Abstract
The presence of water in building stones is one of the main factors in deterioration. Capillary rise is the most usual mechanism of water penetration into building materials. In this study, the kinetics of the capillary rise phenomenon was studied for three porous building stones: two tuff stones and andesite. For each of the examined natural stones the capillary water absorption, pore size distribution, mineralogical–petrographic (optical microscope, XRD, SEM), chemical (XRF) and mechanical–physical properties were determined. The mechanism of capillary water absorption depends mainly on the pore size and the shape of the pore system. The pore size distribution was determined by means of high pressure mercury porosimetry. İscehisar andesite, Ayazini tuffs and Seydiler tuffs have pore sizes ranging from about 0.01 to 10, 0.01 to 20 and 0.01 to 4 µm, respectively. The capillary water absorption of the building stones was determined on the basis of TS EN 1925. The effects were analyzed with a static and dynamic capillary by water absorption under normal and salty water conditions of porous building stones. Water content was determined by weighing after 1, 3, 5, 10, 15, 30, 60, 480, 1440 min until the free saturation was reached and the liquid uptake stopped. The results indicated considerable differences in the water absorption shown as a function of elapsed time. NaCl crystals are observed under SEM in pores and surface of the tested stones. According to the results, capillary absorption of salty water value is bigger than pure water in all stones at the end of test. It could be shown that primarily moisture properties, i.e., capillary and sorptive water uptake and salt crystallisation can be addressed to the deterioration processes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Natural stone has become the expression to describe the versatile, durable and aesthetically plausible building materials. From the very beginning of civilization, important structures and monuments were built from or were principally based on natural stone. The use of local stone resources was mostly in balance with the local environment. Although highly durable when properly applied, no stone type can be considered immortal and most of the stone varieties are affected by a polluted atmosphere (Prikryl and Torok 2010).
Water is an important weathering factor for building materials. Specifically, many chemical reactions take place in building materials only in the presence of water. Also, the transport, crystallization, and hydration of salts are controlled by water. In addition, atmospheric precipitation or air humidity carry pollutants into building materials, leading to their deterioration, and biological decay can occur only in the presence of water (Karoglou et al. 2005).
Capillary absorption is the best property to evaluate the penetration of water into stone materials (Peruzzi et al. 2003). Capillary water absorption is also sometimes called sorptivity or rising damp. The capillary water absorption is related to the durability, porosity, pore size distribution and water absorption. Capillary water absorption is one of the most significant physical properties of building stone. The negative influences of water on many physical and mechanical properties of stone are well known. The higher the capillary water absorption and porosity, the worse are the negative consequences. Structural–petrographic features, such as type, size, distribution and position of mineral components, homogeneity, and the size, shape and system of interconnected pores in the natural stone are also important (Tomašić et al. 2011).
The speed and quantity of capillary water absorption and its retention in the pores have a significant impact on the durability of individual varieties of natural stone. If the absorbed capillary water is retained for a longer period of time at temperatures lower than 0 °C, ice crystallizes. With the growth of ice crystals and the increased volume of the ice, the durability is significantly reduced. This is also true with salt of varying origins, which can enter the stone in different ways. If, during the process of construction/cladding, the system of pores is differently oriented, both the absorption speed and the quantity of absorbed water as well as its retention will change, affecting the long-term integrity of the stone (Tomašić et al. 2011).
The crystallization of soluble salts in porous materials is one of the major causes of rock decay in nature and weathering of stone buildings and other engineering structures. Water can reach a building material through capillary rise of ground moisture, rain, and condensation of air humidity. The mechanism of capillary rise is important in masonry, because it carries soluble salts at the masonry. The maximum salt concentration, and thus the maximum deterioration, is observed in a specific zone of the masonry, which depends on the type of the building material and on the environmental conditions (Arnold 1982).
The building stones used in villages and cities often reflect the regional geology of the surrounding area. Andesite and tuffs have been used as building stones possibly since Roman, Seljuk and Ottoman periods. In Afyonkarahisar (Turkey), there are numerous buildings constructed by andesite and tuff stones. These building stones are still being used as local construction materials in the Afyonkarahisar. This work contributes to the research efforts dedicated to characterize selected stones, including the determination of their microstructure and petrographic characteristics. Furthermore, the aim of this study was to describe capillary water absorption and durability estimators for assessing the resistance of stone to weathering at the static, dynamic and salty water conditions. A proper understanding of the role of water absorption and salts in the structural performance of porous stones is of fundamental importance in the design of conservation procedures and strategies for the safeguard of cultural heritage.
Background
Several investigators have studied to define the size of pores and the petrographic and structural textural characteristics of stone, all of which are important for capillary water absorption. The capillary flow kinetics of water in porous media was analysed theoretically by Washburn (1921). In his simple model, the porous medium is represented by a collection of parallel tubes, each having the same radius. The porosity, the pore size distribution, the connectivity and permeability of the void space to different fluids, the shape and position of pores, the velocity of fluid movement during wetting, drying or circulating inside the material etc. are the most important of these characteristics. These properties, which determine the behavior and movement of water in the stone, the specific surface exposed to chemical reactions, the stresses building up by the movement or formation of foreign liquid or solid substances, were proved to be fundamental by several further investigations (Hoffmann and Niesel 1992; Valdeón et al. 1992; Jeannette 1997; Ordoñez et al. 1997; Iñigo et al. 2000; Mosquera et al. 2000; Nicholson 2001; Chabas and Jeannette 2001; Peters et al. 2001; Peruzzi et al. 2003; Sidraba et al. 2004; Karoglou et al. 2005; Ioannou et al. 2009; Kovacs 2009; Vázquez et al. 2010; Tomašić et al.2011; Sengun et al. 2014; etc.).
Peruzzi et al. (2003) conducted a study on two different methodologies including absolute and relative capillary index value. Franzen and Mirwald (2004) observed an increase in the drying rate in rough stones due to the enlarged evaporation surface. Sidraba et al. (2004) tested capillarity and drying relative to the bedding planes of Roman travertine used in the Baltic Region, particularly in Latvia. The coefficient of capillarity is about two times higher parallel (4.3 g/m2 s0.5) compared with perpendicular (2.58 g/m2 s0.5) to the bedding planes. They demonstrated that changing the orientation of the bedding planes in masonry building can considerably modify their resistance to deterioration. Ioannou et al. (2009) tried to model the capillary water absorption behavior of porous limestone using different liquids. They observed that the large pore structure reduces the capillary water absorption ability in contrast with to small pore structure. Vázquez et al. (2010) found a linear correlation between fractographic characteristics, capillarity and wave velocities in granites. Sengun et al. (2014) investigated capillary water absorption coefficients of 118 different natural stone types and textural features were determined and related with other rock properties (bulk density, apparent porosity, total porosity, seismic velocity, etc.). They observed strong relationships with higher correlation coefficients between capillary water absorption coefficient and open porosity.
Some water contains, creates or attracts harmful materials in the stone, such as salts that crystallize inside or outside the stone. Deterioration patterns vary from efflorescence to cracks and scaling due to sub-florescence. Numerous works have been studied on different porous stone and reported the influence of salts content of ground water on the deterioration (Theoulakis and Moropoulou 1988, 1999; Beck et al. 2003; Géraud et al. 2003; Angeli et al. 2006; Moreno et al. 2006; Franzen and Mirwald 2009; Wedekind et al. 2013).
Theoulakis and Moropoulou (1988) have studied the patterns and causes of decay of the building stone of the Medieval city of Rhodes and the relevant mechanism. They determined soluble salts can also cause decay of building stones. Moreno et al. (2006) undertook a detailed study of salt damage capillary by water absorption on a sixteenth to seventeenth century church building by sampling from many heights and locations for analysis to build up a full picture of salt damage. The presence of salts within sandstone may affect the properties of the stone. Lazzarini et al. (2008) reported on exfoliation and flaking, powdering and alveolic weathering for the Montemerlo trachyte in Venice (Italy) mainly related to salt deterioration. Franzen and Mirwald (2009) studied the effect of three different salt mixtures on the absorption of moisture. The data indicate that the presence of Mg-sulphates increases moisture uptake compared to Na salts.
Materials and methods
Stone types
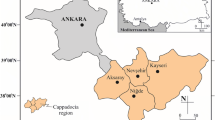
In the study area, andesite and tuffs occupy a vast area to the north of Afyonkarahisar Tuffs and andesite samples from three different locations in Afyonkarahisar, Turkey were sampled. The location map showing the position of their quarries is given in Fig. 1. Ayazini and Seydiler tuff is rhyolitic with white, cream and grey colour. İscehisar Andesite is an extrusive rock and intermediate in composition between rhyolite and basalt. It is medium to dark coloured vesicular volcanic rock, mostly fine grained with typically porphyric texture. The groundmass is fine-grained, frequently glassy. The Andesite stone quarry is located in Agin Mountain north of İscehisar.
Chemical characterization
Major element oxides were determined by X-ray fluorescence (XRF) spectrometry using a Rigaku/ZSX Primus II X-ray fluorescence spectrometer at the Mining Engineering Department Natural Stone Laboratory of the Afyon Kocatepe University (Turkey).
Mineralogical and petrographic characterization
For determination of the mineralogical and petrographic analyses of the andesite and tuff, a polarizing optical microscope, X-ray diffractometry (XRD) and scanning electron microscope (SEM) (in TUAM Laboratory in Afyon Kocatepe University) were used.
Petrographic characteristics (texture and mineralogy) of stone samples were examined under polarized optical microscopy using a Leica DM 2500P equipped with digital camera. Observations were performed both under transmitted and reflected (polarized) light.
The mineralogical composition of the stone samples was determined by powder X-ray diffraction. X-ray diffraction (XRD) analyses were conducted by a Panalytical X-pert MRD series difractometer with Cu-Kα radiation and X-celerator detector in laboratory of Çanakkale Seramik.
Scanning electron microscopy (SEM) operating in back-scattered electron mode was applied to study the chemical composition of the minerals at the building stone surfaces. The samples were investigated under a LEO 1430-VP variable pressure scanning electron microscope fitted with a wolfram filament and energy dispersive X-ray (EDX) microanalysis system. The carbon film coated samples were studied at 15 kV in a high vacuum. The spectra were acquired in a range of 5–20 kV.
Pore structure characterization
Mercury intrusion test allows measuring the volume and dimension of micro, macro and mesopores in solid porous materials. In this study, mercury porosimetry was employed for the determination of porosity and pore-size distribution. Mercury porosimetry relies on capillary theory and the non-wetting property of mercury to determine porosity, pore-size distribution and pore surface area by forcing mercury into matrix samples under pressure. A “Quantachrome Corporation Poremaster 60” in the Central Laboratory of Middle East Technical University (Ankara, Turkey) was used for the mercury intrusion porosimetry measurements with the test conditions of a surface tension mercury-vacuum of 480.00 erg/cm2 and a contact angle mercury-tuff of 140°.
Mechanical and physical characterisation
For mechanical and physical characterisation, both destructive (uniaxial compression) and non-destructive (ultrasound) tests were carried out. The P-wave velocity was determined using an Ele ultrasonic tester, two transducers (a transmitter and a receiver) and the measurements (with 54 kHz) were performed three times on the all stone samples. The laboratory tests were performed according to TS EN (Technical Specification European Standard) standards, at the Afyon Kocatepe University (Turkey). Sample for physico-mechanical tests and related standards are given Table 1. Cubic-shaped samples were prepared for the experiments. The mechanical and physical properties of the sample stone were determined from six samples and experiments.
Capillary water absorption
Water absorption by capillarity was determined using the standard method in accordance with TS EN 1925 (Natural stone test methods—determination of water absorption coefficient by capillarity). Six samples were used in the form of 70 mm cubes for each building stones and distilled water was used. The procedure of the test is as follows: dried cubes are placed in water of 3 (±1) mm heights (Fig. 2a). Before weighing, the face was slightly dampened with a damp cloth. The absorbed water, as a function of time (time interval: 1, 3, 5, 10, 15, 30, 60, 480, 1440 min), was recorded during the test. The level of the water was maintained at that level during the measurement, adding water when it was necessary and closing the tank to avoid evaporation in case of slow capillary absorption. The continuous method was used to determine the capillary water absorption. The results were plotted as the mass of water absorbed per area of sample throughout imbibition versus the square root of time.
Dynamic experiments were performed with two fluids, pure water and salty water, in a porous building stones. Experiments were carried out at atmospheric pressure. Static and dynamic water absorption by capillary experiments was conducted using shown in Fig. 2. Besides water, the test has been performed using salt solution to see whether the ions in water can significantly affect the process of capillary action. Salty water test has been performed with the sodium chloride solution (14 % by weight NaCl). This test was intended to study the building stones durability in the conditions of the salinity. All experiments were conducted at a temperature of 22 °C (±2 °C) in a temperature controlled environmental laboratory. Experimental equipment has been designed in this work to quantify the dynamic effect of water in porous building stones. The apparatus used in this experiment is shown in Fig. 2b. Similar experiments were carried out for carbonate stone by Tomašić et al. (2011).
Experimental investigation and results
Chemical analysis
The results of X-ray fluorescence (XRF) analysis are presented in Table 2. According to results of chemical analysis; there is not much diversity in the major element content for all analyzed tuff rock samples. For instance, the SiO2 contents range from 58.30 to 73.50 wt%, the Al2O3 content from 13.60 to 15.80 wt%, the K2O content ranges from 5.70 to 7.00 wt%, the Na2O content from 2.29 to 3.78 wt%, and the Fe2O3 content from 0.52 to 4.96 wt%. The contents of MgO and TiO2 are all low.
Igneous rocks can be classified according to chemical or mineralogical parameters. If the mineral mode cannot be determined, frequently occurs for the volcanic rocks, then a chemical classification of total alkalis versus silica (TAS) is applied. The chemical parameters which was decided to use were silica (SiO2) weight per cent and total alkalis (Na2O + K2O) wt% because they appeared to be the best and were already widely used (Le Bas and Streckeisen 1991). All rocks samples are classified according to Na2O + K2O − SiO2 diagrams (Le Bas et al. 1992). It is seen that Ayazini and Seydiler tuff samples are located in rhyolitic area while İscehisar andesite is located in trachy-andesite area.
Petrography and mineralogy
Petrographic and mineralogical properties of the tuffs were studied using a polarizing optical microscope, scanning electron microscope (SEM) and X-ray diffractometry (XRD) (Fig. 3).
Macroscopic and microscopic fabric images of the tested rocks. Illustration of the volcanic rock samples described in detail. From top to bottom: İscehisar andesite, Ayazini tuff and Seydiler tuff. Macroscopic view (a, d, g), thin section under polarized light (b, e, h), SEM micrograph (c, f, i). Pj plagioclase, Q quartz, H hornblende, S smectite, VC volcanic glass, various size of P pore
Polarizing optical microscope analysis
Standard thin sections were prepared for petrographic descriptions. Petrographical analyses of each rock sample were performed on these thin sections under a polarization microscope. The İscehisar andesite has a pinkish to reddish matrix with altered hornblende minerals distributed randomly inside the rock. Gas cavities are also observed in the rock. Petrographically, the İscehisar andesite is defined as trachy-andesite with semi-porphyritic texture and micro-crystalline inter-granular matrix. The glass content of the matrix is considerably low and rich in very fine grained opaque minerals. Abundant phenocrysts are plagioclase, amphibole and rarely clino-pyroxene, minerals (Fig. 3b).
The Ayazini tuff is a light grey to white pyroclastic rock with clearly acid composition (rhyolitic tuff). It is characterized by an almost total lack of macro crystals and a fine granular matrix appearance. Only a few platy anhedral feldspar phenocrysts and some scarce idiomorphic hornblende crystals were observed macroscopically. The Ayazini tuff contains crystals of quartz, feldspar, hornblende and opaque minerals (Fig. 3e). Various rock fragments and pumice are also present. The crystals, rock fragments and pumice are embedded in a tuffaceous matrix. In the tuffaceous matrix, volcanic glass shards are rather common. The optic microscope data of these tuffs that are examined petrographically also conform to XRD data.
The Seydiler tuff is composed of a mineral assemblage of various crystals including quartz, feldspar; mafic mineral is biotite and rock particles with glass cement (Fig. 3h). The crystal component of the tuffs is similar to that found in the pumice, being largely embayed quartz and plagioclase, but with the addition of biotite and some K-feldspar. Quartz phenocrysts are generally rounded. Groundmass is mainly constituted by feldspar, various rock fragments and pumice. The matrix is fine-grained and contains iron oxides. Physical weathering causes fracturing of feldspars, especially along their cleavage planes, within the both tuffs.
SEM analysis
Photomicrographs of the minerals and texture identified using the SEM are shown in Fig. 3. Presence of feldspar, quartz and smectite is detected in the İscehisar andesite. Likewise, Ayazini and Seydiler tuffs are composed of them as well. It was determined that flaky morphology smectite is the main clay mineral in both tuffs. In general, smectite develops in fissures, fractures and dissolution voids of the volcanic glass. Alteration of feldspars results in formation of smectites. Formation of smectite is closely related to hydrolysis of volcanic glass and alteration of feldspar. SEM images of all tested stones show the presence of numerous pores.
X-ray diffraction analysis
The results of the optical microscopy were also confirmed by the XRD analyses. In order of abundance, the following minerals were identified: XRD analyses of the İscehisar andesite reveal that there exists majority amount of andesine and sanidine, small amount of muscovite and montmorillonite minerals (Fig. 4a). The analyses of the Ayazini tuff reveal that feldspar, quartz, cristobalite and illite (mica) are present within the tuff. XRD analyses for the Ayazini tuff indicated illite (mica)-type clay minerals. A typical XRD pattern for an Ayazini tuff samples are depicted in Fig. 4b. The Seydiler tuff samples are composed of feldspar, quartz, hornblende and illite (mica) (Fig. 4c). Cristobalite is present only in Ayazini tuff samples and is the dominant silica phase. In addition to these minerals, an important component of amorphous materials (volcanic glass) constitutes the both tuffs.
Pore-size distribution
Pore size distribution and porosity of a rock are responsible for water and moisture uptakes as well as water transport. Generally, pores are divided based on their size into different classes: micropores (<0.1 μm), capillary pores (0.1 μm to 1 mm), and macropores (>1 mm) (Graue et al. 2011). Capillary suction is practically relevant to materials for pore diameters between 1 μm and 1 mm, the so-called capillary pores. For macropores, those with a diameter >1 mm, fluid flow characteristics emerge (Siegesmund and Dürrast 2011).
Pore-size distribution of İscehisar andesite
Cumulative intrusion curves and the pore-size distribution for the İscehisar andesite sample are given in Fig. 5. The results from mercury intrusion porosimetry (MIP) shows, that İscehisar andesite have a pore size distribution with most of the pores in the range from 0.1 to 10 µm. Approximately 50 % of the pores are smaller than 0.1 μm in diameter and the majority between 0.1 and 4 μm (Fig. 5).
The SEM pictures show that the İscehisar andesite is porous; there are crystals on their surfaces with different sizes. SEM image of the surface of the İscehisar andesite are given in Fig. 6. Observed the large subspherical pore in Fig. 6a, b. Mega pores reaching up to 20–30 µm in diameter were also observed.
Pore-size distribution of Ayazini tuff
The cumulative intruded pore volume curves for Ayazini tuffs, obtained from MIP, are provided in Fig. 7. Ayazini tuffs have pore sizes ranging from about 0.01–100 µm. Thus, the size of pores varies widely from nanometer to micrometer. Mercury porosimetry results show that most of the pores (>80 %) have a pore access diameter between 0.01 and 10 µm. Mega pores reaching up to 100 µm in diameter were also observed. SEM images of Ayazini tuffs show the presence of numerous pores. SEM image of the slightly collapsed pumice fragments show irregular vesicle shapes from pipes to pods. Slot pores comprise a honeycomb like structure, bounding the surfaces of flanking grains. Microchannels exhibit elongated shapes; in general, the walls of the microchannels appear to be irregular (Fig. 8).
Pore-size distribution of Seydiler tuff
Mercury intrusion porosimetry of tuff from Seydiler, plots report pore-size distributions, as relative pore volume is provided in Fig. 9. Seydiler tuffs have pore sizes ranging from about 0.010 to 4 µm and varying widely from nanometer to micrometer. Mercury porosimetry results show that most of the pores (>90 %) have a pore access diameter of between 0.1 and 4 µm, mega pores reaching up to 10 µm in diameter were also observed. Seydiler tuffs have a smaller porosity compared to that of the Ayazini tuffs. Seydiler tuff differs very clearly from the two others with most pores beneath 1 µm and a higher total pore volume.
SEM photo-micrograph of Seydiler tuff showing volcanic glass porosity and amounts of spheroidal pore were observed (Fig. 10). Spherical shapes are associated with platy minerals. This pore type has different sizes which are distributed within the Seydiler tuff sample. Pores are present mainly in volcanic glass matrix, which makes up the intergranular cement of the tuff rock.
Mechanical and physical properties of building stones
The physical properties of rocks like porosity, density and water absorption by weight allow a better understanding of the variations on the mechanical properties. Density and porosity are often related to the strength of rock material. A low density and high porosity rock usually has low strength. Porosity is one of the governing factors for the permeability. Porosity provides the void for water to flow through in a rock material. High porosity therefore naturally leads to high permeability (Guruprasad et al. 2012).
Three types of building stone, got from different quarries in Afyonkarahisar region of Turkey, were used in this study. The mechanical and physical properties of these building stones were determined in accordance with the related standards. The mechanical and physical properties of the building stones were determined from 6 samples in each stones and experiments; the results are given in Table 3.
Bulk densities of the İscehisar andesite samples range from 2449.65 to 2684.32 kg/m3, with a mean value of 2643.38 kg/m3. The İscehisar andesite shows the highest apparent density followed by the Seydiler tuff and the lowest value corresponds to the Ayazini tuff. The open porosities of the İscehisar andesite samples range from 6.35 to 9.10 vol%, with a mean value of 7.24 vol%. The İscehisar andesite has the lowest water absorption value, 3.30 %; while the values of the Ayazini tuff and the Seydiler tuff, 16.14 and 5.94 %, respectively. Water absorption values of all types of rock samples were inversely related to porosity, density and uniaxial compressive strength. Measurements were made on Ayazini tuff dry samples P-wave velocities from 1.43 to 1.90 km/s with a mean value of 1.73 km/s. Porosity and P-wave velocities show moderate correlation: samples with higher porosities have slower elastic wave velocities. The uniaxial compressive strength of İscehisar andesite has average values in the order of 56.86 N/mm2. The comparison between the results of uniaxial compressive strength on samples of the tuffs rocks shows a lower in the values from İscehisar andesite.
Capillary water absorption
Capillary action, also known as capillarity, is a result of the intermolecular attraction within the liquid and solid materials. Adhesion of water to the walls of a vessel will cause an upward force on the liquid at the edges and result in a meniscus which turns upward. The surface tension acts to hold the surface intact, so instead of just the edges moving upward, the whole liquid surface is dragged upward. Greater surface tension and increased ratio of adhesion to cohesion also result in greater rise. However, increased density of the liquid will cause it to rise to a lesser degree.
For the ingress of chlorides into building stones the continuous network of the capillary pore system of the rocks, the coarse pore system of the solid/water interface and, eventually, micro cracks provide the paths along which the transport of ions occurs. The ingress of chlorides due to the capillary action of the pore system absorbing a chloride-containing solution is a convective flow of ions, and the amount of chloride introduced is given by the volume of absorbed solution and its chloride concentration. According to these considerations, a reduction of the capillary suction should be expected for salt solutions with increasing salt concentration as compared to pure water.
A porous dimension stone experiences water absorption while exposed to rain when being part of a building masonry or cladding as well as when in contact with groundwater. In general, a porous medium in contact with liquid water will absorb it by capillarity. This is a spontaneous process related to the capillary absorption force originated by the pores in the material between the diameters of 10 μm and 1 mm. It is the result of a balance between the surface tension of the liquid water and the adsorption forces of the pore wall, usually a polar mineral surface. Water at these polar surfaces exhibits a characteristic wetting angle that finally results in the tendency of the water to enter the pore system, the so-called capillary action or capillary suction. The mechanism of capillary water absorption depends mainly on the pore size and the geometry of the pore system (Siegesmund and Dürrast 2011).
The determination of the water absorption by capillarity is described by the TS EN 1925 (Natural stone test methods—Determination of water absorption coefficient by capillarity). The coefficient of capillary uptake (C) is calculated (1) from the first section:
m i weight of sample in i moment (g), m d weight of dry sample (g), A the surface of the sample in the water (m2), t i time (s).
Table 4 gives the average capillary water absorption (C) per stone, water uptake and solution type. When the capillary water absorption is considered in terms of volume absorbed, the initial apparent differences between the water solutions are greatly reduced. Nonetheless, the differences between the different water solutions are significant in term of the capillarity absorption coefficient.
The results indicated considerable differences in the water absorption shown as a function of elapsed time. All the samples showed generally high capillary absorption capacity. This can be attributed to their relatively high porosity and percentage of capillary pores, which determine and control capillary water uptake. Movement of water is restricted to interconnected pores (effective porosity) and it depends upon the pore structure how freely the pore water can move, whether the water in pores becomes bulk or adsorbed, what the maximum degree of saturation is, etc.
According to porosity analysis data for İscehisar Andesite, Ayazini and Seydiler tuffs, respectively, the open porosity contents are 7.24, 25.77 and 11.61 (%). Ayazini tuffs have pore sizes ranging from about 0.010 to 20 µm. Mercury porosimetry results show that most of the pores (>80 %) have a pore access diameter of between 20 and 0.010 µm. Capillary water absorption graphics showed that average value of İscehisar Andesite, Ayazini and Seydiler tuffs are 7–31, 6–33 and 36–87 % respectively. These results correspond with those of water uptake porosity and MIP.
The highest capillarity level recorded for the İscehisar andesite was 5.049 kg/m2 s0.5 in static salty water conditions following 1440 min (24 h). As can be seen in Fig. 12, the lowest capillarity recorded for İscehisar andesite was 3.777 kg/m2 s0.5 in dynamic water conditions following 1440 min (24 h). In this case, all tested building stones have a medium and high porosity, a high capillary water uptake value, which might suggest certain sensitivity to frost-related weathering.
Figures 11 and 12 show the results of the routine average water uptake experiment for three stone samples. Whatever the type of stone, the capillary absorption is always faster with the saturated solution of sodium chloride than pure water. The difference between the salt and pure water solution likely reflects the increased density and viscosity of the saturated sodium chloride solution but these factors have a relatively small effect. The differences between the salt solution and pure water are much smaller. It should be pointed out that except for water the initial absorption is slightly higher for the less porous İscehisar andesite than for the tuff stones reflecting difference in their pore-size distribution.
Photos of the capillary water absorption of sample rocks time interval (1, 3, 5, 10, 15, 30, 60, 480, 1440 min). Graphic representation of capillary water absorption of under static water conditions for samples İscehisar andesite (a), Ayazini tuff (b) and Seydiler tuff (c), as a function of the square root of time (s0,5)
Rocks with a high amount of capillary pores are expected to have a high water uptake value, which means they have the capacity to rapidly absorb water by capillary uptake in the pore spaces. Stones with low capillary absorption have a water uptake value of <0.5 kg/m2 s0.5, those with medium absorption range from 0.5 to 3.0 kg/m2 s0.5 and stones showing strong water suction have water uptake values >3.0 kg/m2 s0.5 (Graue et al. 2011). Accordingly, İscehisar andesite, Ayazini and Seydiler tuffs show a high capillary water absorption value (3–18 kg/m2 s0.5) in this study.
Andesite, Ayazini and Seydiler tuffs have high capillary water absorption values for salty water considering pure water in both dynamic and static conditions. For instance, in static conditions Iscehisar Andesite has capillary water absorption values of 4.115 kg/m2 s0.5 in water while having 5.049 kg/m2 s0.5 in salty water. Likewise, in dynamic conditions the values are 3.777 and 4.397 kg/m2 s0.5 respectively. Therefore; in static conditions, there is 18.5 % increase of capillary action for salty water while it is 23.5 % in dynamic conditions. Similarly, the increases of capillary action in salty water for Ayazini tuffs are 8.58 and 27.75 % respectively (Fig. 13). Also, Seydiler tuffs have the same values of 21.28 and 20.40 %. The reason behind this is the increase of surface tension caused by salt ions.
A high porosity in connection with a high water uptake is considered as having a high damage potential. High water uptake values combined with a high saturation values are the first indicators for a possible susceptibility to weathering, or in other words, frost damage and salt crystallization in the pore spaces.
The capillary absorption capacity of a porous stone is defined by its water uptake coefficient (w value). This is a process driven by the capillary forces that originate in the micro pores and capillary pores (Klopfer 1985; Graue et al. 2011). High water uptake values combined with a high water absorption values are the first indicators for a possible susceptibility to weathering, or in other words, frost damage and salt crystallization in the pore spaces.
Iscehisar andesite has the lowest values of water absorption (3.30 %) and capillary water absorption (4.115 %) while Ayazini tuffs have the highest values, 14.515 and 16.14 % respectively. On the other hand, Seydiler tuffs have values of 7.331 and 5.94 %. This is consistent with both porosity and total porosity values. It is probable that Ayazini tuff is most likely to be suffered by rock weathering the most due to having higher porosity values.
From Fig. 11 some interesting conclusions can be drawn. The volumetric capillary water absorption measured by İscehisar andesite sample increases to approximately 30 % in static water conditions following 60 min. Similarly, the volumetric capillary water absorption measured for Seydiler tuff sample increases to 40 % after a period of about 60 min. Ayazini tuff shows a continued increase in moisture content to approximately 100 % from the beginning of the test after a period of about 60 min. The dramatic water uptake of Ayazini tuff is attributed to a well-connected pore system with a pore size range. The Ayazini tuff sample showed capillary water absorption rises from 50 to 100 % moisture content from 10 to 60 min. This is because of the horizontal pore structure that can be observed on the SEM photos. The horizontal pores allow a faster capillary flow in the horizontal direction. On the contrary, the İscehisar andesite sample shows continuous increase in water content after 1440 min that is still 80 % of the saturation. This is because İscehisar andesite has bigger pores that do not act as capillary.
The values for water uptake under normal and salty conditions were also determined as well as the degree of capillary water absorption. Salts have to be dissolved in liquid water to move inside the porous stone. Dissolution of salt crystals can be achieved by import of liquid water or condensation of vapor water into the pores. The results of the capillary water absorption per stone and impregnation water and solution type experiment are given in Fig. 14. The three tested stones, İscehisar andesite, Ayazini tuff and Seydiler tuff, exhibit a very typical capillary uptake curve at different solution type.
According to the results obtained in Fig. 14, capillary absorption of salty water value is bigger than of pure water in all stone at the end of test. As it can be observed for all salinity cases, water content increases with the chloride concentration. This could be explained by the modification of water activity, which is due to the presence of salt and other ions (Na).
Salt ions usually increase surface tension considering pure water. For instance, adding NaCl which ionizes in water increases surface tension. The reason behind this fact is ion–dipole bonds which are constructed between ions and water. There are hydrogen bonds between the molecules of water. The newly ion–dipole bonds are stronger than these hydrogen bonds. In other words, there is a stronger formation of intermolecular interaction. Furthermore; while adding salt to water, its density increases. This is another reason why surface tension gets bigger.
All materials, solids and liquids, have characteristic surface energies, which reflect the fact that the atoms and molecules which form the surface are in a less favourable (less stable) location than in the interior, therefore, to create more surface costs energy. A consequence of this is that the surface is in tension. The notion of a surface tension, which goes back to Thomas Young, and the notion of a surface or interfacial energy, are essentially the same. A liquid drop, in the absence of other forces acting on it, will form a sphere because this is the shape of minimum surface area. The surface is in tension and acts like a stretched skin. The pressure of the liquid forming the drop is greater than the ambient pressure by an amount Δp. In this simple case, we can see that to create extra surface area dA = 8πrdr requires energy σdA, where σ is the surface tension, and to change the size of the drop requires work = ΔpdV = Δp·4πr2dr. Equating these gives us Δp = 2σ/r: the equilibrium excess pressure inside the drop depends on the radius and the surface tension of the liquid. This is the law of Young–Laplace. For a spherical droplet, the centre of curvature lies within the liquid and the excess pressure is positive (Hall and Hoff 2012).
Solids also have surface tensions or surface energies, though these are harder to measure and have less obvious consequences, because of the rigidity of the solid. When a liquid comes into contact with a solid surface, we have to consider three surface (strictly, interfacial) energies: σSA, the surface energy of the solid in contact with air; σLA, that of the liquid in air; and σSL, that of the solid in contact with liquid (and equally of liquid in contact with solid). First if σSA − σSL > σLA then it pays for the liquid to spread over the solid surface, because the gain in replacing solid/air surface with solid/liquid surface more than compensates for the associated cost of creating additional liquid/air surface (Hall and Hoff 2012).
The change in the surface tension of water can be seen with the help of capillarity as well. To see this, two identical capillary tubes can be sunk inside salty and pure water. After this, the higher capillary action can be seen for salty water. Impurities affect surface tension appreciably. It is observed that impurities, which tend to concentrate on the surface of liquids, compared to its bulk lower the surface tension. Substances like detergents, soaps, alcohol lower the surface tension of water, while inorganic impurities present in the bulk of a liquid such as NaCl tend to increase the surface tension of water. Since the capillary uptake is the result of surface tension of fluids, this elevation inside the tube proves that the surface tension of salty water is higher than pure water.
The Ayazini tuff initially shows, over a short period of time, a very fast capillary water absorption which is followed by a longer period of stopped absorption. This can be explained by the information provided by its pore size distribution, i.e., the range of pore sizes that allows water to access the open porosity (25.77 %). The pore size distribution is very wide, from <0.01 μm to almost 100 μm. In addition, the highest percentage of its total effective porosity is refilled through very wide conduits (1–100 μm).
Investigation of the effects of salt crystallization by SEM-EDX analysis
NaCl is commonly found in building materials and other elements of our cultural heritage and its main source is linked to sea spray. In cold climates, NaCl can lead to durability problems when used as a de-icing salt. Under room conditions, the sodium chloride–water system has one stable phase: halite (NaCl) (Winkler 1997). It has been widely observed and reported that cycles of crystallization, dissolution, and re-crystallization of salts due to changing environmental conditions cause progressive deterioration of porous materials. The extent of deterioration however varies widely from one salt to another. Field observations showed that all soluble salts may produce very strong decay (Arnold and Kueng 1985).
Although chloride species are quite common in the salty environment, they are very abundant compounds in the ground water. Halite (NaCl) is the main type of salt belonging to these species. Salt crystallization in the tested stone samples was studied by SEM equipped with an Energy Dispersive X-ray System (EDX). Halite (NaCl) crystals are observed under SEM in pores and surface of the tested building stones. In the microscopic images (SEM) of the tested building stones from the surface of the samples, in all the cases, large quantities of new mineral phases were observed. Halite appears as dendritic aggregates as well as partially dissolved subhedral crystals, partially dissolved anhedral crystals and prismatic euhedral crystals. The sodium chloride was mainly found in the form of discontinued coatings or cubic crystals merging into larger aggregates.
An experimental approach has been used to understand of sodium chloride crystallization. It is well known that NaCl crystallization in aqueous solutions is controlled by the relations (2, 3):
where [Na+] and [Cl−] are the ion activities and K is the equilibrium constant for a definite temperature and pressure. The ion activity product (IAP) determines whether a salt can precipitate. Supersaturation is actually achieved when the actual ion activity product (Na+)(Cl−] = IAP is greater than the equilibrium constant K: IAP3 K (Theoulakis and Moropoulou 1999).
SEM-EDX images observation of salt crystals developed in the İscehisar andesite sample show aggregates of submicrometer sized halite crystals filling pores (Fig. 15). Some salt crystals that have been occured earlier, observed to be dissolved in the İscehisar andesite and Ayazini tuff samples (Fig. 16). Some crystals merged into aggregates and appeared as prismatic cubic crystals in the surface of the Seydiler tuff samples (Fig. 17). The experiments in the present study clearly indicate that saline water is one of the most aggressive factors of deterioration of porous building stones. Salt crystallization mechanisms lead to deterioration forms related to salty environment conditions. Their chemical analyses by EDX indicated that sodium (31.24 wt%) and calcium (68.45 wt%) were the major components in İscehisar andesite sample. In the case of Ayazini tuff stone, the main elements found are chlorine (55.55 wt%), sodium (31.85 wt%), oxygen (10.25 wt%), with some traces of calcium (0.92 wt%) and aluminum (0.51 wt%). In the Seyfiler tuff stone, silicon and oxygen are the major elements (69.96 and 20.80 wt%, respectively) with some presence of sodium (2.05 wt%) and chlorine (1.63 wt%).
SEM photo-micrographs of after completing the test of capillary water absorption in salty water condition the sample of İscehisar andesite. SEM images of the halite crystals in the large pores of the İscehisar andesite sample (a), energy dispersive spectrum (EDX) of halite (NaCl) (b), cubic halite crystals merging into larger aggregates and observed to be dissolved (c), SEM image of İscehisar andesite (d)
SEM photo-micrographs of after completing the test of capillary water absorption in salty water condition the sample of Ayazini tuff. The sodium chloride (halite) was found in the form of crystals merging into aggregates (a), energy dispersive X-ray spectroscopy (EDX) of halite (NaCl) (b), form of partially dissolved subhedral crystals of halite (c, d)
SEM photo-micrographs of after completing the test of capillary water absorption in salty water condition the sample of Seydiler tuff. The sodium chloride (halite) was mainly found in the form of discontinued coatings, crystals merging into aggregates and appeared as prismatic cubic crystals (a), energy dispersive X-ray spectroscopy (EDX) of halite (NaCl) (b), prismatic euhedral crystals of halite (c, d)
Conclusions
İscehisar andesite, Seydiler and Ayazini tuffs have been used as building stones. The first one is used as cover material in floors and walls having dimension of 2–3 cm thickness slab. Moreover, it can be used in staircases, paving stones and park and garden walkways. On the other hand, Ayazini and Seydiler tuffs have been commonly used as construction stones in buildings and garden walls. Also, they have been used in restoration of historical buildings in Afyon such as Afyon castle, many other mosques, e.g., Mevlevi Mosque. However, since Ayazini tuffs have high capillarity, they should not be used in wet environments.
Water is one of the major factors that contribute to the deterioration of building stones. Transportation of water in porous building stones such as tuff and andesite takes place in open pores mainly due to capillary water absorption. In this paper, an experimental procedure was aimed at testing the capillary water absorption of building stone samples. Water saturation of these stones affected by weathering water and chloride has been presented. Three different types of natural stones, commonly used in Afyonkarahisar (Turkey), have been analyzed. The selected stone samples were characterized by mineralogical–petrographic (optical microscope, XRD, SEM), chemical (XRF) and mechanical–physical properties and their microstructure were studied. The pore size distribution study was carried out with mercury intrusion porosimetry (MIP) testing. This study was centered on the determination of values of capillary water absorption under normal and salty water conditions.
The principal results of the present work can be summarized as follows:
These analyzed properties were the capillary water absorption. The specimens were weighted after 1, 3, 5, 10, 15, 30, 60, 480, 1440 min from the beginning of the test. The water absorption per unit area versus the square root of the time initially showed a linear behavior. The slope of this line corresponds to the water absorption value.
Since the effective porosity contents in the Ayazini tuff are 25.77 %, capillary water absorption of approximately 50 % was reached after only 10 min, with no pressure; 95 % was reached after 30 min and 100 % was reached after 60 min. The liquid transgresses the entire thickness of the specimen (70 mm) in a short time. If chloride solution enters the pores and micro cracks, a typical chloride profile could be established close to the surface in contact with the salt water. The results have been confirmed by direct observation of salt migration by SEM analyses.
The pore size distribution of the İscehisar andesite and Seydiler tuff samples ranges from 0.1 to 10 and 0.01 to 4 µm respectively, which are within the medium range of capillary active pore size. On the contrary, Ayazini tuffs show the pore size distribution in the range 0.01–100 µm. These tuffs uptake water rapidly and have a high capillary water absorption value. A high water uptake is considered as having a high damage potential.
Salt localization in porous building stones was described and interpreted using SEM and the analyses were performed with an energy dispersive X-ray (EDX). According to the results obtained in SEM-EDX images, NaCl crystals are observed to develop in the pore space within the stone of all samples, demonstrating that the actual conditions are in favour of salt crystallization. Salt crystals appears as dendritic aggregates as well as partially dissolved subhedral crystals, partially dissolved anhedral crystals and prismatic euhedral crystals, cubic crystals.
The role of pore size distribution in the damage caused by salt crystallization is very significant, influencing both crystallization pressure and pore size distribution in the stone. The water uptake determines the quantity of water that can enter into the pores and micro cracks of the stones. This will control the quantity of the ice, and thus the degree of damage. A high porosity in connection with a high water uptake is considered as having a high damage potential. High water uptake values combined with a high saturation coefficient are the first indicators for a possible susceptibility to weathering. Laboratory test results indicate that the Ayazini tuff is not appropriate for use as building stone in a humid environment as that of the Afyonkarahisar area. Therefore, it is recommended that, this stone should only be preferred for decorative cladding of indoors of the buildings and should not be used for floor covering. However, for the outdoor use of this tuff, the block surfaces need to be treated with chemicals against weathering. The materials has to be used always in the correct position in the building, and when it is applied properly and the building maintenance works it lasts several centuries (without chemical treatment).
References
Angeli M, Bigas JP, Menendez B, Hébert R, David C (2006) Influence of capillary properties and evaporation on salt weathering of sedimentary rocks. In: Fort R, Alvarez de Buergo M, Gomez-Heras M, Vazquez-Calvo C (eds) Heritage, weathering and conservation. Taylor & Francis/Balkema, AK Leiden, The Netherlands, pp 253–259
Arnold A (1982) Rising damp and saline minerals. In: Gauri KL, Gwinn JA (eds) Fourth international congress on the deterioration and preservation of stone objects. University of Louisville, Louisville, pp 11–28
Arnold A, Kueng A (1985) Crystallization and habits of salt efflorescences on walls I: methods of investigation and habits. In: Félix G (ed) Proceedings of the fifth international congress on deterioration and conservation of stone, 25–27 September 1985, Lausanne. Presses polytechniques romandes, Lausanne, pp 255–267
Beck K, Al-Mukhatar M, Rozenbaum O, Rautureau N (2003) Characterization, water transfer properties and deterioration in tuffeau: building material in the Loire valley, France. Build Environ 38:1151–1162
Chabas A, Jeannette D (2001) Weathering of marbles and granites in marine environment: petrophysical properties and special role of atmospheric salts. Environ Geol 40(3):359–368
Franzen C, Mirwald PW (2004) Moisture content of natural stone: static and dynamic equilibrium with atmospheric humidity. Environ Geol 46:391–401
Franzen C, Mirwald PW (2009) Moisture sorption behaviour of salt mixtures in porous stone. Chem Erde Geochem 69:91–98
Géraud Y, Surma F, Mazerolle F (2003) Porosity and fluid characterization of granite by capillary wetting using X-ray computed tomography. In: Mess F, Swennen R, Van Geet M, Jacobs P (eds) Applications of X-ray computed tomography in the geosciences. Geological Society, London, Special Publications, vol 215, pp 95–105
Graue B, Siegesmund S, Middendorf B (2011) Quality assessment of replacement stones for the Cologne Cathedral: mineralogical and petrophysical requirements. Environ Earth Sci 63:1799–1822
Guruprasad B, Ragupathy A, Badrinarayanan TS, Rajkumar KB (2012) The stress impact on mechanical properties of rocks in hydro fracturing technique. IJEST 4(2):571–580
Hall C, Hoff, WD (2012) Water transport in brick, stone and concrete, 2nd edn. Taylor & Francis, London and New York, CRC Press, p 362
Hoffmann D, Niesel K (1992) Pore structure of rendering as a feature of its weathering. In: 7th international congress on the deterioration and conservation of stone, Lisbon, pp 611–620
Iñigo AC, Vicente MA, Rives V (2000) Weathering and decay of granitic rocks: its relation to their pore network. Mech Mater 32:555–560
Ioannou I, Andreou A, Tsikouras B, Hatzipanagiotou K (2009) Application of the sharp front model to capillary absorption in a vuggy limestone. Eng Geol 105:20–23
Jeannette D (1997) Importance of the pore structures during the weathering process of stones in monuments. In: Paquet H, Clauer N (eds) Soils and sediments, mineralogy and geochemistry. Springer, Berlin, pp 177–190
Karoglou M, Moropoulou A, Giakoumaki A, Krokida MK (2005) Capillary rise kinetics of some building materials. J Colloid Interface Sci 284:260–264
Klopfer H (1985) Feuchte. In: Lutz P et al (eds) Lehrbuch der Bauphysik. Teubner, Stuttgart, pp 329–472
Kovacs T (2009) Durability of crystalline monumental stones in terms of their petrophysical characteristics. Dissertation, Università di Bologna
Lazzarini L, Antonelli F, Cancelliere S, Conventi A (2008) The deterioration of Euganean trachyte in Venice. In: 11th International congress deterioration and conservation of stone. Nicolaus Copernicus University Torun, Poland, pp 153–162
Le Bas MJ, Streckeisen AL (1991) The IUGS systematics of igneous rocks. J Geol Soc Lond 148:825–833
Le Bas MJ, Le Maitre RW, Woolley AR (1992) The construction of the total alkali-silica chemical classification of volcanic rocks. Mineral Petrol 46:1–22
Moreno F, Vilela SAG, Antunes ÂSG, Alves CAS (2006) Capillary-rising salt pollution and granitic stone erosive decay in the parish church of Torre de Moncorvo (NE Portugal)-implications for conservation strategy. J Cult Herit 7(1):56–66
Mosquera MJ, Rivas T, Priet B, Silva B (2000) Capillary rise in granitic rocks: interpretation of kinetics on the basis of pore structure. J Colloid Interface Sci 222:41–45
Nicholson DT (2001) Pore properties as indicators of breakdown mechanisms in experimentally weathered limestones. Earth Surf Proc Landf 26:819–838
Ordoñez S, Fort R, García del Cura MA (1997) Pore size distribution and the durability of a porous limestone. Q J Eng Geol 30:221–230
Peruzzi R, Poli T, Toniolo L (2003) The experimental test for the evaluation of protective treatments: a critical survey of the ‘capillary absorption index’. J Cult Herit 4:251–254
Peters RR, Klavetter EA, George JT (2001) Measuring and modeling water imbibition into tuff. In: Evans DD et al (ed) Flow and transport through unsaturated fractured rock, Geophys Monogr Ser, vol 42, 2nd edn. American Geophysical Union, Washington, DC, pp 75–86
Prikryl R, Torok A (2010) Natural stones for monuments: their availability for restoration and evaluation. In: Prikryl R, Torok A (eds) Natural stone resources for historical monuments. Geological Society, vol 333, London, Special Publications, pp 1–9
Sengun N, Demirdag S, Akbay D, Ugur I, Altindag R, Akbulut A (2014) Investigation of the relationships between capillary water absorption coefficients and other rock properties of some natural stones, V. Global Stone Congress, 22–25 Oct 2014, Antalya/Türkiye
Sidraba I, Normandin KC, Cultrone G, Scheffler MJ (2004) Climatological and regional weathering of Roman travertine. In: Proceedings of the international conference lux lapis (light and stone) 2002 architectural and sculptural stone in cultural landscape. Charles University in Praque, The Karolinum Press, Praque, pp 211–228
Siegesmund S, Dürrast H (2011) Physical and mechanical properties of rocks. In: Siegesmund S, Snethlage R (eds) Stone in architecture, 4th edn. Springer, Berlin, pp 97–225
Theoulakis P, Moropoulou A (1988) Mechanism of deterioration of the sandstone of the Medieval city and the castle of Rhodes. In: Proceedings of the 6 international congress on deterioration and conservation of stone, Torun
Theoulakis P, Moropoulou A (1999) Salt crystal growth as weathering mechanism of porous stone on historic masonry. J Porous Mater 6(4):345–358
Tomašić I, Lukić D, Peček N, Kršinić A (2011) Dynamics of capillary water absorption in natural stone. Bull Eng Geol Environ 70:673–680
TS EN 13755 (2009) Natural stone test methods—determination of water absorption at atmospheric pressure. Turkish Standards Institute, Ankara, Turkey, p 10
TS EN 14579 (2006) Natural stone test methods—determination of sound speed propagation. Turkish Standards Institute, Ankara, Turkey, p 14
TS EN 1925 (2000) Natural stone test methods—Determination of water absorption coefficient by capillarity. Turkish Standards Institute, Ankara, Turkey, p 10
TS EN 1926 (2007) Natural stone test methods—determination of uniaxial compressive strength. Turkish Standards Institute, Ankara, Turkey, p 19
TS EN 1936 (2010) Natural stone test methods—determination of real density and apparent density and of total and open porosity. Turkish Standards Institute, Ankara, Turkey, p 10
Valdeón L, Esbert RM, Grossi CM (1992) Hydric properties of some Spanish building stones: a petrophysical interpretation. In: Proceedings of the symposium of materials research society, vol 267. Materials Research Society, pp 911–916
Vázquez P, Alonso FJ, Esbert RM, Ordaz J (2010) Ornamental granites: relationships between p-waves velocity, water capillary absorption and the crack network. Constr Build Mater 24:2536–2541
Washburn EW (1921) The dynamics of capillary flow. Phys Rev 17:273–283
Wedekind W, López-Doncel R, Dohrmann R, Kocher M, Siegesmund S (2013) Weathering of volcanic tuff rocks caused by moisture expansion. Environ Earth Sci 69:1203–1224
Winkler EM (1997) Stone in architecture: properties durability, 3rd edn. Springer, Berlin
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest as this research was conducted without specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Rights and permissions
About this article
Cite this article
Çelik, M.Y., Kaçmaz, A.U. The investigation of static and dynamic capillary by water absorption in porous building stones under normal and salty water conditions. Environ Earth Sci 75, 307 (2016). https://doi.org/10.1007/s12665-015-5132-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-015-5132-x