Abstract
Hydrogeochemistry is an important tool for the evaluation of the effect of human activities on aquifer system. Sixty eight groundwater samples were collected from bore wells during pre- and post-monsoon from Nagpur , a fast growing city in India, to assess the spatial controlling processes of groundwater contamination using principal component analysis (PCA). Groundwater has variable total dissolved solid (TDS) and total hardness (TH) values classifying them from fresh to saline and moderately hard to very hard types. About 36 and 33 % of the total groundwater samples during pre- and post-monsoon, respectively, are not suitable for drinking purpose. The graphical presentation of groundwater chemistry has indicated Ca–HCO3, mixed Ca–Na–HCO3 and mixed Ca–Mg–Cl types. The PCA summarizes the chemical variables of pH, EC, TDS, TH, TA, Na+, K+, Ca2+, Mg2+, HCO3 −, Cl−, SO4 2− and NO3 − into two PC loadings, accounting for 61.33 and 62.09 % of the total variance during pre- and post-monsoon, respectively. The first PC shows high loadings of EC, TDS, TH, Cl−, NO3 −, Ca2+ and Mg2+, which considered as pollution-controlled processes of anthropogenic sources. Second PC has high loadings of Na+ and HCO3 −, which is taken as alkalinity and pollution-controlled processes of geogenic and anthropogenic sources, respectively. The PC scores suggest the causes of variation in the groundwater chemistry. Negative values of chloro-alkaline indices suggest the prevalence of reverse ion exchange irrespective of the season, silicate weathering and anthropogenic activities over the controlling of groundwater quality which further PCA. Thus, the PCA helps as a tool to assess the controlling processes of the groundwater quality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The rapid economic and population growths coupled with haphazard urbanization in the developing countries like India are rapidly depleting surface water supplies. Hence, groundwater is used as an alternative source of water for domestic, agricultural and industrial purposes. Nagpur is one of the fastest growing cities of India and over the period of time, multitudes of problems are cropping up in order to cater to the need of ever-growing demand of drinking and domestic supply, for which the groundwater is the most dependable alternative supply. The groundwater is under stress due to shrinkage of recharged area caused by sudden spurt in developmental activities. This non-basis and unplanned development of groundwater has caused environmental degradation and lowering of water level.
Increased knowledge of geochemical evolution of groundwater leads to improved understanding of hydrochemical system, which results in its effective management for sustainable development. Hydrochemical assessment of groundwater system is based on groundwater chemistry, which, in turn, depends on a number of factors, such as geology, degree of chemical weathering of various rock types, quality of recharge water, and inputs from various sources other than water–rock interaction (Ayenew et al. 2008; Giridharan et al. 2008). Various graphical methods and different indices were attempted by researchers for evaluation of groundwater chemistry and delineation of various hydrogeochemical processes that are involved in the evolution of groundwater quality, with certain limits (Apadaca et al. 2007). Thus, it is preferred to evaluate the controlling processes using multivariate statistical analysis.
Principal component analysis (PCA) provides valuable information on most meaningful parameters, which describe the whole data set, consisting of a large number of inter-related variables, rendering data reduction with minimum loss of original information. This reduction is achieved by transforming the data set into a new set of variables. The principal components (PCs), which are orthogonal (non-correlated), are in decreasing order of importance (Panda et al. 2006). The PCA is the useful tool in identification of a number of reduced latent factors with pollution sources such as spatial (anthropogenic) and temporal (seasonal and climatic) origins of variations that affect quality and hydrochemistry of groundwater (Simeonov et al. 2003; Pekey et al. 2004; Subba Rao 2006, 2007; Shrestha and Kazama 2007; Fernandes et al. 2008; Koklu et al. 2010; Lin et al. 2012; Arunprakash et al. 2014; Singaraja et al. 2014).
Earlier studies carried out in the parts of Nagpur city were mainly concentrated on groundwater quality (Marghade et al. 2010, 2011a, b). Thus, the focus of the present paper is on evaluating the spatial and temporal variations in groundwater quality with respect to natural processes and anthropogenic inputs, using PCA.
Study area
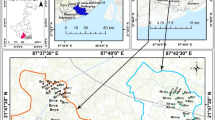
Nagpur (21°03′–21°12′N: 79°′–79°10′E) is situated at an altitude between 300 and 350 m MSL on the western plateau formed in the western slope of Satpura Mountain Ranges in the Central India (Fig. 1), with a population of 23, 98,165 (2011 census). Nag and Pili are the two small rivers flowing towards eastern part and along northern edge of the city. The climate of the area is semi-arid with minimum temperature of 10 °C during winter (December–February) and maximum temperature of 48 °C during summer (March–May). The onset of monsoon is usually from June and it extends up to September. The average rainfall recorded for the period of 30 years is 1200 mm and most of it (70 %) is contributed by southeast monsoon (June to September).
Geologically, the Precambrian crystalline rocks occupy eastern part of the city and represented by gneisses, schists and granites (Fig. 1). These rocks are also exposed along the southeastern and northern part of the city. The Permo-carboniferous Lower Gondwana rocks are represented by Talchir and Kamthi formations. The Talchir Formation comprises alternate layers of shale and sandstone, which are exposed at Nara and Bokara villages along the northernmost boundary of the city. The Kamthi Formation is represented by sandstone and clays, which occupy very limited area in the northern part of the city near Nara, Bokara and Kalamna villages. The sandstones, because of their high primary porosity and permeability, form potential aquifer (Jain et al. 2012). The upper Cretaceous Lameta Formation unconformably overlies the crystalline and Gondwana rocks and consists of sandstones, variegated clays and cherty limestones. It occupies a narrow fringe from north to central part and further southeast of the city around the Deccan traps. Deccan traps cover major part of the study area and occupy western and southern part of the city. They are represented by horizontal basaltic lava flows of uppermost Cretaceous to lower Eocene age. Lava flows are separated by intervening red bole, green bole and inter-trappean beds. The alluvium of recent age, comprising sandy silt, clay, kankar and their admixture, occurs in a limited extent along the rivers Nag and Pili.
Crystalline, Gondwanas and Deccan traps do not possess primary porosity and permeability due to their massive and compact nature, but when jointed, fractured and weathered secondary porosity take place and they form potential aquifers. The Gondwana sandstone, because of its granular nature, possesses both primary porosity and permeability. Presence of fracture and joints has further increased its water bearing capacity. The water bearing capacity of the Lameta Formation depends on the lithological composition. The calcareous sandstone and cherty limestone, which occur as very thin layers, have relatively poor porosity and hence poor yield.
In Nagpur city, groundwater occurs under both unconfined and semi-confined to confined conditions. In open wells (shallow aquifers), it occurs under unconfined condition and in bore wells (deep aquifers) it occurs under confined condition. The occurrence of groundwater in open wells is mostly controlled by degree of weathering, while it is under fractured rocks in deep wells. The thickness of weathered zone is in the range of 1–10 m and the fractured zone is from 10 to 50 m. Considerable variation is seen in depth to water level within the city depending on physiography, stage of groundwater development and topography (Jain et al. 2012). The gentle slope of the area is responsible for the development of sub-surface sluggish drainage conditions. Sewage, wastewaters and industrial effluents drain through the rivers Nag and Pili.
Methodology
Groundwater samples were collected from 68 bore wells during pre- and post-monsoon, where water is used for drinking and domestic purposes. The sampling bottles were first rinsed with distilled water and then with the samples to be collected. The water samples were collected after pumping of hand pump for 5 min. All samples were preserved at 25 °C and transported to the laboratory for analysis immediately, following the methods recommended by APHA (1992).
Electrical conductivity (EC) and pH were measured in the field, using digital meters. Total dissolved solids (TDS) were calculated, using EC values. Total Hardness (TH), calcium (Ca2+) and magnesium (Mg2+) were determined titrimetrically using standard EDTA. Chloride (Cl−) was estimated by standard AgNO3 titration. Carbonate (CO3 2−) and bicarbonate (HCO3 −) were determined using HCl as titration solution. Sodium (Na+) and potassium (K+) were measured by flame photometry. Sulfate (SO4 2−) and nitrate (NO3 −) were determined using UV–Visible spectrophotometer.
To confirm the reliability of the analytical results, the ionic charge balance (ICB) between cations (Ca2+, Mg2+, Na+ and K+) and anions (CO3 2−, HCO3 −, SO4 2− and NO3 −) was computed, using the following formula (Eq. 1), where all the ionic concentrations are expressed in milliequivalent per liter (meq/l). The observed ICB is less than 5 %, which is within the acceptable limit for accuracy of complete chemical analysis of groundwater samples.
The analysis of PCA was performed, using IBM SPSS version 19, to reduce the number of variables in a data set to a smaller number without loss of essential information. It transforms the data matrix into a new set of composite variables or principal components (PCs) based on variance–covariance matrix. The variance and covariance of all involved parameters are determined based on standardized data. The PCs are by definition uncorrelated with each other. The chemical variables used for PCA were pH, EC, TDS, TA, TH Ca2+, Mg2+, Na+, K+, CO3 2−, HCO3 −, SO4 2− and NO3 −.
Results and discussion
Hydrochemistry
The results of the chemical analysis of groundwater samples for pre- and post-monsoon of the study area are summarized in Table 1. The pH values of studied samples indicate mildly acidic to alkaline nature and are well within the acceptable limit of 6.5–8.5 for drinking water (WHO 2011; Table 2). Electrical conductivity (EC) values signify that the amount of total dissolved salts varies between 396 and 2600 μS/cm in pre-monsoon and 365–2750 μS/cm in post-monsoon, reflecting a variation in the enrichment of salts in the water (Subba Rao et al. 2012). Sanchez-Perez and Tremolieres (2003) suggested that the higher EC of the water is a result of ion exchange and solubilization in the aquifer due to influence of anthropogenic sources, such as domestic sewage and septic tanks. Groundwater shows TDS less than 1000 mg/l (i.e., fresh water type) in 91 % in pre-monsoon and 94 % in post-monsoon. The TH values indicate that the groundwater is moderately hard (75–150 mg/l), in 8.8 and 5.9 % samples; hard (150–300 mg/l), in 44.1 and 36.8 % samples and very hard (>300 mg/l), in 47 and 57.3 % of the total samples during pre- and post-monsoon, respectively (Sawyer and McCarty 1967). The majority of the groundwater samples have TH above the desirable limit of 300 mg/l, which causes encrustation on water supply distribution systems. Long-term consumption of extremely hard water leads to cardio-vascular disorders (Durvey et al. 1991).
The studied samples have indicated moderately high Ca2+ and Mg2+ ions (Table 1). Calcic plagioclases and ferromagnesium minerals are present in host rocks and are the possible sources for Ca2+ and Mg2+ ions. The concentration of calcium in groundwater samples is more than the desirable limits of 75 mg/l prescribed for drinking water in about 22 % in pre- and 33.8 % in post-monsoon (Table 2). The Na+ also shows wide variation, which may be inherited by groundwater due to rock weathering and/or dissolution of soil salts by the influences of evaporation and anthropogenic activities (Stallard and Edmond 1983; Meybeck 1987; Subba Rao 2002). The concentration of K+ is low, but at few places it is as high as 199 mg/l in pre-monsoon and 163 mg/l in post-monsoon. Orthoclase feldspars and municipal wastes may be the possible sources of K+ in groundwater.
In groundwater samples, higher concentration of HCO3 − is due to the decay of organic matter in the soil zone (Canter1997; Jeong 2001; Zilberbrand et al. 2001). The dominance of HCO3 − is due to a mineral dissolution (Stumm and Morgan 1996. The concentration of HCO3 − more than 300 mg/l (Table 1) may lead to kidney stones in the presence of higher concentration of Ca2+, especially in dry climatic regions (Subba Rao et al. 2012). The concentration of Cl− exceeds the prescribed potable water limit (250 mg/l) in 16 and 11.7 % of total groundwater samples in pre- and post-monsoon, respectively (Tables 1, 2). Chloride may be derived from the pollution sources such as effluents of industrial and domestic waste, leakage of septic tanks and from natural sources such as rainfall and dissolution of Cl− bearing minerals (Negrel and Roy 1998; Negrel 1999). About 36.8 and 48.5 % of groundwater samples have NO3 − concentration above the recommended level of 45 mg/l (WHO 2011). The higher concentration of NO, beyond this limit, is an indication of anthropogenic pollution caused by poor sanitary conditions. High concentrations of NO3 − can cause methemoglobinemia (blue baby disease).
Groundwater types
Classification of groundwater is generally based on the concentration of various predominant cations and anions or on the interrelationship of ions. The cation and anion data of the groundwater samples collected from the study area are plotted on the Piper’s diagram for chemical classification (Figs. 2, 3). On the basis of this diagram, groundwaters of study area are classified into six types, namely Ca–HCO3, Na–Cl, mixed Ca–Na–HCO3, mixed Ca–Mg–Cl, Ca–Cl and Na–HCO3 (Table 3). Out of these, Ca–HCO3, mixed Ca–Na–HCO3 and mixed Ca–Mg–Cl are the most prevalent groundwater types. About 50 and 36.7 % of samples from pre- and post-monsoon, respectively, belong to Ca–HCO3 type. The study of Piper diagrams shows that alkali (Na+ and K+) significantly exceeds the alkaline earths (Ca2+ and Mg2+) and the strong acids (Cl− and SO4 2−) exceed weak acids (HCO3 − and CO3 2−).
Groundwater types of bore well samples during pre-monsoon in trilinear diagram (Piper 1953)
Groundwater types of bore well samples during post-monsoon in trilinear diagram (Piper 1953)
Mechanisms controlling groundwater quality
Ratios of cations (Na+:Na++Ca2+) and anions (Cl−:Cl−+HCO3 −) of the pre- and post-monsoon groundwater of the study area are plotted against TDS in the Gibbs diagram (Fig. 4), which explains the influences of atmospheric precipitation (rainwater), rock–water interaction (lithology) and evaporation (climate) on groundwater. Most of the groundwater samples fall in a rock domain, while those of few are observed from a domain of evaporation. The rock–water interaction arises due to dissolved ions present in the soil and/or lithology of the study area. The change in the chemical composition from domain of rock towards evaporation domain causes an increase of Na+ and Cl and, consequently, a higher TDS. This is a result of influences of anthropogenic sources on groundwater quality of geogenic origin. Thus, the chemical composition of groundwater samples increases towards domain of evaporation from rock domain.
Mechanism controlling groundwater chemistry of the study area (Gibbs 1970). 1 Evaporation-dominance, 2 precipitation-dominance
Principal component analysis
Principal component analysis was applied to the combined groundwater data sets of the area (Table 1) to examine relations between water properties and to identify the factors that influence the concentration of each parameter. Variables used for the factor analysis in this study are pH, EC,TDS, TH, TA, Na+, K+, Ca2+, Mg2+, HCO3 −, Cl−, SO4 2− and NO3 −. The maximum number of factors to be extracted was fixed by the Kaiser Criterion, which takes into account only factors having eigenvalues higher than 1. Following this procedure, four factors were obtained and rotated according to the Varimax with Kaiser Normalization method, their loading, communalities for first two variables; percentage of the variance of each factor and cumulative percentage of variance for pre- and post-monsoon samples are shown in Table 4.
The first PC shows 41.19 % of the total variance, with the eigenvalue of 5.35 during pre-monsoon and 46.47 % of the total variance, with the eigenvalue of 6.04 during post-monsoon (Table 4). The second PC shows 20.14 % of the total variance, with the eigenvalue of 2.62 during pre-monsoon and 15.63 % of the total variance, with the eigenvalue of 2.03 during post-monsoon. The communalities of chemical variables vary from 0.45 to 1 and 0.31 to 1 during pre- and post-monsoon, respectively, indicating an involvement of different contributions in changing of quality of groundwater. In pre-monsoon, the communalities of all ions except pH and SO4 2− were greater than 0.50, whereas in post-monsoon communality of pH is less than 0.50.
First principal component
The first PC was primarily composed of EC (0.895), TDS (0.873), TH (0.802), Cl− (0.907) and NO3 − (0.799) during pre-monsoon. In post-monsoon, first PC is represented by TH (0.969), Ca2+ (0.797), Mg2+ (0.819) and Cl− (0.738). The positive PC loadings are used to understand the relative contribution of the chemical variables on groundwater quality. The positive PC loadings are classified into five types mainly (a) very low-positive PC loadings (<0.450), (b) low-positive PC loadings (0.450–0.600), (c) medium-positive PC loadings (0.600–0.750), (d) high-positive PC loadings (0.750–0.900) and (e) very high-positive PC loadings (>0.900). On basis of this classification, it is observed that Cl− during pre-monsoon and HCO3 − and TA during post-monsoon have very high-positive PC loadings. While the chemical variables, EC, TDS, TH and NO3 − during pre-monsoon and Mg2+, Ca2+ and Cl− during post-monsoon show high-positive PC loadings.
High-positive PC loadings of Cl− and NO3 − are observed during pre-monsoon (Table 4). There is a significant positive correlation between NO3 − and Cl− (r = 0.54, 0.65), NO3 − and Ca2+ (r = 0.54, 0.69), Cl− and Ca2+ (r = 0.53, 0.69), Cl− and Mg2+ (r = 0.54, 0.48), which indicate that these ions are associated with anthropogenic activities. Therefore, first PC is assumed to be indicative of the contamination source related to human activities.
High-positive PC loadings of Ca2+, Mg2+ and Cl− are observed during post-monsoon season (Table 4). In the study area, Ca2+ and Mg2+ are the dominant cations present in groundwater next to Na+. Similarly, HCO3 − is also present in considerable amount. In Ca2+ + Mg2+ against SO4 2− + HCO3 − scatter diagram (Fig. 5a), most of the points fall along the equiline Ca2+ + Mg2+:SO4 2− + HCO3 −, suggesting that carbonate weathering is not the major hydrogeochemical process operating in study area (Datta and Tyagi 1996; Datta et al. 1996; Rajmohan and Elango 2004). The Ca2+ against HCO3 − scatter diagram (Fig. 5b) shows very weak correlation, which suggests no contribution of calcite weathering on groundwater chemistry. In a plot of Ca2+ and SO4 2−(Fig. 5c), most groundwater samples are not close to the 1:1 line, which suggests that the anthropogenic sources may contribute Ca2+ and SO4 2− ions in groundwater rather than gypsum. This is also supported by the absence of sulfide-bearing minerals in the study area. The contribution of SO4 2− is caused by the uncontrolled usage of fertilizers and soil amendments (Subba Rao 2011, 2012). Further, the source of Cl− is of non-lithological origin. However, along with Na+ it is contributed from the clay products formed by the weathering of rocks due to their sluggish drainage conditions (Hem 1991).
The correlation coefficient (r) computed from the study area shows a strong positive correlation of EC with TDS (r = 0.9, 0.84), Cl− (r = 0.8, 0.91) and NO3 − (r = 0.7, 0.71) during pre- and post-monsoon. This indicates that the groundwater chemistry is mainly controlled by Cl− and NO3 − ions, which mainly come from the anthropogenic activities. A positive correlation between Ca2+ (r = 0.51 and 0.55; p = 0.05) and Mg2+ (r = 0.49 and 0.49; p = 0.05) with TDS during pre- and post-monsoon supports the anthropogenic input mainly domestic and industrial waste (Jalali 2009). The NO3 − has a strong correlation with TDS (r = 0.64 and 0.71; p = 0.05) during pre- and post-monsoon, suggesting anthropogenic input such as decaying organic matter, sewage waste and leakage of septic tanks as a source (Subrahmanyam and Yadaiah 2000; Jalali 2009). A positive correlation between Cl− and NO3 − indicates common source of these ions and also supports anthropogenic inputs (Back and Hanshaw 1966; Piskin 1973; Ritter and Chirnside 1984; Pacheco and Cabrera 1997). In the study area, positive correlation between Cl− and NO3 − (r = 0.54, 0.65, p = 0.005) during pre- and post-monsoon seasons suggests anthropogenic source.
The positive PC scores are categorized into three types for better understanding of the relative intensity of controlling processes on overall quality of groundwater (Tables 5, 6). They are (a) low-positive PC scores (<1.000), (b) medium-positive PC scores (1.000–2.000) and (c) high-positive PC scores (>2.000). Forty five and thirty eight groundwater samples during pre- and post-monsoon, respectively, show negative PC scores in the first PC. This reflects a low dissolution of Mg2+ and Ca2+ ions due to the lesser interaction of water with the source material. This is also supported by non-geodomain-specific control of these ions (Table 1).
Relatively higher interaction between the water and the source material may result in the low-positive PC scores than those of the negative PC scores (Table 6). As a result, the low-positive PC scores show some higher average concentrations of EC (1492, 1277.5 μS/cm), TDS (718.7, 667.5 mg/l), Cl− (174.2, 154.9 mg/l), NO3 − (81.7, 60.8 mg/l), Mg2+ (42.2, 49.9 mg/l) and Ca2+ (61.9, 79.1 mg/l) than those of the respective average concentrations (EC: 1005.7, 1152.8 μS/cm; TDS: 481.5, 598.1 mg/l; Cl−: 95.9, 108 mg/l; NO3 −: 27.3, 58.3 mg/l; Ca2+: 43.2, 51.8 mg/l and Mg2+: 33.7, 33.1 mg/l) of the negative PC scores during pre- and post-monsoon. Further, the medium-positive PC scores show the high average concentrations of EC, TDS, Cl−, NO3 −, Ca2+ and Mg2+ as compared to those of the low-positive PC scores due to the higher rate of dissolution of the ions. Similarly, the higher average concentrations of EC (2215, 2256 μS/cm), TDS (1148.4, 1176.8 mg/l), Cl− (414.2, 349.7 mg/l), NO3 − (239.8, 221.7 mg/l), Ca2+ (120.7, 152.5 mg/l) and Mg2+ (93.2, 78.3 mg/l) are observed from the high-positive PC scores than those of the average concentrations in the medium-positive PC scores during pre- and post-monsoon.
Second principal component
Second PC accounts for about 20.14 % of the total variance during pre-monsoon (Table 4), consisting of high loadings of TA (0.947), Na+ (0.793) and HCO3 − (0.946), whereas in post-monsoon, it accounts for 15.63 % of the total variance, consisting mainly of medium-positive loadings of SO4 2− (0.66).
As high-positive PC loadings of TA, Na+ and HCO3 − observed from the second principal component during pre-monsoon (Table 4), it indicates an interaction of infiltrating recharge water, with the soil and/or lithology. During recharge, water absorbs large amount of CO2, released from the soil due to the decay of organic matter, root respiration, etc. and convert into HCO3 − during weathering reactions (Eqs. 2, 3; Jacks 1973; Berner and Berner 1987). As the pH varies from 6.7 to 8.1 and 6.4 to 8.5 during pre- and post-monsoon, respectively (Table 1), the alkalinity is caused by only HCO3 − ion.
The higher concentration of HCO3 − and Na+ in the groundwater (Table 1) indicates a dominance of mineral dissolution (Stumm and Morgan 1996). The activity of the alkalinity, as a controlling process for HCO3 − and Na+ ions in the water, is further confirmed by high-positive loadings of Na+ (0.793) and HCO3 − (0.946) (Table 4). The significantly high-positive content of Na+ indicates water–rock interaction, because Na+ in groundwater primarily comes from silicate weathering, ion exchange reactions, domestic effluents and atmospheric input (Edmunds et al. 2003; Aiuppa et al. 2003; Valdes et al. 2007; Brenot et al. 2008).
The Na+–Cl− relationship has often been used to identify the mechanism for acquiring salinity in semi-arid regions (Magaritz et al. 1981; Dixon and Chiswell 1992). The dissolution of halite in water releases equal concentration of Na+ and Cl− into the solution, but analytical data from both seasons in Fig. 5d deviates from the expected 1:1 relation, which is probably due to the interference of anthropogenic activities on the groundwater system. About 65 and 50 % of the groundwater samples from pre- and post-monsoon, respectively, lie above the equiline of 1:1, which suggests that the original concentration of Na+ derived by silicate weathering is masked by anthropogenic sources (domestic waste, animal waste, leakage of septic tank, etc.). The cation exchange between Ca2+ or Mg2+ and Na+ may also explain the high Na+ concentration (Stimson et al. 2001). About 64.7 and 60 % samples from pre- and post-monsoon have a value of Na+/Cl− ratio higher than 1, supporting the contribution of Na+ from silicate weathering. These samples also show a deficit in Ca2+ + Mg2+, and this is consistent with a Ca2+–Na+ cation exchange process leading to softening of the water (Hidalgo et al. 1995; Hidalgo and Cruz-Sanjulian 2001). Ca2+ and Mg2+ can exchange Na+ sorbed on the exchangeable sites of the clay minerals, resulting in the decrease of Ca2+ and Mg2+ and the increase of Na+ in groundwaters. This is further supported using chloro-alkaline indices (Schoeller 1965); CA-I and CA-II are calculated as follows:
During this process, the host rocks are the primary sources of dissolved solids in the water. The CA indices of the groundwater samples of the study area (Table 1, 2) reveal that about 63 and 57 % of groundwater samples during pre- and post-monsoon show negative values, indicating cation–anion exchange. This further confirms that the host rocks are the primary sources of dissolved ions in the groundwater, in which the ion exchange is one of the major contributors for higher concentration of Na+ in the groundwater. In remaining samples, the values are positive, confirming base exchange reaction, where exchange occurs between Na+ in the groundwater and Ca2+ or Mg2+ in the aquifer material. The relationship between Na+–Cl− and Ca2+ + Mg2+–HCO3 ––SO4 2− should be linear. Figure 4e shows that most of the groundwater samples of the study area followed a straight line (r = 0.94 in both seasons), which indicates the occurrence of reverse ion exchange (Fisher and Mulican 1997). If the dissolutions of calcite, dolomite and gypsum are the dominant reactions in a groundwater system, then the plot of (Ca2+ + Mg2+) versus (SO4 2− + HCO3 −) will be close to the 1:1 line. The points on the plot shift to right, due to an excess of SO4 2− + HCO3 − and due to ion exchange process (Cerling et al. 1989; Datta and Tyagi 1996; Fisher and Mulican 1997). If reverse ion exchange is the process, it will shift the points to the left due to excess of Ca2+ + Mg2+ over SO4 2− + HCO3 −. The plot of Ca2+ + Mg2+ versus SO4 2− + HCO3 − (Fig. 5a) shows that about 60 and 67 % of groundwater samples from pre- and post-monsoon, respectively, fall left of the 1:1 line irrespective of the seasons, which indicate existence of reverse ion exchange in the study area. The plot of Ca2+ + Mg2+ versus Cl− (Fig. 4f) indicates that Ca2+ and Mg2+ do not increase with increasing salinity which further suggests reverse ion exchange as the main process. Thus, the second principal component is assumed to be indicative of water–rock interaction.
Conclusions
Groundwater samples observed from the Nagpur city are both fresh and brackish, with fresh being dominant. Results show that alkalis slightly exceed alkaline earths and strong acids exceed weak acids. Sodium among cations and HCO3 − among anions are dominant ions in the groundwater and there is no seasonal variation observed in ionic dominance. The PCA summarizes the chemical variables of pH, EC, TDS, TH, TA, Na+, K+, Ca2+, Mg2+, HCO3 −, Cl−, SO4 2− and NO3 − into two PC loadings, accounting for 61.33 and 62.09 % of the total variance during pre- and post-monsoon. The first PC is characterized by high-positive loadings of EC, TDS, TH, Cl− and NO3 − which is considered as a controlled process caused by anthropogenic sources, while the second PC is represented by high-positive loadings of TA, HCO3 − and Na+, which is taken as alkalinity and pollution-controlled processes influenced by both the sources of geogenic and anthropogenic origins, respectively. The PC scores explain the causes in the variation of groundwater quality. The Schoeller indices values are negative except in some locations showing a reverse ion exchange. The high ratio of Na+/Cl− indicates that high Na+ content could be due to the weathering of silicate rocks which is further enhanced by evaporation and anthropogenic input, whereas reverse ion exchange is dominant process resulting in the addition of Cl− in groundwater. The coefficients among various elements indicate the dominance of ion exchange, silicate weathering and anthropogenic input on groundwater chemistry. All these indices support the PCA. Thus, this study demonstrated that the PCA is an effective tool to characterize the dominant controlling processes of groundwater quality.
References
Aiuppa A, Bellomoa S, Bruscab L, Alessandrob WD, Federico C (2003) Natural and anthropogenic factors affecting groundwater quality of an active volcano (Mt. Etna, Italy). Appl Geochem 18:863–882
Apadaca LE, Jeffrey BB, Michelle CS (2007) Water quality in shallow alluvium aquifers, Upper Calordo river basin, Calorado. J Am Water Resour Assoc 38(1):133–148
APHA (1992) Standard method for the examination of water and wastewater, 18th edn. American Public Health Association, New York
Arunprakash M, Giridharan L, Krishnamurthy RR, Jayaprakash M (2014) Impact of urbanization in groundwater of south Chennai City, Tamil Nadu, India. Environ Earth Sci 71(2):947–957
Ayenew T, Demlie M, Wohnlich S (2008) Hydrogeological framework and occurrence of groundwater in the Ethiopian aquifers. J Afr Earth Sci 52:97–113
Back W, Hanshaw B (1966) Hydrochemistry of the Northern Yucatan Peninsula, Mexico, with a section on Mayan water practices. In: Weidie AE (ed) Field seminar on water carbonate rocks of the Yucatan Peninsula, Mexico. New Orleans Geological Society, New Orleans, pp 45–77
Berner EK, Berner RA (1987) The global water cycle, geochemistry and environment. Prentice-Hall, New Jersey
Brenot A, Baran N, Petelet-Giraud E, Negrel P (2008) Interaction between different water bodies in a small catchment in the Paris basin (Brevilles, France): tracing of multiple Sr sources through Sr isotopes coupled with Mg/Sr and Ca/Sr ratios. Appl Geochem 23:58–75
Canter LW (1997) Nitrates in groundwater. Lewis Publisher, New York
Cerling TE, Pederson BL, Damm KLV (1989) Sodium-calcium ion exchange in the weathering of shales: implications for global weathering budgets. Environ Geol 17:552–554
Datta PS, Tyagi SK (1996) Major ion chemistry of groundwater in Delhi area: chemical weathering processes and groundwater regime. J Geol Soc India 47:179–188
Datta PS, Bhattacharya SK, Tyagi SK (1996) 18O studies on recharge of phreatic aquifers and groundwater flow-paths of mixing in the Delhi area. Hydrogeol J 176:25–36
Dixon W, Chiswell B (1992) The use of hydrochemical sections to identify recharge areas and saline intrusions in alluvial aquifers, southeast Queensland, Australia. J Hydrol 130:299–338
Durvey VS, Sharma LL, Saini VP, Sharma BK (1991) Handbook on the methodology of water quality assessment. Rajasthan Agriculture University, India
Edmunds WM, Shand P, Hart P, Ward RS (2003) The natural (baseline) quality of groundwater: a UK pilot study. Sci Total Environ 310(1–3):25–35
Fernandes PG, Carreira P, Oliveira da Silva M (2008) Anthropogenic sources of contamination recognition—Sines coastal aquifer (SW Portugal). J Geochem Expl 98:1–14
Fisher RS, Mulican WF III (1997) Hydrochemical evolution of sodium-sulfate and sodium-chloride groundwater beneath the Northern Chihuahuan desert, Trans-Pecos, Rexas, USA. Hydrogeol J 10:455–474
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 17:1088–1090
Giridharan L, Venugopal T, Jayaprakash M (2008) Evaluation of the seasonal variation on the geochemical parameters and quality assessment of the groundwater in the proximity of River Cooum, Chennai, India. Environ Monit Assess 143:161–178
Hem JD (1991) Study and interpretation of the chemical characteristics of natural waters, 3rd edn. Scientific Publishers, Jodhpur
Hidalgo MC, Cruz-Sanjulian J (2001) Groundwater composition, hydrochemical evolution and mass transfer in a regional detrital aquifer (Baza basin, southern Spain). Appl Geochem 16:745–758
Hidalgo MC, Cruz-Sanjulian J, Sanroma A (1995) Evolucion geoquimica de las aguas subterraneas en una cuenca sedimentaria semiarida (acuifero de Baza- Caniles, Granada, Espana). Tierra y Tecnologia 20:39–48
Jacks G (1973) Chemistry of groundwater in a district in southern India. J Hydrol 18:185–200
Jain PK, Paranjpe SC, Parchure PK (2012) Development, augmentation and management of groundwater resources of Nagpur metropolitan city. Gond Geol Mag 27(2):209–216
Jalali M (2009) Geochemistry characterisation of groundwater in an agricultural area of Razan, Hamadan, Iran. Environ Geol 56:1479–1488
Jeong CH (2001) Effect of land-use and urbanization on hydrochemistry and contamination of groundwater from Taejon area, Korea. J Hydrol 253:194–210
Koklu R, Sengorur B, Topal B (2010) Water quality assessment using multivariate statistical methods—a case study: Melen River System (Turkey). Water Resour Manag 24:959–978
Lin CY, Abdullah MH, Praveena SM, Yahaya AHB, Musta B (2012). Delineation of temporal variability and governing factors influencing the spatial variability of shallow groundwater chemistry in a tropical sedimentary island. J Hydrol 432–433:26–42
Magaritz M, Nadler A, Koyumdjisky H, Dan N (1981) The use of Na/Cl ratio to trace solute sources in a semiarid zone. Water Resour Res 17:602–608
Marghade D, Malpe DB, Zade AB (2010) Assessment of groundwater quality in landfill area of Nagpur city, central India. J Environ Hydrol 18 paper 18:1–12
Marghade D, Malpe DB, Zade AB (2011a) Geochemical characterization of groundwater from northeastern part of Nagpur urban, Central India. Environ Earth Sci 62:1419–1430
Marghade D, Malpe DB, Zade AB (2011b) Major ion chemistry of shallow groundwater of a fast growing city of Central India. Environ Monit Assess 184:2405–2418
Meybeck M (1987) Global chemical weathering of surficial rocks estimated from river dissolved loads. Am J Sci 287:401–428
Negrel P (1999) Geochemical study of a granitic area—the margeride mountains, france: chemical element behavior and 87Sr/86Sr constraints. Aquatic Geochem 5(2):125–165
Negrel P, Roy S (1998) Rain chemistry in the Massif Central (France): a strontium isotopic and major elements study. Appl Geochem 13:941–952
Pacheco JA, Cabrera AS (1997) Groundwater contamination by nitrates in the Yucatan Peninsula, Mexico. Hydrogeol J 5(2):47–53
Panda UC, Sundaray SK, Rath P, Nayak BB, Bhatta D (2006) Application of factor and cluster analysis for characterization of river and estuarine water systems-a case study: Mahanadi River (India). J Hydrol 331:434–445
Pekey H, Karaka SD, Bako˘glu M (2004) Source apportionment of trace metals in surface waters of a polluted stream using multivariate statistical analyses. Mar Pollut Bull 49:809–818
Piper AM (1953) A graphical procedure in the geochemical interpretation of water analysis. Trans Am Geophys Union 25:914–928
Piskin R (1973) Evaluation of nitrate content of groundwater in Hall County, Nebraska. Groundwater 11(6):4–13
Rajmohan N, Elango L (2004) Identification and evolution of hydrogeochemical processes in the groundwater environment in an area of the Palar and Cheyyar River Basins, Southern India. Environ Geol 46:47–61
Ritter WF, Chirnside AEM (1984) Impact of land use on ground water quality in Southern Delaware. Groundwater 22(1):38–47
Sanchez-Perez JM, Tremolieres M (2003) Change in groundwater chemistry as a consequence of suppression of floods: the case of the Rhine floodplain. J Hydrol 270(1–2):89–104
Sawyer CN, McCarty PL (1967) Chemistry for sanitary engineers. McGraw-Hill, New York
Schoeller H (1965) Qualitative evaluation of groundwater resources. In: Methods and techniques of groundwater investigations and developments, UNESCO, pp 54–83
Shrestha S, Kazama F (2007) Assessment of surface water quality using multivariate statistical techniques: a case study of the Fuji River Basin, Japan. Environ Model Soft 22(4):464–475
Simeonov V, Stratis JA, Samara C, Zachariadis G, Voutsa D, Anthemidis A, Sofonioub M, Kouimtzis TH (2003) Assessment of the surface water quality in Northern Greece. Water Res 37:4119–4124
Singaraja C, Chidambaram S, Prasanna MV, Thivya C, Thilagavathi R (2014) Statistical analysis of the hydrogeochemical evolution of groundwater in hard rock coastal aquifers of Thoothukudi district in Tamil Nadu, India. Environ Earth Sci 71(1):451–464
Stallard RF, Edmond JM (1983) The influence of geology and weathering environment on the dissolved load. J Geophy Res Oceans 88:9671–9688
Stimson J, Frape S, Drimmie R, Rudolph D (2001) Isotopic and geochemical evidence of regional-scale anisotropy and interconnectivity of an alluvial fan system, Cochabamba Valey, Bolivia. Appl Geochem 16:1097–1114
Stumm W, Morgan JJ (1996) Aquatic chemistry. Wiley, New York, p 1022
Subba Rao N (2002) Geochemistry of groundwater in parts of Guntur district, Andhra Pradesh, India. Environ Geol 41(5):552–562
Subba Rao N (2006) Seasonal variation of ground water quality in a part of Guntur district, AP, India. Environ Geol 49:413–429
Subba Rao N (2011) High-fluoride groundwater. Environ Monit Asses 176:637–645
Subba Rao N, Prakasa Rao J, Subrahmanyam A (2007) Principal component analysis in groundwater quality in a developing urban area of Andhra Pradesh. J Geol Soc India 69:959–969
Subba Rao N, Subrahmanyam A, Ravi Kumar S, Srinivasulu N, Babu Rao G, Surya Rao P, Venkatram Reddy G (2012) Geochemistry and quality of groundwater of Gummanampadu sub-basin, Guntur District, Pradesh, India. Environ Earth Sci 67:1451–1471
Subrahmanyam K, Yadaiah P (2000) Assessment of the impact of industrial effluents on water quality in Patancheru and environs, Medak district, Andhra Pradesh, India. Hydrogeol J 9(3):297–312
Valdes D, Dupont JP, Laignel B, Ogier S, Leboulanger T, Mahler BJ (2007) A spatial analysis of structural controls on Karst groundwater geochemistry at a regional scale. J Hydrol 340(3–4):244–255
WHO (2011). “Guidelines for drinking-water quality”—4th ed. Geneva, Switzerland
Zilberbrand M, Rosenthal E, Shachnai E (2001) Impact of urbanization on hydrochemical evolution of groundwater and unsaturated-area gas composition in the coastal city of Tel Aviv, Israel. J Contam Hydrol 50:175–208
Acknowledgments
We are thankful to Director, D. Laxminarayan Institute of Technology, Nagpur for the facilities. DBM is thankful to UGC SAP-DRS for the financial help. We also thank anonymous reviewers and editor of the journal for their valuable suggestions in bringing the manuscript in the present form.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marghade, D., Malpe, D.B. & Subba Rao, N. Identification of controlling processes of groundwater quality in a developing urban area using principal component analysis. Environ Earth Sci 74, 5919–5933 (2015). https://doi.org/10.1007/s12665-015-4616-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-015-4616-z