Abstract
Chemical properties and hydrogeological characterization of water resources were studied in the Hammemt Plain, which is located in the northwest of the Tezbent mountain range, City of Tébessa, northeast of Algeria, which drains carbonate aquifers through various large karst springs. The physical and chemical characteristics of spring and well water samples were studied for 2 years in order to assess the origin of groundwater and determine the factors driving its geochemical composition. The ionic speciation and mineral dissolution/precipitation were calculated where we found that water wells, characterizing groundwater circulation at shallow depths, are moderate to highly mineralized waters of the Na-HCO3 type. In contrast to the shallow environment, the CO2-rich, deeper waters are of the Ca-HCO3 type, and undergo significant changes in their baseline chemistry along flow lines with increasing residence time. The main factors controlling the groundwater composition and its seasonal variations are geology, because of the presence of different carbonate formations, additionally elevation, and the rate of karst development. Supersaturation with respect to calcite indicates CO2 degasing occurs either inside the aquifer in open conduits or at the outlet in reservoirs. Undersaturation with respect to calcite shows the existence of fast flow and short residence-time conditions inside the aquifer. The main springs and wells of the Tazbent Mountain have been studied by means of stable isotope measurement, where the values are varied in 18O; between −8.2 and −7.76 for spring samples and from −8.26 to −7.15 for well samples and in 2H between −52.92 and −49.11 for spring samples and from −55.47 to −47.44 for well samples, showing that the groundwater recharge is of meteoric origin and suggests the absence of the evaporation effect on the isotopic composition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water stored in karst aquifers represents an important source of drinking water in many countries around the world where the importance of water quality in human health has recently attracted a great deal of interest. In the developing world, 80 % of all diseases are directly related to poor drinking water and unsanitary conditions (Olajire and Imeokparia 2001). These aquifers are also used for irrigation and for producing electricity (hydropower plants). For all these reasons, water resources must be managed in a sustainable way (Bakalowicz 2005; Ravbar 2007). Since the signification of karst aquifers as important water resources and valuable ecosystems is growing worldwide, these hydrological systems are receiving rapidly increasing attention from the scientific, engineering, and regulatory communities. Due to the many challenges related to their characterization and management, such aquifers require good knowledge and comprehension of groundwater low characteristics (Goldscheider and Drew 2007; Bonacci et al. 2009. One of the advantages of bigger karst springs is sufficient amounts of water also during the time of low waters. On the other hand, these springs have large catchments and their effective protection against pollution is a great challenge. As a result, the water quality of these springs is often not good (Ravbar and Kova 2006). The study area lies in the semi-arid region of Algeria and is susceptible to the various threats common in both growing urban areas and developing agricultural areas. The Tazbeent plateau and the surrounding villages (Chéria, Hammamet) have seen a great deal of growth in the past decade with the establishment of new industries and farms (Rouabhia Aek et al. 2009). It is situated 20 km from Tebessa city. It extends on about 160 km2 and is characterized by a precipitation of <300 mm/year (Fig. 1).
Groundwaters play a dominant role in this part of Algeria. Because of the lack of permanent surface water reservoirs owing to the hard climatic conditions, it constitutes the most widely available source of fresh water. In this region, groundwaters are used for domestic, agricultural, and industrial purposes.
The present investigation concerns the whole Tazbent Plateau. The main objectives are the identification of chemical processes that are responsible for the groundwater chemistry and the reconstruction of the origin and recharge mechanisms of groundwaters.
Description of the study area
The study area is a part of a large mountain which forms a small portion of the great plio-quaternary tectonic plateau of Chéria. The study area is located between 7°45′–8°05E and 35°15–35°30N. The region is bound by Djebel Doukkane, Djebel Gourigueur, and Troubia to the East and West, respectively, by Djebel Gâaga and Bouziane to the North and by administrative boundary to the South (Fig. 1).
Annual precipitation in the study area ranges between 250 and 300 mm, and thus, the area is considered to be a semi-arid area. The temperature can rise in the summer to 40 °C. This situation of dryness accentuates the drawdown of water resource, especially during the last decade, because the renewal of this resource is very weak.
The dry climate, the atmospheric dust, and low intensity of precipitation can also affect the groundwater quality generally causing degradation of the water chemistry (Liana et al. 2014).
Geological and hydrogeological setting
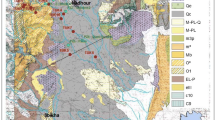
The study area is a part of the oriental Saharan Atlas near the Algerian-Tunisian borders and precisely constitutes a part of the Mellègue Mountains. The stratigraphic characteristics marking the study area show a diversity of facies of predominantly limestone, marly limestone, dolomitic limestone, and marl; these formations are thick and rich in fossils. Then they will generally from upper Cretaceous to Quaternary. Strong fracturing and the predominance of limestone and marly limestones alternating in series have led to intense reossification and to the formation of rock aquifers.
The Eocene limestone Formations, which are about 100 m thick in the study area (Fig. 2a), only constitute a limited aquifer. The dense and thick-bedded limestone in the Djebel Tazbennt has numerous joints and fractures. Karst phenomenons seem to be limited to solution features and small shelter caves. Contrary to the Eocene limestone, the Maestrichian carbonate formation constitutes the main aquifer (about 300 m thick). In this formation, deep caves are known (Fehdi et al. 2011). The existence of some very important springs, with mean flow rates up to 3300 l/s, indicates a water karst system. The Eocene limestone formations and Maestrichian carbonate are hydraulically disconnected in most parts of the synclines, since Marly Formations under the Tezbent Mountain form impermeable boundaries (Fig. 2b).
On top the Plio-Quaternary formations host a shallow unconfined aquifer. It consists of alluvial sediments, gravels, silts, and lacustrine limestone (Vila 1980). This aquifer plays a very important role in supplying drinking water to the local population. Overexploitation of this resource has caused a progressive degradation of the water quality in the irrigated area with the occurrence of high salinity zones (Wilcox 1948).
Methodologies
Sampling and analysis
Groundwater samples were collected into pretreated polyethylene sampling bottles using the standard method of sampling technique (APHA 1999) for geochemical analysis at different depths from six production wells (the selected wells are used for domestic, agricultural, and domestic agricultural purposes), penetrating the shallow Plio-quaternary aquifer and at four springs during the 2012–2013 hydrological year (Fig. 1). Temperature, conductivity, and pH were measured in the field. Electrical conductivity and water temperature were measured using an Orion 240 conductivity meter and prob. The pH was measured using an Orion 250 pH-meter regularly calibrated using two laboratory standards.
Chemical analyses were undertaken at the hydrochemistry Laboratory of Tébessa University. Major cation concentrations (Ca2+, Mg2+, Na+, and K+) together were determined by using atomic adsorption spectrophotometry. Calibrations for cation analysis were performed using appropriately diluted standards, and both laboratory and international reference materials were used as checks for accuracy.
The analysis of Bicarbonate was undertaken by titration to the methyl orange endpoint. Concentration of chloride was determined by titration and precipitation of AgCl until silver chromate appeared. Sulfate was determined by precipitation of BaSO4 and then measuring the absorbency with a spectrophotometer (Ademoroti 1996).
In order to investigate the isotopic characteristics of wells and springs waters, 10 samples were collected (Table 3). Sampling was carried out in 150-ml glass bottles and analyzed in the laboratory of the Department of Hydrology, University of Freiburg, Germany.
Results and discussion
Chemical data are presented in (Table 1).The following discussion will illustrate the significance of these results in the context of groundwater origin, water types, water–rock interactions, and origin of water chemistry.
General hydrochemical characteristics
The different water samples have been classified according to their chemical composition using the Piper diagram (Piper 1944). This classification is based on the concentration of the four major anions bicarbonate, sulfate, chloride, and nitrate and on the four major cations sodium, potassium, calcium, and magnesium.
Using the software diagrams (Simler 2004), the water samples of the study aquifer system are plotted on a Piper diagram (Fig. 3) and on a Scholler–Berkaloff diagram (Fig. 4) to make a comparison between the different water types and to show the effect of mixing. The Piper diagram shows that the overall chemical character falls within the following water types:
-
Moderate to high mineralized waters of Na-HCO3 type.
-
Deeper waters of the Ca–HCO3–SO4 type.
The first water type changed continuously due to the influence of many factors:
First, the water rock interaction of the aquifer material with mainly carbonate facies; and secondly, the influences of human activities such as irrigation return flow and overexploitation of the aquifer system. The result is indicated by a rapid increase in sodium, sulfate, and chloride concentrations in the aquifer. By following the direction of groundwater flow, the water changes its chemistry and becomes more saline, whereby the high sodium concentration is usually an indication of cation-exchange process.
Water rock interaction process
Interactions between groundwater and surrounding host rocks are mostly the main processes responsible for the observed chemical characteristics of groundwaters in the study area (Rouabhia Aek et al. 2009). Evaluation of such processes requires the description of the mean mineral assemblage of the rocks in which groundwater is found, and the identification of chemical reactions responsible for the geochemical evolution of groundwaters (Salameh 2001). From available studies in the literature, such reactions generally include chemical weathering of rock-forming minerals, dissolution–precipitation of secondary carbonates, and ion exchange between water and clay minerals (Djabri 1987; Fehdi et al. 2008).
Two approaches, mathematical and graphical, are generally used for the resolution of hydrogeochemical problems. The mathematical approach is often used for the calculation of saturation indices with respect to mineral phases, thus providing some indication upon the equilibrium state between groundwater and surrounding minerals rock assemblage (Deby and Huckel 1923). In this present paper, different parameters calculated based on the chemical analyses of the water samples include pH-equilibrium, PCO2, ionic strength, and saturation indices for calcite, aragonite, dolomite, gypsum, and anhydrite.
Saturation indices express the extent of chemical equilibrium between water and mineral phases in the matrix of the aquifers and could be regarded as a measure of dissolution and/or precipitation processes relating to the water–rock interaction.
The degree of saturation can be evaluated according to the following equation:
KIAP = The ionic activity product of the ions, Ksp = The solubility product of the mineral, SI = The saturation index.
If SI < 0. The water is undersaturated with respect to a certain mineral which means that the water is still able to dissolve that specific mineral.
If SI > 0. The water is oversaturated with respect to that mineral and the mineral will precipitate.
If SI = 0. Water is in equilibrium.
The computer geochemical program PHREEQC-2 version 2.10 (Appelo et al. 1999) was used to calculate the ionic speciation of the waters, the ionic activities, the theoretical PCO 2 , and most importantly, the saturation indices of calcite, aragonite, dolomite, gypsum, and anhydrite. The significant results are that all the groundwater samples were found to be saturated with respect to calcite, dolomite, and aragonite, but undersaturated with respect to gypsum and anhydrite (Table 2).
Figure 5 shows the distribution of the computed PCO 2 values for all samples. These values vary between −2227 and −1233 being significantly higher than that of atmosphere (10−3.5) atm. Such elevated values suggest that the groundwater system is open to soil CO2. The source of dissolved CO2 can be determined on the basis of 13C analyses (anthropogenic and biogenic origin).
In the diagram calcite saturation index (ISC)–dolomite saturation index (ISD) (Fig. 6), the representative points of the water samples are aligned generally along a regression line whose Eq. (1) is
Most of the water samples are oversaturated with respect to Calcite (ISC > 0) and undersaturated with respect to Dolomite ISD < 0 with the exception of three water samples where ISC and ISD are negative. These wells are P2 (ISC = −1.14; ISD = −0.21), P4 (ISC = −0.87; ISD = −0.06), and P6 (ISC = −1.07; ISD = −0.12).
The waters of the study area were divided into two groups according to the values of ISD.
-
Family 01 (ISD > −0.5):
It covers 05 waters samples which are SG, SAM, SAN, well 01, and well 05. These waters have saturation indices of calcite and dolomite very close to equilibrium state.
-
Family 02 (−2 < ISD < −0.5):
The values of saturation indices reflect an undersaturation state with respect to dolomite and an oversaturation with respect to calcite significantly stronger compared to the family 01.
Origin and recharge mechanisms of groundwater
The use of stable isotopes is very important in the field of evaluation of the resources (Fontes et al. 1976, 1986, 1989; Fontes 1980). Stable isotopes of oxygen and hydrogen in groundwater of an active hydrological cycle derive from and reflect the initial isotopic composition of the recharging rainwater. Within the coordinate system of δ18O and δ2H, it is possible to discern a meteoric line, the slope of which is characteristic of a certain hydrological system (Gat and Carmi 1970).
In this paper, the δ18O and δ2H values of precipitation samples, used to establish the local meteoric water lines, are obtained from the Global Network for Isotopes in Precipitation database managed by the AIEA, for a period of 6 years (September 1992–December 1998) for Tunis–Carthage and Sfax meteorological stations (Tunisia) (Fig. 7).
δ 2H vs δ 18O relationship for groundwater in the study area. Red dots springs samples; blue dots Well samples DMM meteoric water line (Craig 1961) ; meteoric water lines for Sfax and Tunis (Celle et al. 2001)
The meteoric water lines defined for Sfax and Tunis (Fig. 7) have been calculated using a least squares regression (Celle et al. 2001):
Δ2H = (6.4 ± 0.5) δ18O + (5.2 ± 1.7); Tunis (1992–1998, n = 26).
Δ2H = (6.7 ± 0.3) δ18O + (3.5 ± 1.3); Sfax (1992–1998, n = 45).
The water lines of Tunis and Sfax for the period 1992–1998 are close and thus seem to be representative of the precipitation isotopic content in Tunisia. The comparison between the Tunisia and the global meteoric water line (Craig 1961) shows evidence of the evaporation that affects Tunisian precipitation as the rain falls.
Groundwater isotopic signatures
Stable isotope compositions of water collected from wells and springs are presented in Table 3. Stable isotope values were found to vary between −8.2 and −7.76 for spring samples with a mean of −8.006 ± 0.2 ‰ (n = 5) and from −8.26 to −7.15 for well samples with a mean of −7.71 ± 0.15 ‰ (n = 5) in 18O and from −52.92 to − 49.11 for spring samples with a mean of −51.51 ± 0.15 ‰ (n = 5) and from −55.47 to −47.44 for well samples with a mean of −51.46 ± 0.15 ‰ (n = 5) in 2H.
The oxygen-18 and deuterium contents for all the investigated groundwater are plotted in the classical O18–H2 diagram (Fig. 8), together with the so-called global meteoric water line (d H2 = 8d O18 + 10), defined by Craig (1961) and the local meteoric water lines (Tunis and Sfax).The plot of data points in such diagram provides some indication upon the origin and the recharge processes of groundwaters.
Figure 8 shows that nearly all of the groundwater samples are plotted close to the Tunis and Sfax Meteoric line, and indicate no significant isotopic modifications by evaporation, which means that the recharge of the aquifer is quite rapid, and the recharging meteoric water does not occupy the soil zone of the recharge area for a long time.
The similarity of δ2H versus δ18O for both wells and spring groundwater samples is not surprising in karst regions and suggests the rapid recharge (fast infiltration process) of the precipitation to the groundwater throughout highly karst carbonate at high elevations (Fig. 9) and indicates that groundwater is of meteoric origin (Araguas and Diaz Teijeiro 2005). The deuterium excess values vary very slightly from 6 ± 1.5 %, indicating that the catchment area of Tezbent plateau springs are recharged by local originated atmospheric water vapor that reflects low evaporation rates.
The total dissolved solid (TDS) values provide a good indication of the interaction time between the water and reservoir rock. In Fig. 9, the ion concentrations increase from springs toward groundwater samples collected from wells at Tezbent plateau. While wells samples are represented with high TDS values, samples springs are represented with high δ18O values. It must be emphasized that, although water springs originate in higher recharge areas, their ion concentrations are lower than those of wells because of the interaction time of groundwater with a shallow aquifer.
Conclusion
The region of Tazbent is a sedimentary terrain consisting of limestone, clay, alluvium, and marl. The chemical composition of the groundwater is controlled by dissolution processes and anthropogenic inputs. The results indicate that calcium and magnesium are derived mainly from dissolution of carbonate precipitates along with ion exchange process in the groundwater.
Two hydrogeological systems were defined in the study area: (1) a deep water system, which is related to extensive and deep circulation of meteoric water in the regional flow system where the influence of shallow waters is relatively small; and (2) a shallow system, which is related to shallow circulation, and is affected very rapidly by recent rainfall events.
The ionic speciation and mineral dissolution/precipitation were calculated. The significant results are that all the groundwater samples were found to be saturated with respect to calcite, dolomite, and aragonite but undersaturated with respect to gypsum and anhydrite.
All the underground flow systems are fed by meteoric water. The studied water samples collected from springs and wells are fed from recharge at the highest elevations.
References
Ademoroti CMA (1996) Standard methods for water and effluents analysis. Fodulex Press Ltd, Ibadan, pp 32–34
APHA (1999) Standard methods for the examination of water and wastewater. 20th ect. APHA, AWWA, WPCF, New York
Appelo C, Williemsen A, Beekmanhe Grippioen (1999) Calculations and observations on salt water intrusion, II. Validation of a geochemical model with laboratory experiments. J Hydrol 120:225–250
Araguas LJ, Diaz Teijeiro MF (2005) Isotope composition of precipitation and water vapour in the Iberian Peninsula: first results of the Spanish network of isotopes in precipitation. In: international atomic energy agency—isotopic composition of precipitation in the Mediterranean Basin in relation to air circulation patterns and climate. Int At Energy Agency 1453:173–190
Bakalowicz M (2005) Karst groundwater: a challenge for new resources. J Hydrol 13:148–160
Bonacci O, Pipan T, Culver DC (2009) A framework for karst ecohydrology. Environ Geol 56:891–900
Celle JH, Zouari K, Travi Y, Daoued A (2001) Caractérisation isotopique des pluies en Tunisie. Essai de typologie dans la région de Sfax. C.R. Acad. Sci Paris Sci de la Terre et des Planètes 333:625–631
Craig H (1961) Standard for representing concentrations of deuterium and oxygcn-18 in natural waters. Science 133:1833–1834
Deby and Huckel (1923) Zur Theorie der Elektrolyte. I. Gefrierpunktserniedrigung und verwandte Erscheinungen [The theory of electrolytes. I. Lowering of freezing point and related phenomena] Physikalische Zeitschrift 24:185–206
Djabri L (1987) Contribution to the hydrogeological study of the subsidence plain of Tebessa NE Algeria. Attempt of modeling. Doctorate Thesis, University of Franche Comté, France
Fehdi Ch, Aek Rouabhia, Baali F (2008) The Hydrogeochemical characterization of Morsott-El Aouinet aquifer. Environmental Geology, Springer-Verlag, Berlin; Heidelberg, Northeastern Algeria. doi:10.1007/s00254-008-1667-4
Fehdi C, Baali F, Boubaya D, Rouabhia A (2011) Detection of the sinkholes using 2D electrical resistivity imagining in the Cheria Basin(north east of Algeria. Arab J Geosci 4:181–187
Fontes J (1980): Environmental isotopes in ground water hydrology. In: Fritz J, Fontes J (eds) Handbook of environmental isotope geochemistry. Elsevier, Amsterdam, l (A):75
Fontes JCh, Yousfi M, Allison GB (1986) Estimation of long term, diffuse groundwater discharge in the northern Sahara using stable isotope profiles in soil water. J Hydrol 86:315–327
Gat J, Carmi L (1970) Evolution of the isotopic composition of atmospheric waters in the Mediterranean Sea area. J Geophys Res 75:3039–3048
Goldscheider N, Drew D (2007) Methods in Karst hydrogeology. Taylor & Francis, London, p 264
Liana M et al. (2014) Groundwater geochemistry observations in littoral caves of Mallorca (western Mediterranean): implications for deposition of phreatic overgrowths on speleothems
Olajire AA, Imeokparia FE (2001) Water quality assessment of Osun river: studies on inorganic nutrients. Environ Monit Assess 69(1):17–28
Piper AM (1944) A graphic procedure in geochemical interpretation of water analysis. Trans Amer Geophys Union, 25(6):914–928; Richmond,VA
Ravbar N (2007) The protection of karst waters. Carso-logica 6. ZRC publishing, Postojna-Ljubljana, p 254
Ravbar N, Kova G (2006) Karst water management in Slovenia in the frame of vulnerability map ping. Acta Carsologica, Ljubljana, pp 35–2
Rouabhia Aek, Baali F, Fehdi Ch (2009) Impact of agricultural activity and lithology on groundwater quality in the Merdja area, Tebessa, Algeria. Arab J Geosci, Springer-Verlag, Berlin; Heidlerg. doi 10.1007/12517-009-0087-4
Salameh E (2001) Sources of water salinities in the Jordan Valley Area. Jordan Acta hydrochim hydrobiol 6–7:329–362
Simler R (2004) Hydrochemistry Software multilanguage free distribution. Hydrogeology Laboratory of Avignon, Version 2
Vila JM (1980) La chaîne alpine de l’Algérie orientale et des confins Algéro-Tunisiens. Thèse de Doctorat- es -sciences, Université Pierre et Marie curie, Paris VI
Wilcox L (1948) The quality of water for agricultural use. US Dept Agriculture, Tech Bull 962, Washington DC
Acknowledgments
The authors acknowledge the co-operation of the Laboratory of Water and Environment of Tébessa University for their participation in some of the analytical programs. Authors wishes to give special thanks to Prof Elias Salameh of Jordan University, Department of Geology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chemseddine, F., Dalila, B. & Fethi, B. Characterization of the main karst aquifers of the Tezbent Plateau, Tebessa Region, Northeast of Algeria, based on hydrogeochemical and isotopic data. Environ Earth Sci 74, 241–250 (2015). https://doi.org/10.1007/s12665-015-4480-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-015-4480-x