Abstract
The aim of this study was to assess the potential of Indian Sphagnum peat moss, a widespread terrestrial bryophyte, for the uptake of Ni(II) ions from aqueous solution. Experiments were carried out as a function of contact time, pH, adsorbent dosage, and initial concentration of Ni(II). Batch experiment results showed that peat moss removed 99.5 % of Ni(II) from aqueous solution at pH 6. Freundlich model showed satisfactory fit to the equilibrium adsorption data of Sphagnum peat moss. The adsorption kinetics followed pseudo-second-order kinetic model, which demonstrates that chemisorption may be the rate controlling step in the adsorption of Ni(II) onto the Sphagnum peat moss. The interactions between the peat moss and Ni(II) were further studied by Fourier transform infrared spectroscopy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals are the most important pollutants in wastewaters and have become a global concern because of their toxicity, persistence for several decades in the environment, bioaccumulation, and biomagnification in the food chain (Batvari et al. 2008, 2013; Kamala-Kannan et al. 2008). Most of the heavy metals are well known to have toxic effects even at very low concentrations. Nickel (Ni), a heavy metal, is widely used in several industries; thus, large amounts of Ni ions are released into the environment (Revathi et al. 2005). Ni concentration above the tolerable level is a disturbing factor for species survival and stability of the ecosystem.
Numerous studies have reported the toxic effects of Ni ions. Denkhaus and Salnikow (2002) reported that exposure to high concentration of Ni causes skin allergies, lung fibrosis, nausea, tightness of the chest, dry cough, vomiting, chest pain, shortness of breath, rapid respiration, cyanosis, and extreme weakness. Human exposure to high Ni-polluted environments such as Ni refining, electroplating, and welding causes skin allergies, lung fibrosis, and cancer of the respiratory tract (Kasprzak et al. 2003). Hence, it is very important to prevent Ni from accumulating in the environment.
Conventional practices for removing Ni from industrial effluents involve physicochemical methods such as chemical precipitation, chemical oxidation or reduction, electrochemical treatment, evaporative recovery, filtration, ion exchange, and membrane-based technologies. These processes may be ineffective or expensive, especially when the heavy metal ions in the contaminated media are high, i.e., in the order of 1–100 mg of dissolved heavy metal ions per liter (Volesky 1990). Operational problems and high costs related to treatment necessitate research for other methods.
Biological methods such as biosorption/bioaccumulation may provide an attractive alternative to physicochemical methods for the removal of heavy metals from aqueous solution (Vijayaraghavan and Yun 2008). Several low-cost adsorbents such as agricultural byproducts, industrial wastes, polymeric composites, clay, microbial biomass, and algae have been successfully assessed for their ability to remove toxic heavy metals from wastewater/aqueous solutions (Babel and Kurniawan 2003; Vijayaraghavan and Yun 2008; Gupta et al. 2010; Janaki et al. 2014). Sphagnum is one of the most cosmopolitan and ecologically useful peat moss (Brown et al. 2000). The highly porous nature of Sphagnum peat moss and the presence of lignin, cellulose, humic acids, and fulvic acids enhance its application in the removal of various kinds of pollutants, including heavy metals, from aqueous solution. However, adsorption characteristics vary with the type of peat moss. Irish peat moss has been shown to adsorb Cu and Ni from aqueous solution (Gupta et al. 2009), while Northern Ireland peat moss has been shown to remove Pb, Cu, and Ni from aqueous solution (Ho and Mckay 2000). Kalmykova et al. (2008) reported Sphagnum peat mosses adsorption preference for metal ions as Pb > Cu > Ni > Cd > Zn. Thus, the objectives of the present study were to: (1) explore the potential of the Indian Sphagnum peat moss for the removal of Ni(II) from aqueous solution; (2) assess the experimental variables affecting optimal removal of Ni(II); and (3) explore kinetics and isotherm models to identify the possible mechanism of Ni(II) removal.
Materials and methods
Materials
Sphagnum peat moss was collected from Ooty, Nilgiri district, Tamil Nadu, India. The collected peat moss was washed with deionized water and rinsed several times with double distilled water to remove dirt. The peat moss was dried at 70 °C for 24 h, and ground into appropriate particle size using a sterile blender. The grated particles were sieved, and a particle size of 0.2–0.4 mm was used for adsorption studies. All the chemicals used in the experiment were of analytical grade. Stock solution of Ni(II) (1000 mg L−1) was prepared by dissolving a weighed quantity of nickel sulfate (Sigma-Aldrich, Milwaukee, WI, USA) in double distilled water. A working concentration of Ni(II) (50–200 mg L−1) was prepared by diluting the stock solution with double distilled water.
Batch adsorption studies
Batch adsorption was performed in Erlenmeyer flasks containing 100 ml of Ni(II) solution with 0.1 g of peat moss. When necessary, the pH of the solution was initially adjusted using 0.1 N HCl or NaOH. The flasks were subjected to shaking at a constant speed of 150 rpm in a shaking incubator. After the attainment of equilibrium, 4 mL of the mixture was filtered through 0.22 μm membrane, and the residual concentration of Ni(II) was analyzed using UV–Vis spectrophotometer (UV-1800 Shimadzu, Japan) at 440 nm using dimethyl glyoxime as a complexing agent (Vien et al. 1991).
The influence of pH (3–8), adsorbent dosage (0.1–0.4 g L−1), and initial concentration of Ni(II) (50–200 mg L−1) on adsorption rate were also evaluated. For the kinetic experiments, the samples were withdrawn at regular time intervals to determine the equilibrium time.
Data evaluation
The amount of Ni(II) sorbed by the adsorbent was calculated using the mass balance equation:
where V is the sample volume in liters, C o the initial concentration of Ni(II) (mg L−1), C e the equilibrium concentration of Ni(II) (mg L−1), and M the weight of the peat moss (g). However, the percentage of adsorption was calculated using the following equation:
where C o is the initial concentration of Ni(II) (mg L−1) and C e the equilibrium concentration of Ni(II) (mg L−1).
The resulting equilibrium data were modeled using the Langmuir, Freundlich, and Dubinin–Radushkevich (D-R) isotherm.
where q m is the maximum adsorption capacity (mg g−1), q e the equilibrium adsorption capacity (mg g−1), C e the equilibrium adsorbate concentration in solution (mg L−1), and K L the Langmuir constant (L mg−1).
The essential characteristics of the Langmuir isotherm were expressed in terms of a dimensionless separation factor (R L), which is defined as follows:
where b is the Langmuir constant and C o the initial Ni(II) concentration (mg L−1). The value of R L indicates the nature of adsorption to be unfavorable (R L > 1), linear (R L = 1), favorable (0 < R L < 1), and irreversible (R L = 0).
where C e the equilibrium metal concentration in solution (mg L−1), K F (mg g−1) (L g−1)1/n the Freundlich constant related to sorption capacity, and n the heterogeneity factor.
D-R isotherm model was expressed by the following formulation:
where q m is the maximum adsorption capacity, β a coefficient related to the mean free energy of adsorption (mmol2 J−2), ε the Polanyi potential (J mol−1), R the gas constant (8.314 J mol−1 K−1), T the temperature (K), and C e the adsorbate equilibrium concentration (mg L−1). The constant β provides information about the apparent energy (E) of sorption per mole of the sorbate as it migrates to the surface of the biomass from an infinite distance in the solution and can be computed using the relation given as follows (Chowdhury et al. 2011):
Adsorption kinetics was performed using pseudo-first order, pseudo-second order, and Elovich models (Chowdhury et al. 2011; Janaki et al. 2012).
where q e is the amount of metal adsorbed at equilibrium (mg g−1), q t the amount of metal adsorbed at time t (mg g−1), k 1 (min−1) the pseudo-first-order rate constant, k 2 (g mg−1 min−1) the pseudo-second-order rate constant, and α (mg g−1 min−1) and β (g mg−1) the Elovich coefficients representing the initial sorption rate and desorption constant, respectively.
Fourier transform infrared spectroscopy
Sphagnum peat moss was characterized with respect to its surface functional groups using Fourier transform infrared (FTIR) spectroscopy. FTIR was also used to identify functional groups responsible for binding Ni(II) ions. Dry moss or Ni(II)-loaded moss [filtered and dried after contact with an initial Ni(II) concentration of 200 mg L−1 at pH 6] was mixed with KBr at a ratio of 1:100 and compressed into films for FTIR analysis using a Thermo Scientific Nicolet IR100 spectrometer. The absorbance data were obtained in the range of 500–4000 cm−1.
Results and discussion
Effect of contact time
The equilibrium time required for the biosorption of Ni(II) on Sphagnum peat moss was obtained by studying the adsorption of Ni(II) at various initial concentrations (50–200 mg L−1). The results are represented in Fig. 1. The extent of the adsorption efficiency increased sharply with time and attained equilibrium at 35, 55, 70, and 80 min for an initial concentration of 50, 100, 150, and 200 mg L−1, respectively. The gradual increase in the rate of biosorption of Ni(II) at the initial time was due to the availability of more free active sites on the Sphagnum peat moss; gradual occupancy of which reduced the reaction rate and thus decreased the efficiency of adsorption. The time required to attain this state is the equilibrium time, and it reflects the maximum adsorption capacity of the adsorbent. Similar results were reported for Cd and Cu using Sphagnum peat moss collected from Sweden (Kalmykova et al. 2008).
Effect of biosorbent dose
Effect of adsorbent dosage is one of the important parameters that must be optimized during the metal sorption processes. The effect of adsorbent dosage (25, 50, 75, 100, 200, and 300 mg L−1) on removal of Ni(II) is shown in Fig. 2. It was apparent from the graph that marginal increase in Ni(II) removal was observed with increasing biomass concentration (25–100 mg L−1) (Janaki et al. 2014). The rapid increase in the removal of Ni(II) was due to the greater availability of reactive sites on the sorbent. The higher percentage of removal 99.5 % was observed for 100 mg L−1, and further increase in biosorbent dosage did not show appreciable removing efficiency, and it remains almost constant. Thus, further experiments were carried out using 100 mg L−1 of the biosorbent as it exhibits higher percentage of removal.
Effect of pH
pH is one of the vital factors affecting the adsorption process. It influences the degree of ionization of the materials present in the solution and the solution’s chemistry (Crini et al. 2007; Janaki et al. 2012, 2014). Thus, the effect of pH (2–8) was studied at the initial Ni(II) concentration of 100 mg L−1 using 0.1 g L−1 of peat moss. The percentage of Ni(II) removal at different pH is shown in Fig. 3. From the graph, it is inferred that the adsorption of Ni(II) was highly pH dependent. Higher adsorption was observed at pH 6.0 (99.5 %), and the removal rate was altered as the pH increased or decreased. At a low pH value (below 5), the H+ ions competed with metal cation for the exchange sites in the system, thereby partially releasing the metal cations (Ajmal et al. 2003). In addition, cell wall ligands were closely associated with the hydronium ions, while repulsive forces limited the approach of the metal ions. On further increase of pH, more ligands such as amino and carbonyl groups were protonated, leading to attraction between these negative charges and the metals; thus, increasing biosorption onto the peat moss surface was observed at pH 6 (Aksu 2001). The lower uptake at the higher pH (above 7) value was probably due to the formation of anionic hydroxide complexes (Maquieira et al. 1994). However, limited biosorption was observed due to the presence of carboxylate and sulfonate groups present in the anionic hydroxide complexes. The results are consistent with the previous studies reporting the maximum adsorption of Ni(II) ions onto Irish peat moss at pH 6 (Gupta et al. 2009).
Adsorption isotherm
To determine solute solvent interactions and to explore the saturation capacity of the biosorbent, adsorption isotherm was used. In the present study, the experimental data were fitted to the Langmuir, Freundlich, and D-R isotherm models. The Langmuir isotherm is based on the assumptions that intermolecular forces decrease rapidly with distance and that the adsorption proceeds at specific homogenous sites within the adsorbent (Han et al. 2008). It is then assumed that all the sites are energetically equivalent and it predicts existence of monolayer coverage of the sorbent. The biosorption data were analyzed accordingly to the aforementioned Eq. (3), and the important parameters along with the correlation coefficients are presented in the Table 1. The plot of C e vs C e/q e results in a linear graph indicating the applicability of the model and the constants q m and b are calculated from the slope and intercept of the plot. The maximum adsorption capacity (q m) observed for Sphagnum peat moss was 204.08 mg g−1, and this could not be determined experimentally. The constant b (1.8561) represents the affinity between the adsorbent and the adsorbate. The essential characteristics of Langmuir equation is expressed in terms of separation factor R L, and values between 0 and 1 represent the favorability of the adsorption processes.
The Freundlich equation is an empirical equation that assumes adsorption energy to exponentially decrease on completion of the sorptional centers of an adsorbent. Figure 4 represents the Freundlich isotherms for Ni(II) adsorption onto Sphagnum peat moss. The Freundlich constants K F and 1/n can be calculated from the linear plot of log q e versus log C e, and the constants together with the correlation coefficients are presented in Table 1. The values K F and 1/n F were 113.47 and 0.3314, respectively. The magnitude of n represents the favorability of the adsorption processes (i.e., 1/n < 1), and the value (1/n = 0.3314) follows the same trend representing the beneficial adsorption of Ni(II) onto Sphagnum peat moss.
To distinguish between the physisorption and chemisorption, the equilibrium data of Ni(II) were modeled with the D-R isotherm. D-R isotherm assumes about the heterogeneity of the surface energies and helps estimate the characteristic porosity of the biomass and the apparent energy of adsorption. The constants q m and the β are obtained from the linear plots of ln q e and ε 2. The parameters along with the correlation coefficients are listed in Table 1. The high q m values show the high sorption capacity of peat. The apparent energy (E) observed was 0.4377 kJ mol−1, and is lesser than 40 kJ mol−1, indicating that the adsorption process of Ni(II) onto Sphagnum peat moss was a physisorption processes. Similar phenomenon was observed for the adsorption of Cu2+ and Cd2+ from aqueous solution using Cassava waste biomass (Horsfall et al. 2004).
By comparing the linear correlation coefficients (R 2 value) of the three isotherms studied, it was found that Freundlich isotherm described the equilibrium data well with high R 2 value (0.9902). This result confirms that the adsorption system was heterogeneous and that the adsorption proceeds by forming a multilayer.
Kinetic modeling
To determine the sorption mechanism and the potential rate controlling step in the adsorption system, kinetic experiments were performed. The experiments were carried out with initial concentrations of 50, 100, 150, and 200 mg L−1 using 0.1 g of the biosorbent at pH 6.0. Pseudo-first order, pseudo-second order, and the Elovich equation were used to model the kinetic data. First, the kinetic data were modeled with the pseudo-first-order kinetic equation. The rate constants were determined from the linear plot of log (q e − q t ) vs t. The validity of the adsorption model is usually checked by the correlation coefficient (R 2). The R 2 values are more close to 1.0, indicating a better fit to the model. In this study, the R 2 values slightly deviated from unity suggesting that pseudo-first order is not an accurate model to fit the kinetic data. In addition, the calculated adsorption capacities (q e) varied widely with the experimental value. The difference in the q e values may be due to time lag or the formation of boundary layer on the surface of the sorbent. The kinetic data were then modeled with the pseudo-second-order kinetic model. The constants q e and k 2 are calculated from slope and the intercept of the linear plot t vs t/q t (Fig. 5). From the pseudo-second-order parameters, k 2 and q e, the initial adsorption rate h (mg g−1 min−1) can be calculated using the following equation:
The value of h observed for the sorption of Ni(II) onto Sphagnum peat moss was 7.2463 (mg g−1 min−1). The correlation coefficient R 2 (0.9923) values were found to be more closer to unity, and the calculated q e values were closer to experimental q e values indicating better fit to the pseudo-second-order model. It suggests that the chemisorption may be the rate controlling step in adsorption of Ni(II) onto the Sphagnum peat moss. The results are in agreement with previous studies that report the removal of divalent metal ions onto Ireland peat moss to follow pseudo-second-order model (Ho and Mckay 2000).
Further kinetic data were modeled with the Elovich equation, based on the assumption that the actual solid surfaces are energetically heterogeneous. The constants α and β representing the adsorption rate and desorption constant, respectively, can be computed from the linear plots of q t vs ln t. The Elovich constants along with the correlation coefficient are presented in Table 1. The R 2 values were slightly inferior compared to the pseudo-second-order model, suggesting that it is not an appropriate model for describing the kinetics involved.
Infrared spectral analysis
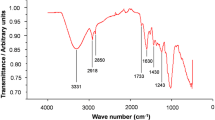
The FTIR spectra for unloaded and Ni(II) (100 mg L−1) loaded Sphagnum biomass (0.2 mm) were analyzed, and percentage transmission was taken to confirm the presence of the functional group that was responsible for the biosorption process (Fig. 6). The peaks at 3419 and 3126 cm−1 could be assigned to N–H and O–H stretching of polymeric compounds, respectively, present in the peat moss (Gardea-Torresdey et al. 1996; Shin et al. 2012; Janaki et al. 2013). The troughs that were observed at 2923, 2852, 1375, and 1319 cm−1 indicated the presence of C–H groups. The band at 1627 cm−1 is associated with C–N vibration. It is observed that after Ni(II) adsorption, the bands assigned to N–H, O–H, and C–H groups have been shifted to lower or higher wave numbers. The results revealed the involvement of several functional groups in the adsorption of Ni(II) ions; and certain chemical bonds are formed between peat moss and Ni(II) ions, which caused the difference in the vibration frequency of these chemical groups. The results are consistent with previous studies reporting the role of similar functional groups in the adsorption of metal ions onto peat moss (Gardea-Torresdey et al. 1996).
Conclusions
The present study showed that Indian Sphagnum peat moss significantly removed (99.5 %) Ni(II) from aqueous solution. The adsorption of Ni(II) is mainly pH dependent, and the maximum removal (99.5 %) was observed at pH 6.0. Results of the FTIR studies indicate the role of N–H, O–H, and C–H groups in the adsorption of Ni(II) ions. The results clearly indicate that Indian Sphagnum peat moss is an effective low-cost adsorbent for the removal of Ni(II) from aqueous solution.
References
Ajmal M, Rao RA, Anwar S, Ahmad J, Ahmad R (2003) Adsorption studies on rice husk: removal and recovery of Cd(II) from wastewater. Biores Technol 86:147–149
Aksu Z (2001) Equilibrium and kinetic modeling of cadmium(II) biosorption by C. vulgaris in a batch system: effect of temperature. Sep Purif Technol 21:285–294
Babel S, Kurniawan TA (2003) Low-cost adsorbents for heavy metals uptake from contaminated water: a review. J Hazard Mater B97:219–243
Batvari BPD, Kamala-Kannan S, Shanthi K, Krishnamoorthy R, Lee KJ, Jayaprakash M (2008) Heavy metals in two fish species (Carangoidel malabaricus and Belone stronglurus) from Pulicat Lake, North of Chennai, Southeast Coast of India. Environ Monit Assess 145:167–175
Batvari BPD, Sivakumar S, Shanthi K, Lee KJ, Oh BT, Krishnamoorthy RR, Kamala-Kannan S (2013) Heavy metals accumulation in crab and shrimps from Pulicat Lake, north Chennai coastal region, south east coast of India. Toxicol Ind Health. doi:10.1177/0748233713475500
Brown PA, Gill SA, Allen SJ (2000) Metal removal from wastewater using peat. Water Res 34:3907–3916
Chowdhury S, Mishra R, Saha P, Kushwaha P (2011) Adsorption thermodynamics, kinetics and isosteric heat of adsorption of malachite green onto chemically modified rice husk. Desalination 265:159–168
Crini G, Peindy HN, Gimbert F, Robert C (2007) Removal of C.I. basic green 4 (Malachite Green) from aqueous solution by adsorption using cyclodextrin-based adsorbent: kinetic and equilibrium studies. Sep Purif Technol 53:97–110
Denkhaus E, Salnikow K (2002) Nickel essentiality, toxicity, and carcinogenicity. Crit Rev Oncol Hematol 42:35–56
Gardea-Torresdey JL, Tang L, Salvador JM (1996) Copper adsorption by esterified and unesterified fractions of Sphagnum peat moss and its different humic substances. J Hazard Mater 48:191–206
Gupta BS, Curran M, Hasan S, Ghosh TK (2009) Adsorption characteristics of Cu and Ni on Irish peat moss. J Environ Manage 90:954–960
Gupta VK, Rastogi A, Nayak A (2010) Biosorption of nickel onto treated alga (Oedogonium hatei): application of isotherm and kinetic models. J Colloid Interf Sci 342:533–539
Han R, Han P, Cai Z, Zhao Z, Tang M (2008) Kinetics and isotherms of neutral red adsorption on peanut husk. J Environ Sci 20:1035–1041
Ho YS, Mckay G (2000) The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Res 34:735–742
Horsfall M, Spiff AI, Abia AA (2004) Studies on the Influence of mercaptoacetic acid (MAA) modification of cassava (Manihot sculenta Cranz) waste biomass on the adsorption of Cu2+ and Cd2+ from aqueous solution. Bull Korean Chem Soc 25:969–976
Janaki V, Oh BT, Shanthi K, Lee KJ, Ramasamy AK, Kamala-Kannan S (2012) Polyaniline/chitosan composite: an eco-friendly polymer for enhanced removal of dyes from aqueous solution. Synthetic Met 162:974–980
Janaki V, Vijayaraghavan K, Oh BT, Ramasamy AK, Kamala-Kannan S (2013) Synthesis, characterization and application of cellulose/polyaniline nanocomposite for the treatment of simulated textile effluent. Cellulose 20:1153–1166
Janaki V, Shin MN, Kim SH, Lee KJ, Cho M, Ramasamy AK, Oh BT, Kamala-Kannan S (2014) Application of polyaniline/bacterial extracellular polysaccharide nanocomposite for removal and detoxification of Cr(VI). Cellulose 21:463–472
Kalmykova Y, Strömvall AM, Steenari BM (2008) Adsorption of Cd, Cu, Ni, Pb and Zn on Sphagnum peat from solutions with low metal concentrations. J Hazard Mater 152:885–891
Kamala-Kannan S, Batvari BPD, Lee KJ, Kannan N, Krishnamoorthy R, Shanthi K, Jayaprakash M (2008) Assessment of heavy metals (Cd, Cr and Pb) in water, sediment and seaweed (Ulva lactuca) in the Pulicat Lake, South East India. Chemosphere 71:1233–1240
Kasprzak KS, Sunderman FW, Salnikow K (2003) Nickel carcinogenesis. Mutat Res 533:67–97
Maquieira A, Elmahadi HAM, Puchades R (1994) Immobilized cyanobacteria for online trace metal enrichment by flow injection atomic adsorption spectrometry. Anal Chem 66:3632–3638
Revathi M, Kavitha B, Vasudevan T (2005) Removal of nickel ions from industrial plating effluents using activated alumina as adsorbent. J Environ Sci Eng 47:1–6
Shin MN, Shim J, You Y, Myung H, Bang KS, Cho M, Kamala-Kannan S, Oh BT (2012) Characterization of lead resistant endophytic Bacillus sp. MN3-4 and its potential for promoting lead accumulation in metal hyperaccumulator Alnus firma. J Hazard Mater 199–200:314–320
Vien D, Colthup NB, Fateley WG, Grasselli JC (1991) The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules. Academic Press, San Diego
Vijayaraghavan K, Yun YS (2008) Bacterial biosorbents and biosorption. Biotechnol Adv 26:266–291
Volesky B (1990) Biosorption and biosorbents. Biosorption of heavy metals. CRC Press, Boston, pp 3–5
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Janaki, V., Kamala-Kannan, S. & Shanthi, K. Significance of Indian peat moss for the removal of Ni(II) ions from aqueous solution. Environ Earth Sci 74, 5351–5357 (2015). https://doi.org/10.1007/s12665-015-4547-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-015-4547-8