact of the formation lithology on the groundwater, the major groundwater sources that feed the Cross River basin from the area are mineralized connate water and water from precipitation.
References
Ahiarakwem CA (2004) Physical and chemical properties of Ubaha spring water, southeastern Nigeria. J Niger Assoc Hydrogeol 14, 15(2):6–8
Chebotarev II (1955) Metamorphism of natural water in the crust of weathering. Geochim Cosmochem Acta 8:198–212
Davis SN, De Wiest RJM (1966) Hydrogeology. Wiley, New York, p 463p
Freeze AR, Cherry JA (1979) Groundwater. Prentice Hall, Englewood Cliffs
Kevin MH (2005) Hydrogeology (principles and practice). Blackwell, Oxford, UK, p 389
Nton ME, Adejumo SA, Elueze AA (2007) Hydrochemical assessment of surface water and groundwater quality. Glob J Geol Sci 5(1, 2):13–23
Nwankwo EB (1988) Hydrogeology of Enugu and environs. Unpublished M.Sc. thesis. University of Nigeria Nsukka
Offodile ME (2002) Groundwater study and development in Nigeria. Mecon Geology and Engineering Services Ltd, Nigeria, p 453
Okogbue CO, Ukpai SN (2013) Evaluation of trace element contents in groundwater in Abakaliki Metropolis and around the abandoned mine sites in the southern part, Southeastern Nigeria. Environ Earth Sci 70:3351–3362
Piper AM (1944) A graphic procedure in the geochemical interpretation of water analysis. Trans Am Geophys Union 25:914–923
Reyment RA (1965) Aspects of the geology of Nigeria. Ibadan University Press, Ibadan, p 148
Sawyer GN, McCarthy DL (1967) Chemistry of sanitary Engineers, 2nd edn. McGraw Hill, New York, p 518
Todd DK (1980) Groundwater hydrology. Wiley, New York
World Health Organization (WHO) (2004) Guidelines for drinking-water quality, 3rd edn
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ukpai, S.N., Nnabo, P.N. & Eze, H.N. Groundwater facie analysis in the upper Cross River basin, southeast Nigeria. Environ Earth Sci 75, 1345 (2016). https://doi.org/10.1007/s12665-016-6139-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-016-6139-7