Abstract
Two hundred and fifty samples were collected from fifty-five (No) boreholes on quarterly basis between March 2011 and October 2012 covering the wet and dry seasons within the Lower Pra Basin for water quality assessment. The analytical results show that groundwater being used by some communities within the basin is contaminated, with Al (19.2 % of boreholes), Se (18.4 % of boreholes), Cd (18 % of boreholes), As (11.6 % of boreholes), Pb (39.6 % of boreholes), Mn (5.6 % of boreholes), Hg (42 % of boreholes) and Fe (21.6 % of boreholes) at levels exceeding the WHO (Guidelines for drinking water quality. Revision of the 1993 guidelines, 2004) guideline limits for drinking water. The stability of iron species for the groundwater system under the prevailing pE/pH condition shows that amorphous Fe(OH)3 significantly controls the concentration of iron in groundwater within the basin. The results also show that pyrite and arsenopyrite oxidation processes in groundwater within the basin are not exclusively responsible for the concentration of iron in the boreholes. Calculated saturation indices (SI) of the iron and manganese species using PHREEQC for Windows show that the groundwaters are generally undersaturated with respect to melanterite (FeSO4·7H2O), siderite (FeCO3), hausmannite (Mn3O4), pyrolusite (MnO2), rhodochrosite (MnCO3), manganite (MnOOH) and pyrochroite [Mn(OH2)], and supersaturated with respect to goethite (FeOOH) and haematite (Fe2O3). Human health risks assessment show that, for both adults and children via ingestion route, the mean HQing levels were found in the order of Zn > Fe = Cu > Pb > Cd >Mn. Results further show that the HQ/HI is the same for both adults and children for all trace metals and are less than 1. Thus, the trace metals considered in this study are not of concern for potential human health risk caused by exposure to non-carcinogenic elements.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The quality of groundwater was hitherto generally considered of “good quality” and therefore served as the primary source of potable water supply to the world’s population, especially in rural areas due to the contamination of surface waters through anthropogenic activities. In view of this, Governments all over the world, especially in Africa (including Ghana), focused on the provision of groundwater to serve the drinking and domestic water needs of their people. However, groundwater has over the last decades been known to be contaminated through natural geochemical and biochemical processes as well as from anthropogenic activities.
One of the environmental concerns in relation to groundwater contamination is trace metal contaminants. Elevated concentrations of trace metal contaminants have widely been reported in groundwater (Smith et al. 2000; Brindha et al. 2010; Armah et al. 2014). Generally, the presence of trace metals in the environment is undesirable, especially if they occur in high amounts due to the non-biodegradable and persistence characteristics of trace metals in the environment. Trace metals constitute the largest class of elements in the periodic table and are conventionally defined as elements with metallic properties and have high atomic numbers with densities greater than 5.0 g/cm3 (Adriano 2001; Thomson 2005). Trace metals occur naturally at levels that are not considered to have any toxic effects to plants, animals and humans. The natural levels of trace metals are normally increased through various anthropogenic processes (Alloway 1995; Al-Chalabi and Hawker 1996). Anthropogenic sources of trace metals include agricultural land uses (fertilizers, animal manures and pesticides), metallurgy (mining, smelting and metal finishing), sewage sludge, scrap disposal and energy production such as power plants, leaded and unleaded petrol (Adriano 2001).
Cases of human poisoning by high proportions of trace metals have been reported. For instance, the World Health Organization (WHO) (1995) has reported that, due to diseases related to polluted water and toxic metals present in water, approximately five million cases of deaths were recorded. In Bangladesh, arsenic concentrations in about 50 % of groundwaters collected from shallow and deep wells were above the maximum permissible level of 0.05 mg/L. Additionally, 35 % of waters from 41 arsenic-affected districts in Bangladesh were found to have arsenic concentrations above 0.05 mg/L (Smith et al. 2000). It has also been confirmed that groundwater in Bangladesh is severely contaminated with arsenic (Smith et al. 2000). As a consequence, a large number of the populations in Bangladesh suffer from the toxic effects of arsenic-contaminated water (Smith et al. 2000). Thus, arsenic is capable of infiltrating groundwater systems irrespective of whether mining has taken place or not. Trace metals can enter the body as part of food, through inhalation and ingestion or by absorption through the skin (Annegarn and Scorgie 2002).
Metallic elements such as Fe, Mo, Cr, Zn, Cu and Co are known to be essential and play very vital roles in metabolic processes in plants and animals at trace levels; however, they are potentially toxic in high concentrations (Chimuka and John 2005). For instance, copper is an essential trace element and forms a part of several enzymes including tyrosinase, which aids the formation of melanin pigment. Additionally, Cu is very important in the utilization of iron; however, in high concentrations, Cu is capable of causing anaemia, liver and kidney damage in addition to stomach and intestinal irritation (Majolagbe et al. 2013). Trace metals such as Pb, Ni and Cd are potentially toxic even at trace concentrations and adversely affect human life under different health conditions. Cadmium is also an unavoidable by-product in the process of Zn refining and is acutely toxic with chronic exposure (Majolagbe et al. 2013). Adverse health effects of cadmium include kidney damage; there is epidemiological evidence that Cd is associated with an increased incidence of cancer of the prostrate (Majolagbe et al. 2013). Lead is known to have adverse impacts on brain development in children (Liu et al. 2007). When in high concentrations, Pb can substitute for calcium in the bones, which results in Pb poisoning (Majolagbe et al. 2013). Children are particularly susceptible to this form of lead poisoning due to the high level of calcium required for the development of skeletal systems (Majolagbe et al. 2013). Several human health risk assessments have been employed previously to evaluate the adverse health risks associated with exposure to toxic metals via oral ingestion and absorption through the skin for children and adults (USEPA 1989; Wu et al. 2009; Li and Zhang 2010; Liang et al. 2011).
The Central and Western regions (where the Lower Pra Basin is located), like any other rural community in Ghana, heavily rely on groundwater as a primary source of water supply due to the widespread contamination of surface water sources through farming and mining activities. Mining is likely to be the most significant source of metals in surface and groundwater within the basin. Exposure of mine waste rocks containing sulphide minerals to the atmosphere often results in the generation of acid mine drainage and subsequently the mobilization of trace metals. Additionally, small-scale mining operations take place within the basin, where artisanal miners indiscriminately use mercury (Hg) and other chemicals, which are detrimental to human health during mining operations. Consequently, potable water sources including some groundwaters within the basin have become contaminated and unsafe for drinking. The major gold ore within the basin is refractory quartz-Fe/As sulphide lode gold (Marston et al. 1992). Junner et al. (1942) reported that pyrite is widespread in several parts of the igneous rocks and quartz veins that underlie the study area. There is therefore high probability of heavy metal pollution of the groundwater, particularly in rocks underlain by the Birimian Supergroup within the Lower Pra Basin.
Despite the appreciable literature on the susceptibility of groundwater within the Lower Pra Basin to trace metal pollution, the results are difficult to interpret due to the limited nature and scattered distribution of similar studies within the basin. Consequently, it has been historically difficult to obtain an explicit understanding of the sources of trace metals in groundwater within the basin. The aim of this study therefore is to obtain sufficient groundwater quality data to assist in the explicit understanding of the spatial distribution of metals in groundwater within the basin as well as distinguish geogenic sources of metals from anthropogenic sources of contamination. The specific objectives of the study are: (1) to assess the levels of the selected trace metals in groundwater within the basin; (2) to identify the sources of the selected trace metals in groundwater within the basin; and (3) to assess the health implications of the selected trace metals (if any) on the consuming public.
Materials and methods
Study area
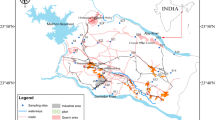
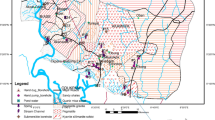
The Lower Pra Basin lies within 05°0′0″ and 06°0′0″N and 01°0′0″ and 02°0′0″W (Fig. 1). The climate falls under the wet semi-equatorial climatic zone of Ghana (Dickson and Benneh 1980). The basin comes strongly under the influence of the moist south-west monsoons during the rainy season. It is quite humid (humidity 60–95 %) with annual rainfall that is in the range of 1500–2000 mm. The average minimum and maximum temperatures are 21 and 32 °C, respectively, for the cooler periods of June–September/October (Dickson and Benneh 2004). There are two wet seasons: the major wet season (April–July) with the maximum rains between June and July, and the minor rainy season (October–November). The Pra Basin is part of the south-western basin system in Ghana and has a drainage area of 23,188 km2 and an estimated mean annual discharge of 214 m3/s (Dickson and Benneh 2004). The basin lies completely within the forest ecological zone of Ghana and has moist semi-deciduous forest with valuable timber species (Dickson and Benneh 2004). Owing to the development of the cocoa industry, the initial forest has been modified to a secondary forest consisting of shrubs, soft woody plants and climbers (Dickson and Benneh 2004). Several trees in the middle and upper layers display deciduous characteristics (Dickson and Benneh 2004). The Lower Pra Basin is predominantly dominated by the forest orchrosols, and to a lesser extent, the forest orchrosol–oxysol integrate (Dickson and Benneh 2004). The orchrosols are highly coloured soils with little leaching characteristics (Dickson and Benneh 2004).
Geology
The basin is predominantly underlain by granitic rocks of the Cape Coast and Dixcove granitoids (Fig. 1). Various portions of the basin are also underlain by metasediments of the Birimian and Tarkwaian (Kesse 1985).
Cape Coast granitoid
The bulk of the Cape Coast granitoid is a granitic to quartz dioritic gneiss. The gneissic rocks are intruded by both acidic and basic igneous rocks, which include white and pink pegmatite, aplites, granodiorites and dykes (Ahmed et al. 1977). These granitoids also contain several enclaves of schists and gneisses (Ahmed et al. 1977). The Cape Coast granitic units are often well foliated, often magmatic and potash-rich granitoids and are normally in the form of muscovite, biotite, granite and granodiorite biotite gneiss, aplites and pegmatites (Ahmed et al. 1977). They are generally associated with Birimian metasediments, and their internal structure is always concordant with those of their host rocks (Ahmed et al. 1977). The Cape Coast granitoid is believed to represent a multiphase intrusion consisting of four separate magmatic pulses (Ahmed et al. 1977). It is believed that the last phase of the Cape Coast granitoid is associated with the upper group of Birimian metasediments (Ahmed et al. 1977). Their general mineralogical composition includes quartz, muscovite, biotite, microcline, albite, almandine, beryl, spessartite, tourmaline, columbite/tantalite and kaolin (Kesse 1985).
Dixcove granitoid
The Dixcove granitoid consists of hornblende granite or granodiorite grading locally into quartz diorite and hornblende diorite (Ahmed et al. 1977). This complex forms non-foliated discordant to semi-discordant bodies in the enclosing country rocks, which are generally Upper Birimian metavolcanics, numerous enclaves of which are found within the granite complex (Ahmed et al. 1977). The Dixcove granitoid is intruded along deep-seated faults in three distinct phases, which follow one another from basic to acid gabbro–diorite–granodiorite (Ahmed et al. 1977).
Birimian supergroup
The Birimian supergroup comprises the Lower and Upper Birimian and are separated from the Tarkwaian by a major unconformity (Kesse 1985). The Lower Birimian is predominantly pelitic in origin having muds and silts with beds of coarser sediments (Kesse 1985). The Upper Birimian is predominantly of volcanic and pyroclastic origin (Kesse 1985). The rocks consist of bedded group of tuffs, sediments and mafic lavas (greenstones), with minor bands of phyllite that include a zone of manganiferous phyllites containing manganese ore (Kesse 1985). The sequence is intruded by batholithic masses of granite and gneiss (Kesse 1985). These predominantly argillaceous sediments were metamorphosed to schist, slate and phyllite, with some interbedded greywacke (Kesse 1985).
The Tarkwaian
The Tarkwaian consists of an overall fining-upwards thick clastic sequence of argillaceous and arenaceous sediments with two well-defined zones of pebbly beds and conglomerate in the lower members of the system (Junner et al. 1942). The Tarkwaian rocks comprise slightly metamorphosed, shallow-water, sedimentary strata, primarily sandstone, quartzite, shale and conglomerate resting unconformably on and derived from rocks of the Birimian supergroup (Junner et al. 1942).
Aquifer characteristics
Boreholes within the basin are generally shallow with depths which ranged 22–96 m and a mean value of 44.42 m. Borehole yield is generally low and largely variable, ranging from 0.4 to 51.7 m3/h with a mean value of 4.55 m3/h. Yields from individual boreholes are usually greatest in fractured schist and granite bedrock aquifers. The fractures in the rocks are generally open. The granite and schist rocks are exposed, whilst the Birimian and Tarkwaian rocks have thick overburdens. The soils develop over the same kind of highly weathered parent material with lateritic to clayey top soil layer, the thickness of which generally ranges from 4 to 14 m, but the soil layer thickness may be greater in some areas. The static water levels of the boreholes generally ranged 0.4–22.4 m with a mean value of 0.37 m. Static water levels in most boreholes are above the top of the aquifer, suggesting that the aquifers are either confined or semi-confined. The gneiss and granite associated with the Birimian rocks are of significant importance in the water economy of Ghana since they underlie extensive and usually well-populated areas (Dappah and Gyau-Boakye 2000). Inherently, they are not permeable, but have developed secondary permeability and porosity as a result of fracturing and weathering (Dappah and Gyau-Boakye 2000). In areas where precipitation is high and weathering processes penetrate deeply along fracture systems, the granite and gneiss usually have been eroded down to low-lying areas (Dappah and Gyau-Boakye 2000). Conversely, in areas where the precipitation is relatively low, the granite occurs in massive poorly jointed inselbergs that rise above the surrounding lowlands (Dappah and Gyau-Boakye 2000). In some areas, weathered granite or gneiss forms permeable groundwater reservoir (Dappah and Gyau-Boakye 2000). Favourable locations for groundwater storage include major fault zones (Dappah and Gyau-Boakye 2000). The Birimian phyllite, schist, slate, greywacke, tuff and lava are generally strongly foliated and fractured (Dappah and Gyau-Boakye 2000). Where they crop out or are near the surface, considerable water may percolate into fractured and weathered bedrock (Dappah and Gyau-Boakye 2000).
Land uses
Farming (cocoa and food crops) and gold mining are the primary land uses within the basin. Large acreages of virgin forest have been removed and replaced with cocoa farms within the basin. In addition, food crops such as cassava, yam, cocoyam, plantain as well as fruits such as banana and oranges are produced together with cocoa for subsistence. Gold mining within the basin is of two types, “large scale” and “small scale” (“Galamsey”). “Large-scale” mining is conducted by heap leach technique or roasting of ore. Oxidized ores derived from sulphides (principally arsenopyrite, realgar, orpiment and pyrite) in the weathered zones are heap leached by cyanidation. Paleoplacer (free milling ore) are mined from deep zones crushed, milled and cyanided (Kortatsi 2007). “Small-scale” mining by artisanal miners involves extracting gold from ochrosols soils mainly from stream floors by mercury amalgamation (Kortatsi 2007).
Sampling and analysis
Two hundred and fifty groundwater samples were collected from fifty-five (No) boreholes on quarterly basis between March 2011 and October 2012 covering the wet and dry season within the basin for sulphate and trace metals assessment. Sampling protocols described by Claasen (1982) and Barcelona et al. (1985) were strictly observed during sample collection. Samples for sulphate determination were collected into high-density linear polyethylene (HPDE) 1-L bottles without preservation, while samples for trace element analyses were collected in high-density linear polyethylene (HPDE) 250-ml bottles. The bottles were cleaned at the Environmental Chemistry and Sanitation Engineering Laboratories of the Council for Scientific and Industrial Research-Water Research Institute (CSIR-WRI) in Accra using detergent and allowing them to stand for at least 24 h. The bottles were then triple rinsed with distilled and de-ionized water. Samples for trace metal analyses were acidified to a pH < 2 using concentrated HNO3. On-site measurements of temperature, pH and electrical conductivity (EC) were carried out using a Hach Sension 1 m, while on-site measurements of redox potential (Eh) were carried out using a Hach Sension 156 m. These measurements were undertaken in a separate sampling bottle after rinsing three times with de-ionized water followed by the sample to be measured. The reading for each on-site parameter was recorded only after stability was achieved in the meter. The possibility of the pump and pump systems retaining stagnant water was marginal for boreholes since most of the boreholes were well utilized. However, the boreholes were pumped/purged before sampling was carried out to avoid taking non-representative samples. Each borehole was pumped/purged for at least 5 min before sampling to avoid mixing water with air during sampling. The temperature, pH, Eh and electrical conductivity were measured at the water point. All samples were stored on ice in an ice-chest and transported to the CSIR-Water Research Institute laboratories in Accra, stored in a refrigerator at a temperature of <4 °C and analysed within 1 week. Samples for sulphate determination were analysed using spectrophotometer at 420 nm. The concentrations of Cu, Fe, Mn, Cd, Zn and Pb were determined using Agilent 240FS atomic absorption spectrometer by direct aspiration of water samples into an air acetylene flame and Al into nitrous oxide acetylene flame. Se and As were determined using a hydride generator attached to the atomic absorption spectrometer, while Hg was determined using AAS-cold Vapour (VGA77) attached to the atomic absorption spectrometer.
The detection limits for the selected trace metals are: Cu (0.02), Cd (0.002), Mn (0.005), Fe (0.01), Pb (0.005), Hg (0.01), As (0.001), Al (0.01), Cd (0.002) and Se (0.005).
Quality control
To ensure the accuracy of the trace metal data, standard reference material (NIVA 1042L) for all trace metals (except Hg, As and Se) from the Norwegian Institute for Water Resources were analysed alongside the samples. In the case of Hg, As and Se, internal control standards were prepared using high-purity commercially prepared reagents. All glasswares used during analysis were thoroughly washed by soaking them in 5 % HNO3 overnight followed by thorough rinsing in distilled water before use. To ensure reproducibility, readings were replicated after every ten samples.
Statistical analysis
In order to determine the mineral reactivity of iron and manganese species responsible for Fe2+ and Mn2+ concentration in groundwater within the basin, calculated saturation indices (SI) were performed using PHREEQC for Windows. To also ensure normality of the data, all trace metal data and sulphate values (except Eh and pH) were log-transformed prior to statistical analyses. The trace metal data were also auto-scaled by calculating the standard scores (z scores) and ensuring that all z scores were < ±2.5. For concentrations below detection limits, one half of the value of their respective detection limit was substituted and used in statistical analysis.
Results and discussion
Table 1 presents the mean trace metal concentrations in groundwater within the Lower Pra Basin and their GPS co-ordinates, while Table 2 presents the summary statistics for trace metal levels in groundwater within the Lower Pra Basin and guideline limits for trace metal constituents in drinking water. Table 3 presents the trace metal doses and their implications for human health alongside the per cent (%) of population with possible potential exposure to contamination based on WHO (2004). Table 4 presents Fe2+/SO4 2− molar ratios for groundwater within the Lower Pra Basin, while Table 5 presents the saturation indices for iron species in groundwater calculated using PHREEQC for Windows and Table 6 presents the saturation indices of manganese species in groundwater calculated using PHREEQC for Windows. Mean concentrations of trace metals were compared with the WHO (2004) guideline limit. The results show that the mean concentrations of Fe, Pb and Hg were above the WHO (2004) guideline limit, while the mean concentrations of Cd, Cu, Mn, Zn, Se, Al and As were below the WHO (2004) guideline limit. The quotient (the ratio of the mean concentration to the WHO guideline limit) of trace metals in groundwater was either many times above or below the WHO guideline limit (Table 2). The results also show that Al (19.2 % of boreholes), Se (18.4 % of boreholes), Cd (18 % of boreholes), As (11.6 % of boreholes), Pb (39.6 % of boreholes), Mn (5.6 % of boreholes), Hg (42 % of boreholes) and Fe (21.6 % of boreholes) exceeded the WHO (2004) guideline limit. It is thus recommended that much greater attention should be paid to managing contaminants of particular concern (Fe, Pb and Hg) in groundwater within the basin.
Iron (Fe)
Fe2+ concentrations in groundwater within the basin ranged 0.005–2.53 mg/L, with a mean value and standard deviation of 0.34 (±0.46) mg/L. The WHO (2004) guideline limit for iron in drinking water is 0.3 mg/L (Table 2). The results obtained in this study have indicated that 21.6 % of boreholes had iron concentrations above the WHO (2004) guideline limit for drinking water (Table 3). The relatively high levels of Fe2+ found in 21.6 % boreholes within the basin could be due to the presence of pyrite and arsenopyrite in the rock matrix of the Birimian rocks, which is associated with some portions of the geology of the Lower Pra Basin (Kesse 1985). The oxidation and dissolution of pyrite and arsenopyrite are expected to be a substantial source of Fe2+ and SO4 2− in groundwater within the basin. Equations 1 and 2 show how iron and sulphate are released by the oxidation of pyrite and arsenopyrite, respectively:
Ideally, these reactions produce high sulphate concentrations, low pH and Fe2+/SO4 2− molar ratios of 0.5 (for pyrite oxidation) and 1.0 (for arsenopyrite oxidation). On the contrary, the generally very low SO4 2− concentration of 0.04–0.95 mmol/L (except Zion camp with a sulphate concentration of 3.68 mmol/L) coupled with the fact that no groundwater satisfied the Fe2+/SO4 2− molar ratios of 0.5 and 1.0 for the stoichiometry of pyrite and arsenopyrite oxidation, respectively (Table 4) suggests that pyrite and arsenopyrite oxidation processes in groundwater within the basin are not exclusively responsible for the concentration of iron in the boreholes.
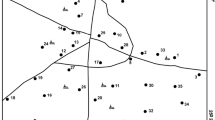
The stability of iron species for the groundwater system under the existing pE/pH condition (at 25 °C and 1 atmosphere) within the Lower Pra Basin is presented in Fig. 2, while the calculated saturation indices (SI) of the iron species: goethite (FeOOH), melanterite (FeSO4·7H2O), haematite (Fe2O3), siderite (FeCO3) and amorphous Fe(OH)3 as calculated using PHREEQC for Windows are presented in Table 5. Figure 2 shows that groundwaters plot in the Fe2+/Fe (OH)3 field, suggesting that amorphous Fe(OH)3 significantly controls the concentration of iron in groundwater within the Lower Pra basin. From Eq. 1, acid mine drainage in groundwater within the basin produces principally Fe(OH)3, which is, perhaps, the source of iron in groundwater within the basin. Additionally, the groundwaters are generally undersaturated with respect to melanterite and siderite (Table 5) and therefore capable of dissolving more melanterite and siderite depending on equilibrium or pE/pH conditions. The groundwaters are also generally supersaturated with respect to goethite and haematite (Table 5). Supersaturation of these minerals suggests that the groundwaters may have reacted with these minerals long enough to attain equilibrium and therefore not capable of dissolving more of these minerals (Kortatsi 2004). Thus, the groundwaters are capable of precipitating these iron minerals to reduce Fe2+/SO4 2− ratio (Kortatsi 2004). However, none of the boreholes had Fe2+/SO4 2− ratio close to or equal to 0.5 (pyrite oxidation) or 1.0 (arsenopyrite oxidation). Petrographic evidence exists for the presence of pyrite and arsenopyrite within the basin, especially in the Birimian rocks (Kesse 1985); therefore, the reduction of Fe2+/SO4 2−ratios due to the continuous precipitation of goethite and haematite is possible. Iron (Fe2+) in groundwater within the basin thus may have been derived significantly from continuous dissolution of amorphous Fe(OH)3, melanterite and siderite in the presence of organic matter until the water has attained equilibrium with these minerals (Kortatsi 2004). Though the organic matter content in the soil zone of the boreholes was not measured in this study, the brown colouration of streams and rivers within the basin throughout the study period is an indication of the presence of organic matter in the soil zone (Kortatsi 2004). Additionally, iron in groundwater within the basin may have been derived from leaching of minerals such as actinolite, chlorite, ankerite, hornblende, mica and biotite present in the rock matrices of the basin as a result of attack on these minerals by aggressive carbon dioxide (CO2)-charged groundwaters.
Copper (Cu)
The results have shown that most of the boreholes had Cu concentrations below detection limits. The WHO (2004) guideline limit of copper in drinking water is 2.0 mg/L (Table 2). Based on the WHO (2004), Cu concentrations in groundwater within the basin pose neither physiological nor aesthetic problems to groundwater quality within the basin. Cu2+ concentrations in groundwater within the basin could be attributed to the presence of oxygen and organic matter, thereby enhancing the process of aerobic degradation of organic matter, which, invariably, is intimately related to the deposition of Cu2+ (Das and Nolting 1993). A principal source of Cu in groundwater within the basin is perhaps, corrosion of interior copper plumbing (IPCS 1998; US NRC 2000).
Manganese (Mn)
Manganese occurs in groundwater within the basin mainly as manganese and manganiferous oxides, particularly in areas underlain by Birimian rocks (Junner et al. 1942; Kesse 1985). Saturation indices of the manganese species using PHREEQC for Windows (Parkhurst and Appelo 1999) (Table 6) have shown that groundwater within the basin is generally undersaturated with respect to all of the common manganese oxides and hydroxides such as hausmannite (Mn3O4), manganite (MnOOH), pyrochroite [Mn(OH2)], pyrolusite (MnO2) and rhodochrosite (MnCO3). Undersaturation occurs when: (1) the rock is depleted with respect to the mineral concerned, (2) the water has limited contact time with the rock to react to equilibrium, and (3) the ions exist in other forms in water (Kortatsi 2004). However, petrographic evidence exists for the presence of manganese and manganiferous oxides within the rocks of the basin, and therefore, sub-saturation due to depletion of the minerals in the rock is not likely. Reactions involving manganese are similar to those involving iron. Thus, if the contact time is largely sufficient for groundwater to be supersaturated with respect to most of the iron oxides as is the case of groundwater within the Lower Pra Basin, then the contact time for groundwater to be supersaturated with respect to manganese oxides and hydroxides should similarly be sufficient. Undersaturation with respect to the common manganese oxides and hydroxides therefore suggests that manganese exists in other forms, perhaps insoluble manganese complexes formed with other chemical constituents different from the common oxides and hydroxides.
Manganese dissolves under mildly reducing conditions to produce mobile divalent manganous ion (Mn2+). When exposed to air, the manganous ion is oxidized to hydrated oxides, which forms black colouration and stain on plumbing fixtures and laundry textiles. The growth of certain problematic bacteria that concentrate manganese and give rise to taste, odour and turbidity problems in distributed water is also supported by manganese (Griffin 1960; Wolfe 1960). Exposure to manganese at levels common in groundwater is associated with intellectual impairment in children (Bouchard et al. 2011). As is the case of iron, the sensory effect that it produces results to the rejection and abandoning of boreholes. The WHO (2004) guideline limit for manganese in drinking water is 0.4 mg/L (Table 2). Manganese concentrations in groundwater within the basin ranged 0.003–2.28 mg/L with a mean value and standard deviation of 0.162 (±0.34) mg/L. Results show that 5.6 % of boreholes had manganese concentrations above the WHO (2004) guideline limit for drinking water (Table 3). Boreholes with manganese concentration above the WHO (2004) guideline limit have the tendency of being abandoned in spite of the high cost of drilling. There is therefore the need to treat these boreholes by reducing the levels of manganese. Construction of iron removal treatment systems attached to these boreholes might be a possible solution as most of the boreholes with high iron content incidentally had high manganese content. Ferric hydroxide that may form in the iron removal plant during aeration has the capacity to absorb manganous ions, and therefore, the removal of both iron and manganese would take place (Kortatsi 2004).
Cadmium (Cd)
Cadmium concentration in groundwater within the Lower Pra Basin ranged 0.001–0.003 mg/L, with a mean value and standard deviation of 0.001 (±0.0) mg/L. Based on the result from this study, approximately 18 % of groundwater within the basin had cadmium concentrations in excess of WHO (2004) guideline limit for drinking water of 0.003 mg/L (Table 3). Sources of cadmium contamination include plating operations and the disposal of cadmium-containing wastes (Smith et al. 1995). Other sources of cadmium in drinking water are corrosion of galvanized pipes, erosion of natural deposits, discharge from metal refineries, runoff from waste batteries and paints. However, there are no metal refineries and runoffs from waste batteries and paints are rare within the basin. This leaves corrosion of galvanized fittings used for the construction of the boreholes as one of the probable source through which cadmium can be released in significant quantities into groundwater within the basin.
Zinc (Zn)
Zinc concentrations in groundwater within the Lower Pra Basin ranged 0.003–0.395 mg/L, with a mean value and standard deviation of 0.021 (±0.02) mg/L. This study therefore shows that zinc concentrations in groundwater within the Lower Pra Basin do not pose quality problem for groundwater supply and development within the basin.
Lead (Pb)
Lead concentrations in groundwater within the Lower Pra Basin ranged 0.003–0.062 mg/L with a mean value and standard deviation of 0.017 (±0.02) mg/L. The results have indicated that approximately 39.6 % of boreholes within the basin had lead levels in excess of WHO (2004) guideline limit of 0.01 mg/L (Table 3). The relatively high levels of lead in groundwater occurred in Assin Breku, AssinNyankomase, Brofoyedru Habitat, Sabina, Somnyamekordur, Nkrafo, Ayitey, Obirikwaku, Obobakokrowa, Akonfude and Mamponso communities, which are underlain by rocks of the Cape Coast/Dixcove granitoid and by Birimian rocks. This suggests that consumers living in these communities may be potentially at risk of possible metabolic poisoning, particularly children under 5 years and pregnant women are at potential risk of elevated lead levels in the blood stream (Moskowitz et al. 1986).
Selenium (Se)
Sources of selenium contamination include: discharge from petroleum and metal refineries; erosion of natural deposits; and discharge from mines. However, there are no petroleum and metal refineries within the basin. The most likely source of selenium in the basin is discharge from mines (small-scale mining activities) through which selenium can be released in significant quantities into groundwater. The concentrations of selenium in groundwater within the basin ranged 0.001–0.01 mg/L with a mean value and standard deviation of 0.005 (±0.001) mg/L. The WHO (2004) guideline limit of selenium in drinking water is 0.01 mg/L (Table 2). Based on WHO (2004), approximately 18.4 % of boreholes within the basin had selenium levels above the WHO (2004) guideline limit. Selenium could pose a serious threat to groundwater management and development in these communities.
Aluminium (Al)
Aluminium concentrations are often controlled by the precipitation of amorphous hydroxide [Al(OH)]5 (Šrácek and Zeman 2004). Thus, water with lower pH (acidic) is often accompanied by high concentration of aluminium (Šrácek and Zeman 2004). As expected, relatively higher aluminium concentrations in groundwater within the basin are associated with boreholes with lower pH values in some communities even though no distinct pattern was established (Fig. 3). Aluminium concentrations in groundwater within the Lower Pra basin ranged 0.005–0.727 mg/L, with a mean value and standard deviation of 0.136 (±0.17) mg/L. Aluminium speciation using PHREEQC for Windows (Parkhurst and Appelo 1999) suggests a phenomenon of Al3+ as the thermodynamically favoured under the prevailing pH/Eh conditions. Generally, aluminium (Al3+) appears to have only little deleterious effect on human. However, aluminium toxicity has been associated with central nervous system disorders including Alzheimer’s disease and dialysis dementia (Moskowitz et al.1986). The greatest problem associated with aluminium metal is the incidence of discolouration it produces in drinking water and its distribution systems that increases when aluminium concentrations exceed 0.2 mg/L, thereby rendering the drinking water aesthetically unacceptable (WHO 2004). On this basis, aluminium concentrations in groundwater within the Lower Pra Basin seem to pose a major quality problem to borehole water owing to the fact that, approximately, 19.2 % of the boreholes had Al3+ concentration exceeding the WHO (2004) guideline limit for drinking water (Table 3).
Arsenic (As)
Groundwater within the Lower Pra Basin generally had As concentrations, which ranged 0.001–0.019 mg/L, with a mean value and standard deviation of 0.005 (±0.002) mg/L. Based on these values, 11.4 % of the shallow boreholes (depths <100 m) had arsenic concentrations in excess of the WHO (2004) guideline limit of 0.01 (p) mg/L (Table 3). The generally low concentrations of arsenic in groundwater within the basin notwithstanding the presence of pyrite and arsenopyrite minerals in the rocks within the basin suggest a level of co-precipitation of arsenic with ferric oxyhydroxide in the creeks before possible infiltration into the aquifer (Kortatsi 2004). The generally very low SO4 2− concentration of 0.04–0.95 mmol/L coupled with the fact that no sample satisfied the iron to sulphate (Fe2+/SO4 2−) molar ratios of 0.5 and 1.0 for the stoichiometry of pyrite and arsenopyrite oxidation, respectively (Table 4) suggests that pyrite and arsenopyrite oxidation processes in groundwater within the basin may not be exclusively responsible for the concentration of As in the boreholes. According to Smedley et al. (1995), arsenic occurs in high concentrations in association with manganese and iron ores, especially sulphide minerals and particularly pyrites. Saturation indices of the iron species melanterite, siderite and amorphous Fe (OH)3, as calculated using PHREEQC for Windows (Table 4) suggests that the groundwaters are generally undersaturated with respect to these minerals and therefore will continuously dissolve in solution depending on the Eh/pH conditions. This suggests that pyrite and arsenopyrite minerals may have undergone extensive oxidation, hydrolysis and co-precipitation of iron and arsenic as Fe3+/ferrihydrite as a result of the introduction of oxygenated air within the aquifer. The generally low levels of As in groundwater within the basin suggest that currently mining has an insignificant impact on As concentrations in groundwater within the basin.
The WHO (1993) restricted the level of As in drinking water based on its carcinogenicity, and taking into consideration its potential nutrient requirement, provisionally to 0.01 mg/L. Thus, the 11.6 % of boreholes which had arsenic slightly in excess of the WHO (2004) guideline limit located in Odumase Camp, Dwendaama, Sienchem, Akonfude and Atu kurom posses potential diseases associated with long-term low-level exposure to consumers in these communities. Nevertheless, Wang and Huang (1994) noted that no morbidity cases were found where As concentrations in drinking water were less than 0.1 mg/L but morbidity increased exponentially as aqueous As increased, and indeed, mild As poisoning was observed in the range of 0.1–0.2 mg/L. Since no borehole within the basin had As concentration exceeding 0.1 mg/L, it suggests that though the concentrations were slightly in excess of the WHO (2004) guideline limit, no morbidity is expected.
Mercury (Hg)
Groundwater within the Lower Pra Basin generally had Hg concentrations which ranged 0.001–0.01 mg/L with a mean value and standard deviation of 0.003 (±0.002) mg/L. Based on these values, approximately 42 % of the shallow boreholes (depths <100 m) had mercury concentrations significantly in excess of the WHO (2004) guideline limit of 0.001 (p) mg/L (Table 3). However, there is no petrographic evidence of Hg in the rocks within the basin, neither are there industrial activities within the basin apart from small-scale mining activities that can release mercury in significant quantities into groundwater. The temporal variation of Hg in groundwater within the basin (Fig. 4) presents a trend which suggests that the concentrations of Hg in the boreholes are significantly in excess of the WHO (2004) guideline limit of 0.001 mg/L for drinking water during the rainy seasons (June–October). However, the concentrations of Hg become 0.0 mg/L or below detection limit during the dry seasons (January–March). This observation suggests that Hg concentrations in groundwater within the basin increase during the rainy season. Assuming that the mercury concentration in groundwater within the basin was derived from the rocks, it would have been expected that there would be a uniform and even distribution in Hg concentrations within the basin regardless of the season (wet or dry) all year round (Kortatsi 2004). However, the results suggest a relation between the recharge regimes of polluted surface water and Hg concentrations, in which case during the rainy season surface water resources polluted with Hg as a result of the small-scale mining (“Galamsey”) activities recharge groundwater through infiltration and therefore reach the groundwater table. Subsequently, groundwater within the basin is potentially under threats of mercury contamination due to contaminated surface water resources, perhaps as a result of the indiscriminate use of mercury amalgamation through small-scale mining activities. The implication of the significantly higher Hg concentrations (in excess of WHO 2004 guideline limit for drinking water) mostly during the wet season is that consumers in the affected communities within the basin are potentially at risk from mercury poisoning and its associated health hazards such as kidney failure, brain and nervous breakdown, gastrointestinal tract irritation, ulceration and diarrhoea (WHO 1980).
Health effects of trace metals through drinking water consumption within the Lower Pra Basin
Several human health risk assessment studies on toxic trace metals in aquatic ecosystems have previously been undertaken (USEPA 1989; Wu et al. 2009; Li and Zhang 2010; Liang et al. 2011). Human lives may be exposed to toxic metals through three main pathways such as direct ingestion, inhalation through mouth and nose, and dermal absorption through skin exposures. According to USEPA (1989), Wu et al. (2009), direct ingestion and dermal absorption are often common for water exposure. A report contained in the USEPA Risk Assessment Guidance for Superfund (RAGS) methodology has indicated that the numeric expressions for risk assessment can be presented as in Eqs. 3 and 4(USEPA 1989):
where D ing is the exposure dose through ingestion of water (μg/kg-day); D derm is the exposure dose through dermal absorption (μg/kg-day); C water is concentration of the estimated metals in groundwater (μg/L); IR is the ingestion rate (L/day, 2.2 for adults and 1.8 for children); EF is the exposure frequency (days/year, 350); ED is the exposure duration (years, 70 for adults and 6 for children); BW is the average body weight (kg, 70 for adults and 15 for children); AT is the averaging time (days, 25,550 for adults and 2190 for children); SA is the exposed skin area (cm2, 18,000 for adults and 6600 for children); ET is the exposure time (h/day, 0.58 for adults and 1 for children); CF is the unit conversion factor (L/cm3, 0.001); and K p is the dermal permeability coefficient (cm/h), 0.001 for Fe, Mn, Cu, and Cd; 0.002; 0.004 for Pb, and 0.0006 for Zn (USEPA 1989; Li and Zhang 2010; Liang et al. 2011).
Assessment of the potential non-carcinogenic risks for exposure to contaminants was done by comparison of the calculated contaminant exposures from each exposure route with the reference dose (RfD) in order to produce the hazard quotient (HQ), defined as follows (USEPA 1989):
where HQing/derm is the hazard quotient via ingestion or dermal contact and is unitless and RfD ing/derm is the oral/dermal reference dose (μg/kg-day). The RfD ing and RfD derm values are contained in the literature (USEPA 1989; Wu et al. 2009; Li and Zhang 2010; Liang et al. 2011). The hazard quotient is a numeric estimation of the systemic toxicity potential posed by a single element within a single exposure route. In order to evaluate the overall potential for non-carcinogenic effects posed by more than one element, the computed hazard quotient for each element is integrated and expressed as a hazard index (HI) (USEPA 1989):
where HIing/derm is the hazard index via ingestion or dermal contact and is unitless. An HQ/HI > 1 is a concern for potential human health risks caused by exposure to non-carcinogenic elements (USEPA 1989).
A summary of the non-carcinogenic health risk assessment for the trace metals assessed in groundwater within the Pra basin is presented in Table 7. The results show that, for both adults and children via ingestion route, the mean HQing levels were found in the order of Zn > Fe = Cu > Pb > Cd >Mn. The human health risks posed by Al, Se, As and Hg were not assessed because their dermal permeability coefficients (K p ) and the oral (RfD ing) and dermal (RfD derm) reference doses were not available. The results also show that the HQ/HI is the same for both adults and children for all trace metals and are less than 1 (i.e. HQ/HI < 1). Thus, the trace metals considered in this study are not of concern for potential human health risk caused by exposure to non-carcinogenic elements.
Conclusions and recommendations
Results from this study show that groundwater in some communities within the basin contains metal concentrations in excess of drinking water guideline concentrations, with Al (19.2 % of boreholes), Se (18.4 % of boreholes), Cd (8.8 % of boreholes), As (11.6 % of boreholes), Pb (39.6 % of boreholes), Mn (5.6 % of boreholes), Hg (42 % of boreholes) and Fe (21.6 % of boreholes) concentrations exceeding the WHO (2004) guideline limits for drinking water. An assessment of the stability of iron species in groundwater under the prevailing pE/pH conditions suggests that amorphous Fe(OH)3 controls the concentration of iron in groundwater within the basin. The results also suggest that the oxidation of pyrite and arsenopyrite within the basin is not exclusively responsible for the concentration of iron in the boreholes, since the Fe2+/SO4 2− molar ratios for pyrite and arsenopyrite oxidation in groundwater within the basin do not match the required stoichiometry for these reactions. The calculated saturation indices (SI) of the iron and manganese species using PHREEQC for Windows have indicated that the groundwaters are generally undersaturated with respect to melanterite (FeSO4·7H2O), siderite (FeCO3), hausmannite (Mn3O4), pyrolusite (MnO2), rhodochrosite (MnCO3), manganite (MnOOH), and pyrochroite [Mn(OH2)], and supersaturated with respect to goethite (FeOOH) and haematite (Fe2O3). Human health risks assessment show that, for both adults and children via ingestion route, the mean HQing levels were found in the order of Zn > Fe = Cu > Pb > Cd >Mn. The HQ/HI is the same for both adults and children for all trace metals and are less than 1 (i.e. HQ/HI < 1). Thus, the trace metals considered in this study are not of concern for potential human health risk caused by exposure to non-carcinogenic elements. In order to safeguard groundwater within the basin, the Government of Ghana should enforce the existing laws on mining (especially small-scale or “galamsey” operations) to deter the small-scale miners from the indiscriminate amalgamation of gold with mercury (Hg) in order to prevent further pollution of the water resources within the basin and therefore ensure sustainability. It is also recommended that the Government of Ghana and other stakeholders within the Water Sector in collaboration with the mandated health institutions should institute health monitoring programs with respect to the health disorders associated with toxic trace metals such as Al, Se, Pb, Cd and Hg in order to prevent the long-term effects of intake of these trace metals in water.
References
Adriano DC (2001) Trace elements in the terrestrial environment. Springer, New York
Ahmed SM, Blay PK, Casto SB, Coakley GJ (1977) Geology of (¼)° Field sheets Nos. 33 Winneba NE 59, 61 and 62 Accra SW, NW and NE. Ghana Geological Survey Bulletin No. 32
Al-Chalabi AS, Hawker D (1996) Retention and exchange behavior of vehicular lead in street dusts from major roads. Sci Tot Environ 187:105–119
Alloway BJ (ed) (1995) Heavy metals in soils, 2nd edn. Blackie Academic and Professional Publishers, NY
Annegarn HJ, Scorgie Y (2002) Air quality—chemistry and physics of the atmosphere. University of the Witwatersrand, MSc Course work Notes
Armah FA, Quansah R, Luginaah I (2014) A systematic review of heavy metals of anthropogenic origin in environmental media and biota in the context of gold mining in Ghana. Int Sch Res Notices. doi:10.1155/2014/252148
Barcelona M, Gibb JP, Helfrich JA, Garske EE (1985) Practical guide for groundwater sampling. Illinois State Water Survey ISWS Contract Report 37
Bouchard MF, Sauvé S, Barbeau B, Legrand M, Brodeur MÈ, Bouffard T, Limoges E, Bellinger DC, Mergler D (2011) Intellectual impairment in school-age children exposed to manganese from drinking water. Environ Health Perspect 119(1):138–143
Brindha K, Elango L, Rajesh VG (2010) Occurrence of chromium and copper in groundwater around tanneries in Chrompet area of Tamil Nadu. Indian J Environ Prot 30(10):818–822
Chimuka L, John OO (2005) Metals in environmental media: study of trace and platinum group metals in Thohoyandou, South Africa. Water S.A. 31:581–586
Claasen HC (1982) Guidelines and techniques for obtaining water samples that accurately represent the quality of an aquifer. U.S. Geological Survey Open File Report 82-1024.
Dappah S, Gyau-Boakye P (2000) Hydrologic framework and borehole yields in Ghana. Hydrol J 8:405–416
Das JD, Nolting RF (1993) Distribution of trace metals from soils and sewage sludge’s Abay refluxing with aqua regia. Analyst 108:277–285
Dickson KB, Benneh G (1980) A new geography of Ghana, Second impression. Longman Group Limited, London
Dickson KB, Benneh G (2004) A new geography of Ghana, Fifth impression, Revised edn. Longman Group Limited, London
Griffin AE (1960) Significance and removal of manganese in water supplies. J Am Water Works Assoc 52:1326
Junner NR, Hirst T, Service H (1942) Tarkwa goldfield. Memoir No. 6. Gold Coast Geological Survey
Kesse GO (1985) The mineral and rock resources of Ghana. A.A/Balkema, Rotterdam/Boston
Kortatsi BK (2004) Hydrochemistry of groundwater in the mining area of Tarkwa-Prestea, Ghana. Unpublished PhD Thesis. University of Ghana, Legon-Accra
Kortatsi BK (2007) Hydrochemical framework of groundwater in the Ankobra Basin, Ghana. AquatGeochem 13:41–74. doi:10.1007/s10498-006-9005-4
IPCS (1998) Copper. Geneva, World Health Organization, International Programme on Chemical Safety, Environmental Health Criteria 200
Li S, Zhang Q (2010) Risk assessment and seasonal variations of dissolved trace elements and heavy metals in the Upper Han River, China. J Hazard Mater 181(1–3):1051–1058
Liang F, Yang S, Sun C (2011) Primary health risk analysis of metals in surface water of Taihu Lake, China. Bull Environ Contam Toxicol 87(4):404–408
Liu OM, Salmijah M, Ismail BS, Aminah A (2007) The impact of traffic causing lead exposure to Malaysian school children. Global J Environ Res 1(2):43–48
Majolagbe A.O., Yusuf, K.A., Duru, A.E. 2013. Trace metals characterization in Environmental media: a case study of cement production area, Ewekoro, Southeast, Nigeria. Eur Sci J. Special Edition Vol 3. ISSN 1857-7881 (Print) e-ISSN 1857-7481
Marston RJ, Woolrich P, Kwesi J (1992) Closely associated stock work and paleoplacer gold mineralization in the early Proterozoic Tarkwaian System of Ghana. Regional Trends in African Geology. In: Proceedings of the 9th international geological conference, Accra. 2nd–7th November 1992. Geological Society of Africa/Geological Society of Ghana
Moskowitz PD, Coveney EA, Hamilton LD, Kaplan E, Medeiros WH (1986) Identifying human population at risk from acid deposition mobilised materials in drinking-water supplies. A preliminary Pilot study. Acid precipitation and Health-Part 1 Water Quality Bulletin 11, No. 1.12-18 and 59
Parkhurst DL, Appelo CAJ (1999) PHREEQC for windows version 2.8.01. A hydrogeological transport model. U.S. Geological Survey Software
Smedley PL, Edmunds WM, West JM, Gardner SJ, Pelig-ba KA (1995) Vulnerability of Shallow Groundwater quality due to Natural Geochemical Environment. Health problems related to Groundwater in the Obuasi and Bolgatanga Areas, Ghana. Report prepared for ODA under the ODA/BGS Technology Development and Research Programme, Project 92/5
Smith LA, Means JL, Chen A, Alleman B, Chapman CC, Tixier JS Jr, Brauning SE, Gavaskar AR, Royer MD (1995) Remedial options for metals-contaminated sites. Lewis Publishers, Boca Raton
Smith AH, Lingas EO, Rahman M (2000) Contamination of drinking-water by arsenic in Bangladesh: a public health emergency. Bull World Health Organization (WHO) 78(8):1093–1103
Šráček O, Zeman J (2004) Introduction to environmental hydrogeochemistry. Faculty of Science, Masaryk University in BRNO, Brno. ISBN 80-210-3586-2
Thomson G (2005) Heavy metals and heavy metals poisoning. World of Chemistry. Thomson Corporation, Stamford
USEPA (1989) Report. EPA/540/1-89/002. 1989. Risk assessment guidance for superfund. United States Environmental Protection Agency, Vol. 1, Human Health Evaluation Manual (Part A). Washington, DC, USA
US NRC (2000) Copper in drinking water, National Research Council. National Academy Press, Washington, DC
Wolfe RS (1960) Microbial concentration of iron and manganese in water with low concentration of these elements. J Am Water Works Assoc 52:133
Wang L, Huang J (1994) Chronic Arsenism from drinking water in some areas of Xinjiang, China. In: Nriagu JO (ed) Arsenic in the environment, Part II: Human Health and ecosystem effects. Willey, New York, pp 159–172
World Health Organization (WHO) (1980) Recommended health-based limits in occupational exposure to trace metals. Technical Report Series, No. 647. Vienna, Austria
World Health Organization (WHO) (1995) Guidelines for drinking water quality. World Health Organization, Geneva 121
World Heath Organization (WHO) (2004) Guidelines for drinking water quality. Revision of the 1993 guidelines. Final Task Group Meeting, Geneva, 2003
Wu B, Zhao DY, Jia HY, Zhang Y, Zhang XX, Cheng SP (2009) Preliminary risk assessment of trace metal pollution in surface water from Yangtze River in Nanjing section, China. Bull Environ Contam Toxicol 82(4):405–409
Acknowledgments
The authors are grateful to the Government of Ghana through the Council for Scientific and Industrial Research-Water Research Institute for providing financial assistance and analytical facilities for this PhD study. We are also grateful to Dr. Anthony Duah a Research Scientist of the CSIR-WRI, for the maps of the study area.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tay, C.K., Hayford, E. Levels, source determination and health implications of trace metals in groundwater within the Lower Pra Basin, Ghana. Environ Earth Sci 75, 1236 (2016). https://doi.org/10.1007/s12665-016-6034-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-016-6034-2