Abstract
Located in Yunnan province, China, Yangzonghai Lake is a fault-controlled highland lake where arsenic concentration suddenly increased 20-fold in 2008, which raised great environmental concerns. It is therefore a nice prototype to study the natural and/or anthropogenic influences on the arsenic geochemistry of the sediment in highland lakes. In this study, lacustrine sediments were recovered from the lake and the differences in the occurrence of arsenic between the surface (0–10 cm depth) and sub-surface sediments (>20 cm) were investigated. The arsenic distribution in the sub-surface sediment was in accordance with the locations of springs, suggesting that arsenic therein was affected by groundwater. The surface sediment, however, was affected by human contamination carried by Yangzong River. In the sub-surface sediment, arsenic concentrations increased with pH values but decreased with redox potentials. Higher grain sizes corresponded to lower arsenic contents. Arsenic was strongly positively correlated with Sb and Bi due to their similar geochemical behaviors. In the surface sediment, however, the human activities increased the arsenic concentration in the sediment and weakened the correlations between arsenic occurrence, pH/Eh values, grain size, and trace metals. Although the average differences in the trace element concentrations between surface and sub-surface sediments over the entire Yangzonghai Lake were less than 15 %, the pairwise differences were statistically significant, and the southern portion of the lake showed the greatest anthropogenic influence. This research will provide important information on the geochemistry and environmental science of arsenic in fault-controlled highland lakes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic is a serious environmental concern because of its high toxicity and global occurrence in the environment. It is ubiquitously present in soils, sediments, water bodies, minerals, and organisms (Rieuwerts et al. 2014; Smedley and Kinniburgh 2002). Arsenic can accumulate in soils and sediments, and thus threaten the local environment (Vaculík et al. 2013). Human beings who are chronically exposed to high concentrations of arsenic through oral, dermal, or respiration pathways may develop serious skin lesions and the risk of cancers may be increased (Gunduz et al. 2010; Melak et al. 2014). Studies on the behavior and occurrence of arsenic in the environment therefore capture global interest.
The sources of arsenic in the environment can be classified as either anthropogenic contribution or natural input. Anthropogenic sources include combustion of fossil fuels (Galiulin and Galiulina 2011), and mining and smelting of metal ores (Gonzalez-Fernandez et al. 2011; Otones et al. 2011). In addition, arsenic was widely used for producing herbicides and pesticides, and these arsenic-bearing chemicals will also contribute arsenic to the water bodies and sediments (De Carlo et al. 2014; Savery et al. 2014, Mandal and Suzuki 2002; Peryea and Creger 1994). Natural releases of arsenic from weathering and erosion of arsenic-bearing minerals and rocks, hydrothermal inputs, or volcanic eruptions (Lee et al. 2009; Petrini et al. 2011; Smedley and Kinniburgh 2013) are also important sources for arsenic in water and lacustrine sediment. For instance, arsenic has a strong affinity for pyrite and therefore is enriched in sulfide-bearing minerals (Nordstrom 2002). These ubiquitously present minerals will release arsenic into ambient environment if the pH and redox potentials are changed (Nordstrom 2002; Smedley and Kinniburgh 2002). In many areas worldwide, the oxidation and dissolution of arsenian pyrite and arsenopyrite in the sediment, associated with microbial processes, have caused high arsenic contamination in adjacent water bodies (Basu and Schreiber 2013; Biswas et al. 2011).
Many arsenic pollution events are primarily driven by natural inputs rather than human contaminations. Bangladesh suffered serious arsenic pollution in groundwater which threatened the health of millions of people. In addition to human activities, scientists have reached agreement that natural input was also responsible for the arsenic contamination in the groundwater of Bangladesh, which was mainly released from eroded Himalayan sediment via anaerobic reduction and dissolution of arsenic-bearing minerals (Fendorf et al. 2010; Nickson et al. 1998; Polizzotto et al. 2008). In Chianan Plain, Taiwan, high arsenic pollution (10–180 μg L−1) in groundwater has caused endemic Blackfoot disease and a number of internal cancers (Chen and Liu 2007; Guo et al. 2014; Nath et al. 2008). The arsenic therein has been proven to be dissolved from alluvial and deltaic sediments due to the strongly reducing conditions with Eh values of −75 ~ −140 mV (Guo et al. 2014; Sengupta et al. 2014). Geothermal input of arsenic also caused arsenic pollution in some areas, such as the Simav Plain, Turkey, where geothermal fluids exhibited arsenic concentrations of higher than 590 μg L−1 (Gunduz et al. 2010).
As a result, arsenic pollution in groundwater was usually associated with sediment and sediment served as a medium to adsorb and release arsenic to and from water bodies. Therefore, it is important to research the arsenic geochemistry of lacustrine sediment. In pristine areas, arsenic in the lacustrine sediment is strongly controlled by ambient pH value, redox condition, sedimentation process, minerals, and so on (Fendorf et al. 2010; Halim et al. 2009; Polizzotto et al. 2008; Selim Reza et al. 2010). First, arsenic will be released from arsenic-bearing pyrite (FeAsS) into groundwater under oxidizing conditions but will be precipitated as arsenic sulfide in sediment under reducing conditions (Couture and Van Cappellen 2011). Arsenic speciation is affected by pH values, and lower Eh values are needed to form reductive species if the pH values are increased (Carbonell-Barrachina et al. 2000; Smedley and Kinniburgh 2002). Second, oxide or hydroxide minerals such as goethite can adsorb inorganic arsenic from water. The decrease in grain size will usually increase the adsorption ability because the specific surface area and adsorption sites are enhanced (Canales et al. 2013; Mirlean et al. 2012). However, the adsorption capacity will be decreased with reduced redox potentials due to the destruction of metal oxides and hydroxides (Erbs et al. 2010; Mandal and Suzuki 2002). Third, the competitive adsorption between arsenate and other anions (e.g., phosphate, carbonate, silicate) on minerals will also affect the release and adsorption of arsenic to and from water bodies (Markley and Herbert 2010; Miao et al. 2012; Zhu et al. 2011). In addition, under some anaerobic conditions, micro-organisms will oxidize As (III) or reduce As(V) and thus change the speciation and mobility of arsenic in water and sediment (Islam et al. 2004; Liao et al. 2011).
Yangzonghai Lake is a typical highland lake in Yunnan province, China. It is the primary water source for agriculture, fishery, and drinking water for its surrounding counties. In June 2008, arsenic concentration in the lake water dramatically increased more than 20-fold from less than 0.005 mg L−1 to higher than 0.1 mg L−1 (Wang et al. 2010). The adjacent residents and related agriculture were strongly influenced by this arsenic pollution event (Liu et al. 2012). Although the lake water has been treated by precipitation and the arsenic concentration has been decreased to original level (<0.01 mg L−1), the precipitated arsenic in the sediment could be released into the water body again if the microbial activity increased (Liu et al. 2014). In addition, scientists have studied the occurrence of arsenic in the lake water, sediments, and organisms to assess the magnitude of arsenic contamination and tried to figure out the pollution sources. For instance, Wang et al. (2010) investigated the As concentrations in the sediment, lake water, and aquatic organisms, and assessed the risk of arsenic pollution of Yangzonghai Lake. Zhang et al. (2010, 2012a, b) studied the concentrations of arsenic and related heavy metals (Pb, Cu, Cr, Mn, and Zn) in the lake water and surface sediments, and calculated the geoaccumulation indexes and anthropogenic contributions of these heavy metals. These surveys indicated that human activities have affected the surface sediments with depth of 0–2 cm and the anthropogenic influences decreased with the depth of the sediment. Qi et al. (2010) studied the source and speciation of arsenic in the Yangzonghai lake water, and suggested that arsenic was mainly pollution from a chemical factory. Wang et al. (2005) reconstructed the environmental evolution of Yangzonghai Lake using 210Pb and 137Cs dating and diatom analyses. They found that the average sedimentation rate of the lacustrine sediment was about 1.25 mm a−1, and the coal-burning pollution started from 1960.
Previous studies focused on the arsenic pollution in the lake water and the assessment of contamination level in the lake water and sediments. The occurrence and geochemistry of arsenic in Yangzonghai Lake, however, have not been fully understood because the correlations between arsenic, heavy metals, and geochemical parameters (including pH, Eh, and particle size) in the lacustrine sediment were still unknown, while these correlations are critical to understand the sources and behaviors (e.g., mobility) of arsenic in the water bodies and sediments (Halim et al. 2009; Polizzotto et al. 2008). Furthermore, the effect of anthropogenic influence on the occurrence and behaviors of arsenic in the lake sediments was barely understood. In this study, lacustrine sediment samples were collected from Yangzonghai Lake using gravity-driven core sampler and the samples were separated into sub-surface sediment (depth > 20 cm) and surface sediment (depth of 0–10 cm) depending on the depth of the recovered samples. The spatial distribution of arsenic, the correlations between arsenic and pH, redox potential, grain size, and trace metals in the surface and sub-surface sediments were investigated and compared. Furthermore, the effect of human activities on the occurrence and geochemical behaviors of arsenic was assessed.
Field descriptions
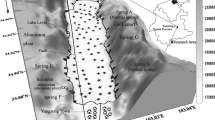
The studied area—Yangzonghai Lake—is located in Yiliang county, Yunnan province, China, with geographical location at longitude of 102°55′–103°02′ and latitude of 24°27′–24°54′ (Fig. 1). The nearly rectangular-shaped lake has a length of about 12 km and width of 3 km and is a natural fault-controlled freshwater lake, where the Xiaojiang fault developed along the north–south direction of the lake. One major water supply for the Lake is groundwater, which can be characterized by the ubiquitous occurrence of various springs (important springs with large runoff are marked on Fig. 1) around the lake. The groundwater has been proven to be an important factor in determining the elevation of water table and the quality of the lake water (Wang et al. 2010). The major surface water input of the lake is Yangzong River that flows through Yangzong town and recharges water at the south of the lake. The main output is Tangci Canal that runs through the Tangci town. The major chemical industry was situated at the southwest bank of the lake, which was supposed to have input large amount of arsenic-containing wastewater into the lake (Qi et al. 2010).
Sampling and methods
Sampling methods
The lacustrine sediment samples were collected by self-made gravity-driven core sampler with inner diameter of 52 mm and length of 0.9 m during August 5th to August 12th, 2010. The sample sites were nearly evenly located on the lake and the coordinates were recorded by a hand-GPS (Tables S1, S2 in supplementary material; Fig. 1). The recovered samples were separated into two or three parts according to the length of the samples. The upper part with depth of 0–10 cm, which in contact with the lake was designated the surface section, and the lower part with depth of >20 cm was designated as sub-surface sample. The middle parts with depth of 10–20 cm were not used in this study. If the recovered sediment core was longer than 30 cm, sediment with depth of 20–30 cm was designated as the sub-surface section. On the contrary, only sediment with depth of <10 cm was used as surface sediment if the core length was lower than 20 cm. According to the previous study, the sedimentation rate of the lacustrine sediments in Yangzonghai lake was about 1.25 mm a−1. Sediment at depth of 10 cm was formed at 1960s, and that of 20 cm was formed at mid-nineteenth century (Wang et al. 2005). In addition, the watershed of Yangzong river and the counties surrounding the lake was undeveloped before 1970s. Therefore, the sub-surface sediments with depth of >20 cm should not have been subjected to anthropogenic influence, while the surface samples may have been affected by human activities.
To analyze the pH and Eh values of the sediment, the pore water of each part was extracted by the commonly used Rhizon sampling method as soon as the sediment cores were recovered (Seeberg-Elverfeldt et al. 2005). The pH and Eh values of the pore water samples were measured immediately using a pH3110 (WTW) pH meter with accuracy of ±0.005 for pH and ±1 mV for Eh values.
Analyses
The concentrations of As, Sb, Hg, Bi in the sediment samples were analyzed by an AF-610A atomic fluorescence spectroscopy (AFS) (Beijing Rayleigh Analytical Instrument Co., Beijing, China). The operation conditions were maintained as follows: negative high voltage of 270 V, atomizer height of 8 mm, Ar flow rate of 300 mL min−1, lamp current of 30 mA, 170 °C atomization temperature, and recording time of 18 s. The concentrations of Fe, K, Mn, Ca, Mg, Al were determined by a SPECTRO XEPOS XEP01 X-ray fluorescence spectrometer (XRF) (Spectro Analytical instruments, Germany). The operation voltage and current were maintained at 50 kV and 50 mA, respectively.
The Ag concentration was investigated by Inductively Coupled Plasma—Atomic Emission Spectrometry (ICP-AES) (JY ULTIMA 2C, Longjumeau, France) in a simultaneous mode. The operation conditions were: 1100 W of output power, 18 L min−1 Ar flow rate, auxiliary Ar flow of 0.8 L min−1, 1 L min−1 nebulizer Ar flow, and nebulizer pressure of 0.3 MPa. The contents of Pb, Zn, Ni, V, Cd, Co were determined by a 7500ce inductively coupled plasma-mass spectrometry (ICP-MS) (Agilent, USA) with operation conditions of 1350 W in RF Power, 6.0 mm of sampling depth, 1.02 L min−1 carrier gas (Ar) and 4.5 mL min−1 Helium flow rate. Reagent blanks, duplicate samples, and reference materials (Dorm-2, Dogfish muscle, NRC, Canada) were used for quality controls. The estimated errors of the analyses as relative standard deviation were between 2 and 8 %.
The grain size distributions of the samples were analyzed using an LS 13 320 laser particle size analyzer (Beckman Coulter, USA). About 1 g sample was mixed with 20 mL deionized water, the mixture was then treated with 10 mL 30 % H2O2 and 10 mL 1 mol L−1 HCl to remove the impurities such as organic substances and carbonate. The product was dispersed by 5 mL 0.5 mol L−1 [NaPO3]6 solution and then was determined by the laser particle size analyzer. The average particle size was calculated and used in this study.
Statistical tests
The correlation coefficients among heavy metals in both surface and sub-surface lacustrine sediments were calculated by SPSS 19.0 Windows version using Pearson correlation coefficients with 2-tailed test of significance. Paired t test was used to test the statistical significance of differences in heavy metal concentrations between surface and sub-surface sediments. The surface data without corresponding sub-surface values were discarded before the statistical tests. The test was calculated by SPSS 19.0. The df values for all elements were 55 and 2-tailed significances were calculated. Wilcoxon signed-rank test was used to investigate the statistical significance of the differences in the correlation coefficients between As and other elements in surface and sub-surface sediments. This test was calculated by SPSS 19.0 and 2-tailed asymptotic significance values were obtained.
Results and discussion
Metal concentrations of the lacustrine sediment
The elemental concentrations of lacustrine sediment samples are shown in Table S1 (supplementary material), Table S2 (supplementary material), and Fig. 2. Compared with sub-surface sediments with depth of >20 cm, surface sediment samples (0–10 cm) exhibited relatively higher average concentrations of trace elements (e.g., As concentrations in the surface sediment, 19.95 mg kg−1, while in sub-surface sediment 18.05 mg kg−1; Pb 38.74 mg kg−1 in surface and 37.26 mg kg−1 in sub-surface sediments) with the exception of Mn. Although the differences in the concentrations between surface and sub-surface sediments were less than 15 %, the pairwise statistical tests suggested that these differences were significant for most heavy metals. As shown in Table 1, the 2-tailed p values obtained from paired t test indicated that the differences in the concentrations of As, Sb, Bi, Cd, Zn, Mn, and V between surface and sub-surface sediments were extremely significant with significance of <0.01; Hg and Co were significant at 0.05 level, while Ag, Pb, Ni, and Fe were not statistically significant. Therefore, except for Ag, Pb, Ni, and Fe, the differences in the concentrations of all other heavy metals (especially As) between surface and sub-surface sediments were statistically significant and unlikely to be from the result of measurement errors. As a result, the increase of heavy metals in the surface sediments was most likely the result of the enhanced accumulation of trace metals in recent decades due to the fast development of agriculture and industry in adjacent areas which delivered contaminants into the lake (Galal-Gorchev 1991). The low average difference between the surface and sub-surface sediment may be attributed to the short duration of anthropogenic contamination, because while significant human activities started from 1960s, the most rapid human activities have occurred since the 1990s when many hotels, golf courts, real estates, and industrial plants were established around the lake (Yang et al. 2012). The concentration of Cd in the surface sediment (0.68 mg kg−1) was 30 % higher than that in the sub-surface sediment (0.52 mg kg−1), which may be attributed to a very high input of Cd-bearing pesticides or industrial wastewaters.
Although the average differences in arsenic concentrations between surface and sub-surface sediment were less than 15 %, the spatial distribution patterns of arsenic were dramatically different. As shown in Fig. 3, the distribution of arsenic in the sub-surface lacustrine sediment showed two maximums according to the contour map. The first appeared at the northeastern part of the lake with maximum arsenic concentration of about 30 mg kg−1. It is accompanied by the appearance of cold spring A. The second was located at the lower middle part of the west bank showing maximum concentration of more than 28 mg kg−1. This position is also in accordance with the appearance of a cold spring E. Because of the good agreement between arsenic concentrations and the appearance of cold springs, combined with the fact that the sub-surface sediments should be formed at about mid-nineteenth century that without significant anthropogenic disturbance according to the sedimentation rate of Yangzonghai lake sediment (Wang et al. 2005), we can conclude that arsenic in the sub-surface sediment was mainly controlled by spring water, that is, groundwater. Groundwater with high arsenic concentrations has been found around the world (Nordstrom 2002). It is usually associated with arsenic-rich sediments or minerals (Guo et al. 2014; Smedley and Kinniburgh 2002; Welch et al. 2000). In Yangzonghai area, the cold springs around the Lake are developed in arsenic-bearing strata with arsenic concentrations of as high as 25 mg kg−1 (Table S3 in supplementary material). The arsenic in these strata could be leached out by groundwater or be released into groundwater if the redox or pH conditions were changed (Al-Abed et al. 2007; Nickson et al. 2000). The generated arsenic-rich groundwater can be subsequently inputted into the lake and then precipitated into the sediment. This hypothesis can be substantiated by the source sampling near the springs. As shown in Table S3 (supplementary material), the onshore sediment and soil samples near spring A and E exhibited much higher As concentrations than that near spring B and D; accordingly, the As contents in the lacustrine sediments near spring A and E were also significantly higher than that near spring B and D. In addition, the geothermal water around Yangzonghai Lake could accelerate the leaching of arsenic from the strata, which can be verified by the As concentrations in the sinter of Spring C which even exceeded 200 mg kg−1 (Table S3 in supplementary material) (Ballantyne and Moore 1988; Sengupta et al. 2014; Welch et al. 2000).
In the northern part of the lake, the distribution patterns of arsenic in the surface and sub-surface sediments were quite similar with analogous contour maps and comparable arsenic concentrations. This indicates that the major arsenic source and sedimentation processes in this area did not change significantly and were still mainly controlled by groundwater. In the southern part of the lake, however, the spatial distribution of arsenic in the surface sediment exhibited a totally different pattern than that of sub-surface sediment. First, arsenic concentrations in the surface sediments near the west bank were decreased, and no maximum can be found near spring E. This phenomenon may be attributed to the dramatically decreased groundwater output of spring E. After the establishment of aluminum plant in 1970s and the rapid development of real estate since 2000s, the groundwater has been massively utilized and the flow rate of this spring has been greatly reduced. Second, maximum arsenic concentration appeared in the southernmost area of the lake, adjacent to the mouth of the Yangzong River. The maximum arsenic contents were up to 30 mg kg−1, significantly higher than that of sub-surface sediment at this area (20 mg kg−1). These phenomena should be ascribed to the influence of human activities. Before 1970s, the Yangzong river watershed was undeveloped and the Yangzong River delivered fresh water with little contaminant into the Yangzonghai Lake. Consequently, arsenic in the sub-surface sediment in Yangzonghai Lake was mainly affected by groundwater. With the fast development of agriculture and industry after 1970s, arsenic-bearing agricultural and industrial wastes such as pesticides and mining wastewater were directly poured into the Yangzong River and subsequently flowed into the lake. The anthropogenic influences, combined with groundwater, shaped the spatial distribution of As in the surface lacustrine sediment. Because the lake is recharged from the south end via Yangzong River and drained from the north end through Tangci Canal, arsenic from human contamination was concentrated at the southern end of the lake. The arsenic pollution by the Yangzong river can be further substantiated by the ratios of the surface to the sub-surface As concentrations. As shown in Fig. 3c, high ratios of >1.6 appeared at the mouth of the Yangzong river. Although high ratios can also be found at the center of the lake and near the spring D, both cases were largely attributed to the low As concentrations in the sub-surface sediments at these areas. Note that arsenic concentrations in the surface sediment near the chemical industry (Fig. 1) that was supposed to be the main source of arsenic contamination did not show significantly higher values than that in the sub-surface sediment, which may due to the short pollution history because this phosphate fertilizer plant started producing arsenic-bearing wastes only after 1996 (Table S3 in supplementary material). In addition, another explanation could be that arsenic input from the chemical industry was quite limited when compared with that from the Yangzong River and groundwater.
Effects of pH and Eh
The pH and Eh values of some lacustrine sediment samples were also determined to assess the effects of pH and Eh on the arsenic concentrations in the sediments. As shown in Table S1 (supplementary material), Table S2 (supplementary material), and Fig. 4, both sub-surface and surface sediments showed highly reducing and acidic conditions with Eh values of less than −180 mV and pH of lower than 5.6. The sub-surface sediment samples exhibited relatively higher Eh but lower pH values than that of surface sediments, which may be affected by the changes in the acidity and redox conditions of the lake water (Liu et al. 2011).
a Comparisons on the Eh and pH values between a sub-surface and b surface sediments; b aqueous Eh–pH system for the lacustrine sediment samples; Arsenic concentration as a function of pH values in the c sub-surface and d surface sediments; Arsenic concentration as a function of redox potentials in the e sub-surface and f surface sediments
It is a consensus that the concentration and speciation of arsenic are strongly affected by the ambient acidic and redox environment (Yamaguchi et al. 2011) (Fig. 4b). According to the arsenic speciation as functions of pH and Eh values in aquatic systems (Smedley and Kinniburgh 2002), the major species of arsenic under high Eh values are As(V) compounds while As(III) species are present under reducing conditions. At even lower redox potentials, arsenic may precipitate as sulfides such as FeAsS, As2S3, and so on (Carbonell-Barrachina et al. 2000; Lee et al. 2005). On the other hand, pH value also plays an important role in determining the arsenic concentration in lacustrine sediment. First, the lower limits for the arsenic speciation boundaries as a function of Eh decrease with increasing pH values. Second, higher pH values will greatly reduce the arsenic mobility in aquatic systems (Lee et al. 2009; Mandal and Suzuki 2002; Nordstrom 2002). Third, arsenic will be adsorbed by oxide minerals under acidic to neutral conditions, and the adsorption capacity increases with pH at values of 4–6 (Mamindy-Pajany et al. 2011). Based on these assumptions, arsenic concentration in lacustrine sediment will generally increase with pH values between 4 and 6 and decrease with redox potentials at extremely reducing conditions.
In this study, the variations of arsenic concentration in the sub-surface lacustrine sediment were consistent with the above theories, such that the arsenic concentration significantly increased with pH values and decreased with redox potentials with correlation coefficients (R 2) of 0.4900 and 0.4383, respectively. These results indicate that arsenic in the sub-surface sediments of Yangzonghai lake was affected by the ambient geochemical conditions (pH and Eh), and the correlation between them was largely undisturbed due to lack of significant human activities. The surface sediment, however, exhibited very weak correlations (R 2 < 0.025) between arsenic concentrations and pH/Eh values, indicating that the occurrence of arsenic in the surface sediment was largely not affected by ambient pH and redox conditions. Considering the fast development of adjacent areas and distorted arsenic distribution map in the surface lacustrine sediment, human activities were probably responsible for the decreased correlations between arsenic and pH/Eh values.
Influence of grain size on the accumulation of arsenic
Generally speaking, arsenic concentrations in lacustrine sediments are negatively correlated with the grain size of the sediment due to the close relationship between grain size and the adsorption capacity for arsenic (Devesa-Rey et al. 2011; Kim et al. 2013). This “grain size effect” is particularly important in stable lakes without significant disturbances of groundwater or human activities. In this study, the grain size effect played a minor role in determining the distribution of arsenic in the lacustrine sediment of Yangzonghai lake. As shown in Fig. 5, both the surface and sub-surface lacustrine sediments from Yangzonghai Lake showed similar particle size distributions with mean values of about 20 μm (Fig. 2). Discarding the data with grain size of larger than 60 μm which may cause bias (Ackermann et al. 1983), arsenic concentration and particle size of the sub-surface sediment showed a weak negatively correlation with coefficient R 2 of 0.1689. The correlation coefficient of the surface sediment, however, was even lower with R 2 of only 0.0042, suggesting that the anthropogenic influences further weakened the already weak correlation between arsenic enrichment and grain size.
Correlations between arsenic and other trace metals
As is usually correlated with other trace metals such as Fe, Mn, Pb, and so on in lacustrine sediment (Gawel et al. 2014; Nickson et al. 1998). In this study, the correlations among As and other trace metals including Sb, Bi, Pb, Cd, Fe, V, and so on were calculated to investigate the coherence among these elements. As shown in Table 2 and Fig. 6, As was strongly positively correlated with Sb, Bi, and Pb in the sub-surface lacustrine sediment with correlation coefficients of higher than 0.5. The significant correlations among these elements can be ascribed to the fact that all these elements are chalcophile elements that are closely associated with sulfides (Goldschmidt 1937; Kiseeva et al. 2013). As exhibited weak correlations with common anthropogenic contaminants such as Hg, Cd, and Ag in the sub-surface sediment, suggesting that these elements were originated from different sources or experienced different geochemical processes (Yao and Gao 2007). Zn, Ni, Co, Fe, Mn, and V showed strong correlations among each other, because these elements have similar geochemical characteristics and may form comparable metal oxides or hydroxides. The weak correlations between these elements and arsenic suggested that arsenic in the sub-surface sediment was feebly associated with the oxides or hydroxides that formed by these elements, which is consistent with previous studies that As and Fe were decoupled in groundwater and sediment in most arsenic-rich areas of China (Guo et al. 2014).
The correlations among arsenic and other trace metals in the surface lacustrine sediment from Yangzonghai Lake, as shown in Table 3 and Fig. 7, exhibited analogous characteristics with that in the sub-surface sediment. Arsenic was most strongly associated with Sb, Bi, and Pb, and showed weak correlations with other elements. A difference between surface and sub-surface sediments can be seen in the correlation coefficients, such that the coefficients between As and Sb/Bi in the surface sediment were lower than that in sub-surface sediment, while the correlation between As and Pb was slightly higher. The correlations among Zn, Ni, Co, Fe, Mn, and V in the surface sediment were much lower than that in the sub-surface sediment. In addition, As and Bi showed enhanced negative correlations with Co, Fe, Mn, and V. Statistical tests suggested that the differences of the correlations between As and other elements in the surface and sub-surface sediments were significant. Because the correlation coefficients were abnormally distributed, we used Wilcoxon signed-rank test, which gave the results that Z = −2.354 and 2-tailed asymptotic significance of 0.019. In addition, if we only assess the differences in the correlations between As and (Sb, Bi, Hg, Cd, Zn, Mn, Co, and V) because the differences in the concentrations of Ag, Pb, Ni, and Fe were not statistically significant between surface and sub-surface sediments, we obtained similar results with Z = −2.243 and asymptotic significance of 0.025. Therefore, the significant differences in the correlation coefficients between surface and sub-surface sediments can probably be ascribed to the enhanced anthropogenic influences in the surface sediment rather than measurement errors. First, arsenic input from human activities diluted the non-anthropogenic geochemical associations among As, Sb, and Bi and therefore weakened the correlations among these elements. The correlations between As and (Zn, Ni, Co, Fe, Mn, and V) were disturbed for the same reason. Second, both As and Pb are common ingredients in many industrial and agricultural products such as pesticides (Peryea and Creger 1994; Bissen and Frimmel 2003). The enhanced anthropogenic activities in recent decades appear to have increased the correlations between As and Pb in the surface sediment.
Conclusion
In summary, arsenic occurrence in the lacustrine sediments from a highland fault-related lake—Yangzonghai Lake—was studied and the differences between surface and sub-surface sediments were investigated in this study. The spatial distribution of arsenic in the sub-surface sediment was consistent with the locations of cold springs, suggesting that the occurrence of arsenic in pre-industrial sediment was mainly controlled by groundwater. Arsenic concentrations in the sub-surface sediment increased with pH values but decreased with redox potentials, while larger average grain size resulted in lower arsenic concentrations. Arsenic was strongly positively correlated with Sb, Bi, and Pb, but weakly associated with Zn, Ni, Fe, and Mn. Arsenic in the surface sediment was affected by human activities associated with the rapid development of adjacent areas in recent decades. The anthropogenic influences distorted the distribution of arsenic in the surface sediment and weakened the correlation between arsenic and pH/Eh values, grain size, and other trace metals. Although the overall differences in trace element concentrations between surface and sub-surface sediments were less than 15 %, pairwise comparisons were strongly statistically significant. The major anthropogenic input was the Yangzong River, as shown by the contour map of arsenic distribution in the surface sediment, with higher As concentrations in the surface sediment near the mouth of the Yangzong River. Currently, the sediment chemistry in the northern part of the lake does not appear to have been disturbed by human activities. This study investigated the natural and anthropogenic sources of arsenic in Yangzonhai Lake and will help the local government for policy making. In the near future, the occurrence of arsenic in the geological bodies around Yangzonghai Lake will be investigated and the detailed trace element correlations will be studied to unveil the trace element geochemistry of this fault-controlled highland lake.
References
Ackermann F, Bergmann H, Schleichert U (1983) Monitoring of heavy metals in coastal and estuarine sediments—a question of grain-size: <20 μm versus <60 μm. Environ Technol Lett 4:317–328
Al-Abed SR, Jegadeesan G, Purandare J, Allen D (2007) Arsenic release from iron rich mineral processing waste: influence of pH and redox potential. Chemosphere 66:775–782
Ballantyne JM, Moore JN (1988) Arsenic geochemistry in geothermal systems. Geochim Cosmochim Acta 52:475–483
Basu A, Schreiber ME (2013) Arsenic release from arsenopyrite weathering: insights from sequential extraction and microscopic studies. J Hazard Mater 262:896–904
Bissen M, Frimmel FH (2003) Arsenic—a review part I: occurrence, toxicity, speciation, mobility. Acta Hydrochim Hydrobiol 31:9–18
Biswas A et al (2011) Groundwater chemistry and redox processes: depth dependent arsenic release mechanism. Appl Geochem 26:516–525
Canales RM, Guan H, Bestland E, Hutson J, Simmons CT (2013) Particle-size effects on dissolved arsenic adsorption to an Australian laterite. Environ Earth Sci 68:2301–2312
Carbonell-Barrachina A, Jugsujinda A, Burlo F, Delaune R, Patrick W Jr (2000) Arsenic chemistry in municipal sewage sludge as affected by redox potential and pH. Water Res 34:216–224
Chen K-Y, Liu T-K (2007) Major factors controlling arsenic occurrence in the groundwater and sediments of the Chianan coastal plain, SW Taiwan. Terr Atmos Ocean Sci 18:975–994
Couture R-M, Van Cappellen P (2011) Reassessing the role of sulfur geochemistry on arsenic speciation in reducing environments. J Hazard Mater 189:647–652
De Carlo EH, Tomlinson MS, deGelleke LE, Thomas S (2014) Distribution and abundance of arsenic in the soils and sediments of O’ahu, Hawai’i. Aquat Geochem 20:87–113
Devesa-Rey R, Diaz-Fierros F, Barral MT (2011) Assessment of enrichment factors and grain size influence on the metal distribution in riverbed sediments (Anllons River, NW Spain). Environ Monit Assess 179:371–388
Erbs JJ, Berquó TS, Reinsch BC, Lowry GV, Banerjee SK, Penn RL (2010) Reductive dissolution of arsenic-bearing ferrihydrite. Geochim Cosmochim Acta 74:3382–3395
Fendorf S, Michael HA, van Geen A (2010) Spatial and temporal variations of groundwater arsenic in South and Southeast Asia. Science 328:1123–1127
Galal-Gorchev H (1991) Dietary intake of pesticide residues: cadmium, mercury, and lead. Food Addit Contam 8:793–806
Galiulin R, Galiulina R (2011) Pollution of the territory of Chelyabinsk and the suburbs with arsenic upon coal combustion. Solid Fuel Chem 45:197–199
Gawel JE, Asplund JA, Burdick S, Miller M, Peterson SM, Tollefson A, Ziegler K (2014) Arsenic and lead distribution and mobility in lake sediments in the south-central Puget Sound watershed: the long-term impact of a metal smelter in Ruston, Washington, USA. Sci Total Environ 472:530–537
Goldschmidt VM (1937) The principles of distribution of chemical elements in minerals and rocks. The seventh Hugo Müller Lecture, delivered before the Chemical Society on March 17th, 1937. J Chem Soc (Resumed), 655–673. doi:10.1039/JR9370000655
Gonzalez-Fernandez O, Queralt I, Carvalho ML, Garcia G (2011) Lead, zinc, arsenic and copper pollution in the alluvial plain of a mining wadi: the Beal Case (Cartagena–La Union Mining District, SE Spain). Water Air Soil Pollut 220:279–291
Gunduz O, Simsek C, Hasozbek A (2010) Arsenic pollution in the groundwater of Simav Plain, Turkey: its impact on water quality and human health. Water Air Soil Pollut 205:43–62
Guo H, Wen D, Liu Z, Jia Y, Guo Q (2014) A review of high arsenic groundwater in Mainland and Taiwan, China: distribution, characteristics and geochemical processes. Appl Geochem 41:196–217
Halim M et al (2009) Groundwater contamination with arsenic in Sherajdikhan, Bangladesh: geochemical and hydrological implications. Environ Geol 58:73–84
Islam FS, Gault AG, Boothman C, Polya DA, Charnock JM, Chatterjee D, Lloyd JR (2004) Role of metal-reducing bacteria in arsenic release from Bengal delta sediments. Nature 430:68–71
Kim C et al (2013) (Micro) spectroscopic analyses of particle size dependence on arsenic distribution and speciation in mine wastes. Environ Sci Technol 47:8164–8171
Kiseeva ES, Litasov KD, Yaxley GM, Ohtani E, Kamenetsky VS (2013) Melting and phase relations of carbonated eclogite at 9–21 GPa and the petrogenesis of alkali-rich melts in the deep mantle. J Petrol 54:1555–1583
Lee M-K, Saunders JA, Wilkin RT, Mohammad S (2005) Geochemical modeling of arsenic speciation and mobilization: Implications for bioremediation. Adv Arsen Res 915:398–413
Lee JU, Lee SW, Chon HT, Kim KW, Lee JS (2009) Enhancement of arsenic mobility by indigenous bacteria from mine tailings as response to organic supply. Environ Int 35:496–501
Liao VHC et al (2011) Arsenite-oxidizing and arsenate-reducing bacteria associated with arsenic-rich groundwater in Taiwan. J Contam Hydrol 123:20–29
Liu S, Shi X, Liu Y, Zhu Z, Yang G, Zhu A, Gao J (2011) Concentration distribution and assessment of heavy metals in sediments of mud area from inner continental shelf of the East China Sea. Environ Earth Sci 64:567–579
Liu WH, Yang CL, Fu Q, Yang T, Yang LX (2012) Analysis on eco-environmental water demand and environmental functions of Yangzonghai Lake. Environ Sci Manag 37(2):121–125
Liu RB, Yang CL, Li SY, Sun PS, Shen SL, Li ZY, Lin K (2014) Arsenic mobility in the arsenic-contaminated Yangzonghai Lake in China. Ecotoxicol Environ Saf 107:321–327
Mamindy-Pajany Y, Hurel C, Marmier N, Roméo M (2011) Arsenic (V) adsorption from aqueous solution onto goethite, hematite, magnetite and zero-valent iron: effects of pH, concentration and reversibility. Desalination 281:93–99
Mandal BK, Suzuki KT (2002) Arsenic round the world: a review. Talanta 58:201–235
Markley CT, Herbert BE (2010) Modeling phosphate influence on arsenate reduction kinetics by a freshwater cyanobacterium. Environ Model Assess 15:361–368
Melak D et al (2014) Arsenic methylation and lung and bladder cancer in a case-control study in northern Chile. Toxicol Appl Pharmacol 274:225–231
Miao AJ, Wang NX, Yang LY, Wang WX (2012) Accumulation kinetics of arsenic in Daphnia magna under different phosphorus and food density regimes. Environ Toxicol Chem 31:1283–1291
Mirlean N, Medeanic S, Garcia F, Travassos M, Baisch P (2012) Arsenic enrichment in shelf and coastal sediment of the Brazilian subtropics. Cont Shelf Res 35:129–136
Nath B, Jean JS, Lee MK, Yang HJ, Liu CC (2008) Geochemistry of high arsenic groundwater in Chia-Nan plain, Southwestern Taiwan: possible sources and reactive transport of arsenic. J Contam Hydrol 99:85–96
Nickson R, McArthur J, Burgess W, Ahmed KM, Ravenscroft P, Rahmanñ M (1998) Arsenic poisoning of Bangladesh groundwater. Nature 395:338
Nickson R, McArthur J, Ravenscroft P, Burgess W, Ahmed K (2000) Mechanism of arsenic release to groundwater, Bangladesh and West Bengal. Appl Geochem 15:403–413
Nordstrom DK (2002) Public health. Worldwide occurrences of arsenic in ground water. Science 296:2143–2145
Otones V, Álvarez-Ayuso E, García-Sánchez A, Santa Regina I, Murciego A (2011) Mobility and phytoavailability of arsenic in an abandoned mining area. Geoderma 166:153–161
Peryea F, Creger T (1994) Vertical distribution of lead and arsenic in soils contaminated with lead arsenate pesticide residues. Water Air Soil Pollut 78:297–306
Petrini R et al (2011) Natural arsenic contamination in waters from the Pesariis village, NE Italy. Environ Earth Sci 62:481–491
Polizzotto ML, Kocar BD, Benner SG, Sampson M, Fendorf S (2008) Near-surface wetland sediments as a source of arsenic release to ground water in Asia. Nature 454:505–508
Qi JY, Xu ZC, Li XP, Fang JD, Huang JH (2010) Study on source and speciation distribution characteristics of arsenic in Yangzonghai Lake Waters. J Anhui Agric Sci 38(20):10789–10792
Rieuwerts J, Mighanetara K, Braungardt C, Rollinson G, Pirrie D, Azizi F (2014) Geochemistry and mineralogy of arsenic in mine wastes and stream sediments in a historic metal mining area in the UK. Sci Total Environ 472:226–234
Savery LC et al (2014) Global assessment of arsenic pollution using sperm whales (Physeter macrocephalus) as an emerging aquatic model organism. Comp Biochem Physiol Toxicol Pharmacol 163:55–63
Seeberg-Elverfeldt J, Schlüter M, Feseker T, Kölling M (2005) Rhizon sampling of porewaters near the sediment-water interfaceof aquatic systems. Limnol Oceanogr 3:361–371
Selim Reza AHM et al (2010) Occurrence of arsenic in core sediments and groundwater in the Chapai-Nawabganj District, northwestern Bangladesh. Water Res 44:2021–2037
Sengupta S et al (2014) Spatial variation of groundwater arsenic distribution in the Chianan Plain, SW Taiwan: role of local hydrogeological factors and geothermal sources. J Hydrol 518:393–409
Smedley PL, Kinniburgh DG (2002) A review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochem 17:517–568
Smedley PL, Kinniburgh DG (2013) Arsenic in groundwater and the environment. In: Selinus O (ed) Essentials of medical geology, Springer, Dordrecht. pp 279–310. doi:10.1007/978-94-007-4375-5_12
Vaculík M, Jurkovič Ľ, Matejkovič P, Molnárová M, Lux A (2013) Potential risk of arsenic and antimony accumulation by medicinal plants naturally growing on old mining sites. Water Air Soil Pollut 224:1–16
Wang YF, Hu SY, Zhu YX, Yin Y, Zhou WP, Appel E, Hoffmann V (2005) The lacustrine sedimentary records of coal-burning atmospheric pollution. Sci China Ser D Earth Sci 48(10):1740–1746
Wang Z et al (2010) Levels, trends and risk assessment of arsenic pollution in Yangzonghai Lake, Yunnan Province, China. Sci China Chem 53:1809–1817
Welch AH, Westjohn D, Helsel DR, Wanty RB (2000) Arsenic in ground water of the United States: occurrence and geochemistry. Ground Water 38:589–604
Yamaguchi N, Nakamura T, Dong D, Takahashi Y, Amachi S, Makino T (2011) Arsenic release from flooded paddy soils is influenced by speciation, Eh, pH, and iron dissolution. Chemosphere 83:925–932
Yang CL, Li SY, Yuan LN, Yang LX, Zhang HZ, Liu WH (2012) The eutrophication process in a plateau deepwater lake: response to the changes of anthropogenic disturbances in the watershed. In: 2012 international conference on biomedical engineering and biotechnology, pp 1815–1821
Yao Z, Gao P (2007) Heavy metal research in lacustrine sediment: a review Chinese. J Oceanol Limnol 25:444–454
Zhang YX, Sun JC, Liu JT, Huang GX, Xiang XP, Chen X, Jing JH, Cui HW (2010) Heavy metal contamination and potential ecological risk assessment of sediments in Yangzonghai Lake. In: 2010 international conference on digital manufacturing and automation, pp 795–801
Zhang Y et al (2012a) Distribution and sources of arsenic in Yangzonghai Lake, China. Huanjing Kexue 33:3768–3777
Zhang Y, Liu J, Chen X, Sun J (2012b) Arsenic in waters and sediments of Yangzonghai Lake, China. Energy Educ Sci Technol Part A 30:309–316
Zhu J, Pigna M, Cozzolino V, Caporale AG, Violante A (2011) Sorption of arsenite and arsenate on ferrihydrite: effect of organic and inorganic ligands. J Hazard Mater 189:564–571
Acknowledgments
This research is supported by the National natural science foundation of China (40973066).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhu, XH., Zhang, PP., Chen, XG. et al. Natural and anthropogenic influences on the arsenic geochemistry of lacustrine sediment from a typical fault-controlled highland lake: Yangzonghai Lake, Yunnan, China. Environ Earth Sci 75, 217 (2016). https://doi.org/10.1007/s12665-015-4924-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-015-4924-3