Abstract
In this study, data on several metals (Cd, Zn, Cu, Ni and Pb) in soil and carabids Chlaenius (Chlaeniellus) olivieri sampled from northeastern Tunisia were presented. Samples were taken at six sites S1, S2, S3, S4, S5 and S6 alongside Kantra River close to three jeans fading industries: Lee Cooper, Denim authority and CRJ. Elemental compositions were assessed through the estimation of geochemical accumulation indices, pollution load index, the contamination factor and bioaccumulation factor (BAF), determined to reveal any potential toxic effects on biota. Significant enrichment of the soil with Cd, Zn, Cu, Ni and Pb was observed in all considered sites and shows a veritable metallic polluted state. The highest concentrations of metals recorded in soil were found at S1. In addition, negative correlation was observed between concentrations of heavy metals in the soil and the species. The BAF values show that C. olivieri is a macro-concentrator for Cd and a micro-concentrator for Zn. The finding provides a rich dataset of metals in several biotas and had operational significance for coexistence of heavy metals in both soil and ground beetles. C. olivieri can be used as a bioindicator in monitoring metals pollution of terrestrial ecosystem. Results can be useful for mitigating the impact of metallic pollution on environmental health and required strategies to prevent such effect.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anthropogenic metallic pollution is a worldwide threat, mainly due to urbanization and the frequently surrounding industries (Boldina-Cosqueric et al. 2010). Unlike other contaminants, metals are non-degradable and persisting in the environments with potential bioaccumulation and biomagnifications into the food chain (Kontas 2007). They pose severe environmental problems and managing ecological effects of affected streams remain important challenges (Luoma and Rainbow 2008). In this fact, metals have given a particular scientific concern as it is a major problem in the environment (Dong et al. 2011); however, in soil pollution, the major problem in the environment and metals exists either as separate entities or in combination in soil (Chandrasekaran et al. 2015). Some heavy metals such as Co, Cu, Fe, Mn, Ni, and Zn are required in low amounts by organisms. Nevertheless, high levels of these elements may become harmful for organisms (Chandrasekaran et al. 2015). Invertebrates are usually useful to assess the effects of anthropogenic activities on the terrestrial ecosystem, as they are in contact with toxic elements in the soil and the leaf litter (Heikens et al. 2001). In addition, metal residue measurements in invertebrates have been done in the framework of biomonitoring programs (Nico et al. 2001). Several studies have demonstrated detrimental effects on invertebrate populations in metal gradients around emission sources (Spurgeon and Hopkin 1996; Kuznetsova and Potapov 1997). Among terrestrial invertebrates carabid beetles are frequently used due to their resistance in highly contaminated areas (Avgin and Luff 2000; Bednarska et al. 2013; Lagisz and Laskowski 2008; Sklaski et al. 2011). As holometabolous insects, they are present in the soil during their life cycle, so they can accumulate pollutants (Cortet et al. 1999). Carabids are well known ecologically and taxonomically and characterized by their higher sensitive detection of environmental changes (Butovsky 2011; Rainio and Niemelä 2003). They were considered as bioindicators of some toxicant effects such as metals (Stone et al. 2002; Wilczek et al. 2003; Jelaska et al. 2007; Simon et al. 2016). However, further studies are needed to provide data on pollutant accumulation in carabids. The conducted study aims to the assessment of: (1) the revelation of spatial distribution of five heavy metals in soil and into beetles collected from Kantra River; (2) a screening of heavy metal contamination has been performed using multiple indices; (3) the natural and/or anthropogenic sources of these metals have been presented; finally (4) the environmental risks in the study area and a description of habitats of the beetles were provided.
Materials and methods
Study area
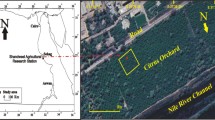
The study area was the Kantra River for the disposal of factories and urban wastewater from the town Ras Jebel situated in northeastern Tunisia. Three jeans fading industries: Lee Cooper, Denim authority and CRJ are installed alongside of this river that present a discharge receptor of these industries. Soil samples were collected from six sites at the Kantra River (Fig. 1).
Site 1 (37°08′26.54N, 9°06′33.54″E) is at the Kantra River bed situated 500 m east from the factory. It is characterized by an abundant vegetation of Rubus allegheniensis and Ilex aquifolium.
Site 2 (37°04′19.78N, 11°06′41.27″E) is at the right bank of the Kantra River 2 km from the factory. Its vegetation is mainly constituted by Nerium oleander and Quercus faginea.
Site 3 (37°21′66.48N, 10°07′42.13″E) is at the left bank of the Kantra River 3 km from the factory. The layer of grass and leaf litter is very thin and fragmented.
Site 4 (36°24′69.11N, 17°05′44.53″E) is 4 km from the factories and situated at the right bank of the Kantra River. The vegetation is composed of Salix pedicellata.
Site 5 (36° 62′95.16N, 22°11′47.78″E) is located at the left bank of the Kantra River 5 km from the factory. Agricultural runoff and irrigation return flow are other pollution sources at this site. The vegetation is formed by the Ficus carica and Populus alba.
Site 6 (36°81′79.68N, 32°24′51.74″E) is 7 km from the factory at the left bank of the Kantra River. Its vegetation is dominated by Celtis australis and Eucalyptus sp.
The control site (site C) was chosen in a relatively safe habitat. It belongs to the Ichkeul Park, at the coordinates 37′08′51.31″N 9°40′03.18″E, altitude: 46 m. It was qualified by high biodiversity and important potential resources (Hollis et al. 1986). Ichkeul has been registered, since 1977, as a Biosphere Reserve under the UNESCO MAB program, in 1979 as a Natural and Cultural Site and in 1980 as a wetland of international importance under the RAMSAR Convention.
Samples collection
Soil sampling was carried out during July 2015 using a 50 × 50 cm plot at each site, using a stainless steel corer (10 cm diameter). Samples were taken from the 0–20 cm upper layer, and were crushed and sieved. Carabids were collected simultaneously with live trapping. Live ground beetles were moved to the laboratory where they were transferred into polyethylene bags and were stored in freezing at −20 °C.
Chemical analyses of soil and animals
One gram of soil was weighed and kept in the oven at 450 °C for 2 h for calcinations. After cooling, 10 ml of hydrofluoric acid and 5 ml of perchloric acid were added. The sample was placed on a heating plate for evaporation towards dryness; 70 ml of hydrochloric acid 13 % are added. Afterward, the sample was filtered in phials of 100 ml.
Heavy metal concentrations in animals were measured in emerging adults reared on contaminated soil from site P (n = 5) and on uncontaminated control soil from site C (n = 5). All specimens were dried at 105 °C using a microwave and each animal was weighed. The digestion was performed by adding 3 ml of HNO3, 3 ml of H2O2 and 1 ml of H2O to each sample. The operating conditions for the microwave digestion system were the following: 720 W for 20 min. After digestion, the extracts were transferred into a graduated polypropylene test tube and diluted with ultrapure water to 50 ml according to Talarico et al. (2014). The soil extracts and animals were analyzed for Cd, Cu, Pb, Ni and Zn.
Metal concentrations in animals and in soil were measured by inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES, Perkin Elmer Optima 8000, France). The reagent blanks were monitored throughout the analysis and were used to accredit the analytical results. Standard reference material IAEA-SL-1 (lake sediment) was analyzed at the same time to ensure quality control and accuracy of the analysis. The concentrations measured for the reference sediment were in agreement with the certified values (accuracy <10 %).
Assessment of soil contamination
In this study, different indices were used to assess the degree of heavy metal contamination in soil and in the ground beetles of the Kantra River (Ras Jebel).
The contamination factor (CF)
Assessed as the ratio obtained by dividing the concentration of each metal in the soil by baseline or background value (concentration in uncontaminated soil):
According to Hakanson (1980), CF values were interpreted as follows:
-
If CF < 1: it means that low contamination exists.
-
If 1 < CF < 3: it means that moderate contamination exists.
-
If 3 < CF < 6: it means that considerable contamination exists.
-
If CF > 6: it means that very high contamination exists.
The pollution load index (PLI)
For the entire sampling site, PLI has been determined as the n-root of the product of the n CF:
For assessing the level of heavy metal pollution this empirical index provides a simple, comparative means. When PLI >1, it means that a pollution exists; otherwise, if PLI <1, there is no metal pollution (Tomlinson et al. 1980).
The geo-accumulation index (Igeo)
It is defined by the following equation: Igeo = Log2 (C n )/1.5 (B n ); where C n is the metals concentration in soil samples and B n is the geochemical background concentration of the metal (n). Factor 1.5 is the background matrix correction factor due to lithospheric effects. The geoaccumulation index consists of seven classes (Müller 1981). Class 0 (practically unpolluted): Igeo ≤0; Class 1 (unpolluted to moderately polluted): 0 < Igeo < 1; Class 2 (moderately polluted): 1 < Igeo < 2; Class 3 (moderately to heavily polluted): 2 < Igeo < 3; Class 4 (heavily polluted): 3 < Igeo < 4; Class 5 (heavily to extremely polluted): 4 < Igeo < 5; Class 6 (extremely polluted): 5 > Igeo (Bhuiyan et al. 2010).
Bioaccumulation factor
The bioaccumulation factor (BAF) under field conditions provides a simple estimation for metal accumulation in carabids, i.e. the ratio between the metal concentration in the beetle body (in mg/kg dry weight) and that in the soil (in mg/kg dry weight). The BAF was used to classify Chlaenius (Chlaeniellus) olivieri as a macroconcentrator (BAF >2), microconcentrator (1 < BAF < 2) or deconcentrator (BAF <1) as proposed by Dallinger (1993).
Statistical analyses
Analysis of variance (ANOVA) was performed to analyze the significant spatial differences (p < 0.05). Multivariate analysis of the River data set was performed using non-parametric multidimensional scaling (NMDS) ordination on the basis of Bray–Curtis similarity. The correlation was analyzed between toxic element concentrations of ground beetles and soil using Pearson correlation (Zar 1996).
Results and discussion
Heavy metals in soil of Kantra River
The concentration of heavy metals (mg kg−1 of dry soil) in soils of the study area P (Table 1) ranged from 0.9 to 3.8 for Cd, 32.9 to 205.2 for Cu, 12 to 45.5 for Ni, 13.8 to 78.3 for Pb and 167.5 to 682.3 for Zn. Besides Zn, the highest concentrations in soil were recorded for Cu, Pb and Ni and the lowest concentration was observed for Cd. Due to wastewater discharges from textile factory, site 1 presents the highest concentrations of heavy metals.
The minimum of Cd concentration (0.9 mg/kg) and Ni (12 mg/kg) were observed in site S4 (site P), and the minimum of Pb (13.8 mg/kg), Cu (32.9 mg/kg) and Zn concentrations (167.5 mg/kg) in site S6 (site P). The highest deposition of Zn in soil might be due to its long-period use in the production of machine tools, fading, paints, and alloying in different industries of the study area that could contaminate soil and modifies the soil structure and so make it less fertile for agriculture. In this study, total metal concentrations are in the following order S1 > S2 > S 3 > S5 > S4 > S6. The increase in some metal concentrations at site 5 may be due to the various anthropogenic activities in the Ras Jebel province releasing important amounts of heavy metals into the river. All considered metals showed significant differences (ANOVA, p < 0.05). The mean values of Cd, Cu, Ni, Pb and Zn were higher when compared with the control site C.
Heavy metals distribution among the sites is indicated in Fig. 2. The mean concentrations of Cd, Cu, Ni, Pb and Zn from the two areas were, respectively, 2.01, 111.48, 32.77, 40.06 and 376.10 from the area P and 0.16, 24.65, 21.84, 5.28 and 35.05 from area C. Total heavy metal concentrations in the soil samples from the Kantra River followed the order: Zn > Cu > Pb > Ni > Cd. Lower metals concentration were observed in sites 4 and 6, probably due to low anthropogenic activities in the area. Urbanization close to the site 4 was limited. The concentrations of heavy metal in the studied soil samples followed the order of sites S1 > S2 > S3 > S5 > S6 > S4 for Cd and Ni. Concentrations of Pb and Zn showed the following order: S1 > S2 > S5 > S4 > S3 > S6; whereas concentration of Cu showed the following order: S2 > S1 > S3 > S5 > S4 > S6. The extent of metals observed in soil of the study area (P) exceeded the permissible standard values proposed by the French association of normalization (AFNOR 1996) (except Pb and Ni), the European norm for non-polluted soils (IEEP 2009) (except Pb and Ni), and the limits for public green areas as established by current Italian legislation (Talarico et al. 2014) (except for Ni and Pb).
Comparison of metal contamination data on the Kantra River with published data on other rivers (Table 2) reveals that the soil are slightly contaminated with heavy metals, while the extent of metal contamination was much worse than the Axios River for Cu and Zn (Table 2).
Indices of soil contamination
The results of the contamination factors (CFS) and load index of pollution (PLI) are shown in Table 3. The highest value of CF for all studied metals was found on the site 1, which receives a huge quantity of metal discharge released by textile plant. In the study area P, the CF values for Cd were >6 in soil of sites 1, 2, 3 and 5, show thus a “higher contamination” but a “considerable contamination” for sites 4 and 6. By the same way, the CF values for Cu were >6 in soil at site 1, which indicates a “high contamination” and denotes a “considerable contamination” for site 3, and a “moderate contamination” for sites 4, 5 and 6. In soil of sites 1 and 2, the values of CF denoted a “considerable contamination” but a “moderate contamination” in site 3 (except Pb), sites 4, 5 and 6 (except Pb), while the CF values for Ni indicated a “low contamination” at all sites. Cd has the CF higher values among the five studied metals in all sites, whereas, Ni had the lowest CF values among them. A higher CF value was recorded at the sites 2 and 3 characterized by discharges of urban and industrial wastewater and at the site 5 which received urban wastewater. The order of the total contamination is as following S1 > S2 > S3 > S5 > S4 > S6. In the area C, CF values of metals for all sites showed a “low contamination” except Cd at site 2 denoting a “moderate contamination”.
The load index of pollution (PLI) varied from 0.28 to 5.12 (Table 3). According to the mean value of PLI (2.73), the Kantra River was moderately polluted. Sites 1 and 2 had the highest PLI, respectively, 5.12 and 4.14, within the study area P, indicating that the soils of these sites were strongly polluted by heavy metals. For PLI, values were between 1 and 2. For other sites it must be classified as moderately polluted. The order of PLI for sites is S1 > S2 > S5 > S3 > S4 > S6. In the area C the mean values of PLI (0.42) <1 show that there is no metal pollution in its soil.
Table 4 presents Igeo values of the studied metals. The Igeo values of Ni at all sites, Pb at sites 4, 5 and 6, Cu at sites 5 and 6, Zn at site 6 were under limits of detection, showing that these sites were safe. The Igeo values for Cu at site 4, Pb at site 3 and Zn at sites 3, 4 and 5 were under 1 which belong to “unpolluted to moderately polluted” class. Among studied metals, Cd, Cu, Zn, Pb and Ni have the highest Igeo values, respectively. The soils of site 1 presents the highest values and “heavily polluted” for the Igeo class for Cd; for soils of sites 2, 3 (except for Cu) and site 4 (except for Cu), were “moderately polluted” for the Igeo class of Cd and Cu, while at sites 5 and 6 and Cu at site 3 were “moderately polluted” for the Igeo class of Pb and Zn. Total Igeo values followed the order of S1 > S2 > S3 > S4 > S5 > S6.
Multivariate statistical analyses
Cluster analysis
The non-parametric multidimensional scaling (NMDS) was applied to analyze the affinities between metals. NMDS gives a dendrogram (Fig. 3) where all metals were grouped into two statistically significant groups at 78 % of similarity. Group 1 includes Cd which is identified as contaminant derived from anthropogenic sources (wastewater discharges of textile plant). Group 2 contains Cu, Pb, Ni and Zn derived from anthropogenic sources (wastewater discharges of textile factory, and domestic wastewaters).
Correlation matrix
In order to establish relationships between metals and determine the common source of heavy metals in the Kantra River, a correlation matrix was calculated for metals in the soils. According to the values of Pearson’ correlation coefficient (Table 5), a significant positive correlation existed between the considered metals. Ni was significantly correlated only with Cu, whereas Zn showed significant correlation with all metals except for Cd. These positive correlations indicate that these heavy metals have the same origin which is probably due to the wastewater discharged from textile factory.
Metal analyses in ground beetles
Based on Pearson correlation, we found negative correlation between all concentrations in soils and C. olivieri (Cd: r = −0.487, p = 0.649; Cu: r = −0.89, p = 0.06; Ni: r = −0.346, p = 0.821; Zn: r = −0.63, p = 0.434), which indicate that Cd, Cu, Ni and Zn are associated with the soil minerals. So, the soil cannot be the source of these elements for Carabids, since they are not directly assimilated by ground beetles. It is known that, the organic matter composition and soil properties modify the behavior of toxic elements in soil (Van Straalen et al. 2001). Since regulation efficiency of heavy metals varies among species, the toxic element concentrations in ground beetles did not reflect to the concentration in the soil (Kramarz 1999). Heikens et al. (2001) examined the Cd, Pb, and Cu concentration in several taxonomic groups and indicated that the bioaccumulation of Cd and Pb is correlated to the total soil concentration, i.e. the internal Pb concentration increased proportionally with soil concentration (Heikens et al. 2001). In addition, the internal Zn and Cu concentration is also related to the metal concentration of soil (Jelaska et al. 2007).
Bioaccumulation factor
Metal residues in animals were determined by the equilibrium between absorption and excretion. Such phenomenon, in the case of heavy metals, may be subject to the regulatory mechanisms that maintain more or less a range of internal concentrations. The rates of absorption and excretion indicate extremely large inter-species variability as reported for Cd by Janssen et al. (1991). As a result, there are large differences between species in their metal concentrations (Pokarzhevskii 1985; Van Straalen and Van Wensem 1986; Janssen and Hogervorst 1993). Unlike persistent organochlorines, metals were not accumulated in food chains (Laskowski 1991), except specialized predators that feed on prey accumulating metal (Van Straalen and Ernst 1991; Butovsky and Van Straalen 1995).
In our study, the element trace levels found in species from the C. olivieri population living in the vicinity of Kantra River are the same as those in the Kantra River soil except for Pb (Table 6). Indeed the highest metal concentrations were measured near to the factory. The BAF values for species from site P show that this species is a macroconcentrator for Cd (BAF = 9.89) and a microconcentrator for Zn (BAF = 0.18). The high bioaccumulation values of Cd (BAF = 9.89) in beetles at contaminated and control soils indicate that the contact with the soils plays an important role in bioaccumulation of such metals. Using BAF values our results demonstrated that ground beetles may accumulate the toxic metal Cd from soil, in indirect or direct way.
Our results are in good agreement with Talarico et al. (2014) who found high BAF values for As and Hg on C. lefebvrei, indicating that this species was a good indicator for As and Hg. In addition, Simon et al. (2016) have also found high BAF values for Cu, Zn and Pb on P. oblongopunctatus and C. violaceus. The metal concentrations in C. olivieri from area P and C are mentioned in Fig. 4 that present high concentrations of Zn, Cu, Cd, and Ni. The residues of metals in animals can be determined by the equilibrium between assimilation and excretion. According to Nico et al. (2001), to keep the internal concentrations within a more or less narrow range, uptake and excretion of heavy metals may be subject to regulatory mechanisms. However, metal kinetic studies in Carabids indicated that the physiological capabilities of species for detoxification by metal excretion may explain observed differences (Kramarz 1999; Lagisz et al. 2005). According to Talarico et al. (2014), various studies suggested that species differences in capability for element uptake and excretion are more important than the trophic level. A similar trend is evident for several studied species of ground beetles, which can discharge absorbed metals by excretion when their internal concentration is higher than a certain threshold (Jelaska et al. 2007; Butovsky 2011; Scheifler et al. 2002; Laskowski 1991).
Nevertheless, there are several other factors, in addition to species physiology, that may influence metal concentrations, such as age and abiotic factors. Different ways in which samples were collected may be considered in comparison between studies. Pitfall trapping is the most used method (Hunter et al. 1987), however, for chemical analysis, aspirators and hand-sorting of litter seems to be better tools for collection of live animals (Nico et al. 2001).
Carabids are known as important predators of the soil fauna (Brunsting and Heessen 1984). The eco-biology of C. olivieri revealed that juvenile and smaller earthworms are their food. According to Van Straalen et al. (2001), the higher concentrations of toxic elements were found in Juvenile earthworms as they consume a huge amount of organic matter from soil. Hence, the food preference of C. olivieri may explain differences in elemental concentrations in ground beetles species.
Conclusion
Using sensitive species can be more informative and efficient test systems allowing for a better assessment of the risk to the environment. In the current research, heavy metals in soil and Carabids C. olivieri collected from the vicinity of factory were important, thus confirming that they threaten environment and human health in such locality. However, additional compartments of contamination, including water, air, and contaminated food, should be studied in detail in the future. The Carabids, C. olivieri can be used as a good indicator for heavy metals. The present data can help in the assessment and establishment of future monitoring programs focusing on metal contamination in Tunisian ecosystems.
References
AFNOR (Association française de normalisation) (1996) Qualité des sols. Recueil des normes françaises, 3e édition. La Défense, Paris
Avgin SS, Luff ML (2000) Ground beetles (Coleoptera: Carabidae) as bioindicators of human impact. Mun Entomol Zool 5:209–215
Bednarska NG, Schymkowitz J, Rousseau F, Van Eldere J (2013) Protein aggregation in bacteria: the thin boundary between functionality and toxicity. Microbiology 159:1795–1806
Bhuiyan MAH, Parvez L, Islam MA, Dampare SB, Suzuki S (2010) Heavy metal pollution of coal mine-affected agricultural soils in the northern part of Bangladesh. J Hazard Mater 173:384–392
Boldina-Cosqueric I, Amiard JC, Amiard-Triquet C, Dedourge-Geffard O, Métais I, Mouneyrac C, Moutela B, Berthet B (2010) Biochemical physiological and behavioural markers in the endobenthic bivalve Scrobicularia plana as tools for the assessment of estuarine sediment quality. Ecotoxicol Environ Saf 73(7):1733–1741
Brunsting AMH, Heessen HJL (1984) Density regulation in the carabid beetle Pterostichus oblongopunctatus. J Anim Ecol 53:751–760
Butovsky RO (2011) Heavy metals in carabids (Coleoptera: Carabidae). In: Kotze DJ, Assmann T, Noordijk J, Turin H, Vermeulen R (eds) Carabid beetles as bioindicators: biogeographical, ecological and environmental studies. ZooKeys, vol 100, pp 215–222
Butovsky RO, Van Straalen NM (1995) Copper and zinc contents in trophic chains of terrestrial arthropods in the Moscow region. Pedobiologia 39:481–487
Chandrasekaran A, Ravisankar R, Harikrishnan N, Satapathy KK, Prasad MVR, Kanagasabapathy KV (2015) Multivariate statistical analysis of heavy metal concentration in soils of Yelagiri Hills, Tamilnadu, India-Spectroscopical approach. Spectrochim Acta Part A Mol Biomol Spectrosc 137:589–600
Cortet J, Gomot-De Vauflery A, Poinsot-Balaguer N, Gomot L, Texier C, Cluzeau D (1999) The use of invertebrate soil fauna in monitoring pollutant effect. Eur J Soil Biol 35:115–134
Dallinger R (1993) Strategies of metal detoxification in terrestrial invertebrate. In: Dallinger RAR, Rainbow PS (eds) Ecotoxicology of metals in invertebrates. Lewis, Boca Raton, pp 245–290
Dong JH, Yu M, Bian ZF, Wang Y, Di CL (2011) Geostatistical analyses of heavy metal distribution in reclaimed mine land in Xuzhou. China Environ Earth Sci 62(1):127–137
Farkas A, Claudio E, Vigano L (2007) Assessment of the environmental significance of heavy metal pollution in surficial soils of the River Po. Chemosphere 68:761–768
Hakanson L (1980) An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res 14(8):975–1001
Heikens A, Peijnenburg WJGM, Hendriks AJ (2001) Bioaccumulation of heavy metals in terrestrial invertebrates. Environ Pollut 113:385–393
Hollis GE, Agnew CT, Battarbee RW, Chisnall N, Fisher RC, Flower R, Goldsmith FB, Phethmean SJ, Skinner J, Stevenson AC, Warren A, Wood JB (1986) Modelling and management of the internationally important wetland at Garaet El Ichkeul, Tunisia. IWRB, Publication No. 4, Slimbridge
Hunter BA, Johnson MS, Thompson DJ (1987) Ecotoxicology of copper and cadmium in a contaminated grassland ecosystem II. Invertebrates. J Appl Ecol 24:587–599
Institute for European Environmental Policy (2009) Report on the Implementation of the Sewage Sludge Directive 86/278/EEC
Janssen MPM, Hogervorst RF (1993) Metal accumulation in soil arthropods in relation to micro-nutrients. Environ Pollut 79:181–189
Janssen MPM, Bruins A, de Vries TH, Van Straalen NM (1991) Comparison of cadmium kinetics in four soil arthropod species. Arch Environ Contam Toxicol 20:305–312
Jelaska LS, Blanusa M, Durbesic P, Jelaska SD (2007) Heavy metal concentrations in ground beetles, leaf litter, and soil of a forest ecosystem. Ecotoxicol Environ Saf 66:74–81
Karageorgis AP, Nikolaidis NP, Karamanos H, Skoulikidis N (2003) Water and soil quality assessment of the Axios River and its coastal environment. Cont Shelf Res 23:1929–1944
Kontas A (2007) Trace metals (Cu, Mn, Ni, Zn, Fe) contamination in marine sediment and zooplankton samples from Izmir Bay (Aegean Sea, Turkey). Water Air Soil Pollut 188:323–333
Kramarz P (1999) Dynamics of accumulation and decontamination of cadmium andzinc in carnivorous invertebrates 1. The ground beetle, Poecilus cupreus L. Bull Environ Contam Toxicol 63:531–537
Kuznetsova NA, Potapov MB (1997) Changes in structure of communities of soil springtails (Hexapoda: Collembola) under industrial pollution of the south-taiga bilberry pine forests. Russ J Ecol 28:386–392
Lagisz M, Laskowski R (2008) Evidence for between-generation effects in Carabids exposed to heavy metals pollution. Ecotoxicology 17:59–66
Lagisz M, Kramarz P, Niklinska M (2005) Metal kinetics and respiration rates inF1 generation of Carabid Beetles (Pterostichus oblongopunctatus F.) originating from metal-contaminated and reference areas. Arch Environ Contam Toxicol 48:484–489
Laskowski R (1991) Are the top carnivores endangered by heavy metal biomagnification? Oikos 60:387–390
Luoma SN, Rainbow PS (2008) Metal contamination in aquatic environments: science and lateral management. Cambridge University Press, Cambridge
Morillo J, Usero J, Gracia I (2002) Heavy metal fractionation in soils from the Tinto River (Spain). Int J Environ Anal Chem 82:245–257
Müller G (1981) Die Schwermetallbelstung der soile des Neckars und seiner Nebenflusse: Eine Estandsaufnahme. Chem Zeitung 105:157–164
Nico M, Van Straalen NM, Ruslan O, Butovsky RO, Andrei D, Pokarzhevskii AD, Andrei S, Zaitsev AS, Cornelis Verhoef S (2001) Metal concentrations in soil and invertebrates in the vicinity of a metallurgical factory near Tula (Russia). Pedobiologia 45:451–466
Pokarzhevskii AD (1985) Geochemical ecology of terrestrial animals. Naouka Publ House, Moscow
Rainio J, Niemelä J (2003) Ground beetles (Coleoptera: Carabidae) as bioindicators. Biodivers Conserv 12:487–506
Scheifler R, Gomot-de Vaufleury A, Toussaint ML, Badot PM (2002) Transfer and effects of cadmium in an experimental food chain involving the snail Helix aspersa and the predatory carabid beetle Chrysocarabus splendens. Chemosphere 48(6):571–579
Simon E, Harangi S, Baranyai E, Braun M, Fábián I, Mizser S, Nagya L, Tóthmérész B (2016) Distribution of toxic elements between biotic and abiotic components of terrestrial ecosystem along an urbanization gradient: soil, leaf litter and ground beetles. Ecol Ind 60:258–264
Sklaski T, Gargasz K, Laskowski R (2011) Does mixed diffused pollution decrease ground beetle diversity? Baltic J Coleopterol 11:1–15
Spurgeon DJ, Hopkin SP (1996) The effects of metal contamination on earthworm populations around a smelting works: quantifying species effects. Appl Soil Ecol 4:147–160
Stone D, Jepson P, Laskowski R (2002) Trends in detoxification enzymes and heavy metal accumulation in ground beetles (Coleoptera: Carabidae) inhabiting a gradient of pollution. Comp Biochem Physiol Part C Toxicol Pharmacol 132:105–112
Talarico F, Brandmayr P, Giulianini PG, Ietto F, Naccarato A, Perrotta E, Tagarelli A, Giglio A (2014) Effects of metal pollution on survival and physiological responses in Carabus (Chaetocarabus) lefebvrei (Coleoptera, Carabidae). Eur J Soil Biol 61:80–89
Tomlinson DC, Wilson JG, Harris CR, Jeffery DW (1980) Problems in the assessment of heavy metals levels in estuaries and the formation of a pollution index. Helgol Wiss Meeresunters 33(1–4):566–575
Van Straalen NM, Ernst WHO (1991) Metal biomagnification may endanger species in critical pathways. Oikos 62:255–256
Van Straalen NM, Van Wensem J (1986) Heavy metal content of forest litter arthropods as related to body-size and trophic level. Environ Pollut Ser A 42:209–221
Van Straalen NM, Butovsky RO, Pokarzhevskii AD, Zaitsev AS, Verhoef SC (2001) Metal concentrations in soil and invertebrates in the vicinity of a metallurgical factory near Tula (Russia). Pedobiologia 45(5):451–466
Vandecasteele B, De Vos B, Tack FMG (2003) Temporal–spatial trends in heavy metal contents in soil-derived soils along the Sea Scheldt River (Belgium). Environ Pollut 122:7–18
Wilczek G, Kramarz P, Babczyska A (2003) Activity of carboxylesterase and glutathione S-transferase in different life-stages of carabid beetle (Poecilus cupreus) exposed to toxic metal concentrations. Comp Biochem Physiol C Toxicol Pharmacol 134:501–512
Woitke P, Wellmitz J, Helm D, Kube P, Lepom P, Litheraty P (2003) Analysis and assessment of heavy metal pollution in suspended solids and soils of the River Danube. Chemosphere 51:633–642
Zar IH (1996) Biostatistical analysis. Prentice Hall, NJ
Acknowledgments
The authors would like to thank Pr. Mohammed Hachicha, Dr. Hamdi Sahraoui, Dr. Dalila Souguir and Dr. Abdelwaheb Aydi for their cooperation and data availability. Pr. David SHEEHAN is gratefully acknowledged as native English speaker for her helpful criticisms on an early version of the manuscript and for English improvements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghannem, S., Khazri, A., Sellami, B. et al. Assessment of heavy metal contamination in soil and Chlaenius (Chlaeniellus) olivieri (Coleoptera, Carabidae) in the vicinity of a textile factory near Ras Jbel (Bizerte, Tunisia). Environ Earth Sci 75, 442 (2016). https://doi.org/10.1007/s12665-016-5373-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-016-5373-3