Abstract
Understanding the geochemical cycling of sulfur in sediments is important because it can have implications for both modern environments (e.g., deterioration of water quality) and interpretation of the ancient past (e.g., sediment C/S ratios can be used as indicators of palaeodepositional environment). This study investigates the geochemical characteristics of sulfur, iron, and organic carbon in fluvial and coastal surface sediments of the Laizhou Bay region, China. A total of 63 sediment samples were taken across the whole Laizhou Bay marine region and the 14 major tidal rivers draining into it. Acid volatile sulfur, chromium (II)-reducible sulfur and elemental sulfur, total organic carbon, and total nitrogen were present in higher concentrations in the fluvial sediment than in the marine sediment of Laizhou Bay. The composition of reduced inorganic sulfur in surface sediments was dominated by acid volatile sulfur and chromium (II)-reducible sulfur. In fluvial sediments, sulfate reduction and formation of reduced inorganic sulfur were controlled by TOC and reactive iron synchronously. High C/S ratios in the marine sediments indicate that the diagenetic processes in Laizhou Bay have been affected by rapid deposition of sediment from the Yellow River in recent decades.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The global biogeochemical cycles of carbon, iron, and sulfur are closely linked during early diagenesis to form sedimentary sulfide-bearing minerals (Morse and Rickard 2004; Canfield et al. 2005; Johnston et al. 2014). Interactions and limiting components of these cycles within different formation environments can dictate the geochemistry of the sulfidic material formed (Morgan et al. 2012). Therefore, inorganic sulfur speciation may be important as a diagnostic tool for sediment conditions during early diagenesis. The interplay of microbial iron and sulfate reduction and inorganic reactions driven by their respective products are the primary controls on the burial of reduced sulfur in anoxic sediments (Morse and Berner 1995). Berner and Raiswell (1983) found differences in the burial ratio of organic carbon to pyrite-S in different depositional environments, suggesting that C/S ratios could be a useful tool for determining palaeodepositional environments in ancient sedimentary rocks. Berner (1982) indicated that the C/S mass ratio in normal marine sediments (deposited under an oxic water column) was between 0.75 and 1.35 (calculated from molar ratio 2.0–3.6). Research into the distribution and transformations of reduced inorganic sulfur (RIS) in freshwater, estuary, peatland, salt marsh, and marine sediments (Berner 1984; Coulson et al. 2005; Bottrell et al. 2010; Sheng et al. 2011, 2013a; Mortimer et al. 2011) has shown that pyrite and ferrous monosulfide are the two major end products in sediments, where dissimilatory sulfate reduction is active (Zhu et al. 2014). Although sulfate and Fe(III) reduction along with the microbially mediated formation of sulfide minerals can increase alkalinity and reduce metal availability (Burton et al. 2005; Mortimer et al. 2011), oxidation of sedimentary sulfide during sediment resuspension may cause rapid deoxygenation and acidification of overlying water, posing an environmental hazard (Morse and Rickard 2004; Sullivan et al. 2002). Therefore, in addition to potentially recording information about the environment of deposition, sulfur geochemistry may play an important role in affecting estuarine sediment and water quality (Anthony et al. 2010; Morgan et al. 2012).
Laizhou Bay is situated in the northern part of Shandong province, east China. It is a typical semi-enclosed inner sea, one of three main bays of the Bohai Sea. This bay is an important production base for fisheries and salt in China, and marine industrial and urban developments have been booming around the bay in recent years. Over 10 rivers, notably the Yellow River, Xiaoqing River, Yuhe River, Dihe River, and Jiaolaihe River drain into the bay with high loads of sediment and industrial and municipal wastewater (Sheng et al. 2013b). The rivers and coastal zone of this area have been polluted by organochlorine pesticides (Zhong et al. 2011), antibiotics (Zhang et al. 2012), polychlorinated naphthalenes (Pan et al. 2011), and trace metals (Wang and Wang 2007). However, there are little data about RIS in the region. In this study, acid volatile sulfur (AVS), chromium (II)-reducible sulfur (CRS), elemental sulfur (ES), total sulfur (TS), reactive iron, total organic carbon (TOC), and total nitrogen (TN) were analyzed in surface sediments from both rivers and the coastal zone. The environmental biogeochemical behavior of different RIS species and relationships between organic carbon, sulfur and iron within the river-estuary-coastal zone system of Laizhou Bay was studied in order to identify the factors controlling the transformation, burial, and preservation of Fe, C, and S and the ratios of C/S, Fe/S that result. This allows both assessment of current and potential future impact on water quality as well as providing evidence about how sediment sulfur geochemistry reflects the depositional environment.
Methods and materials
Samples collection and handling
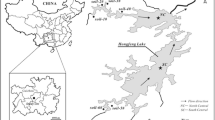
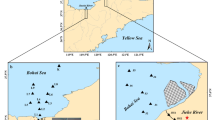
General information on Laizhou Bay and its adjacent region was introduced by Pan et al. (2011) and Zhao et al. (2013). The salinity in surface and bottom water of the offshore area of Laizhou Bay is typically 26–31 psu (increasing gradually seaward from the river mouth offshore) (Qiao et al. 2010). Water and sediment were collected from a total of 63 sampling sites in Laizhou Bay and from rivers draining into it (Fig. 1). All sampling equipment and storage containers were cleaned with distilled water before use. All of the seawater and river water samples were collected (approximately 0–50 cm below the surface) using a stainless steel bucket and were immediately transferred to a 5-L pre-cleaned amber glass bottle. The bottle was rinsed 3 times with a sample prior to sampling. The samples were kept at 4 °C in a cold room before further treatment and analysis in the laboratory. The surface sediments (0–10 cm) from the marine region were collected using a stainless steel grab sampler, and the surface sediments (0–10 cm) from rivers were collected using a plastic spatula. All sediment samples were immediately placed into 250 ml polypropylene vials, which were fully filled with sediment and sealed with gas-tight screw-caps and immediately frozen under nitrogen in an adiabatic box until further analysis. Before analysis, all sediment samples were homogenized (mixing with a glass rod) under a stream of N2 in a sealed chamber.
Sample analysis
The reagents used were all analytical grade or above, and deionized water (milli-Q) was used to prepare reagent solutions. All glass and plastic were soaked in 10 % HNO3 for 48 h and rinsed with milli-Q water several times before use. Water samples were filtered (0.47 μm Whatman® filters) and kept cool and dark prior to analysis for total dissolved organic carbon (TDOC, calculated from the difference between total carbon and total inorganic carbon), and total dissolved nitrogen (TDN) was determined using a Shimadzu TOC-VCPH/SSM-5000A (analytical relative standard deviation (RSD) ≤ 5 %). Salinity was determined by a BANTE531 Portable Conductivity/Salinity Meter (Shanghai, China) (RSD < 0.05 %). Sediment was treated with excess 1 N HCl overnight (stirring once a while) and washed twice with deionized water to remove carbonates, then the samples were dried at ~60 °C for 12 h and ground to ~100 mesh before TOC analysis using a Shimadzu TOC-VCPH/SSM-5000A (RSD ≤ 3 %). TN and TS were determined by an Elementar vario MACRO cube CHNS analyzer (RSD < 2.5 %). Prior to grain-size analysis, each sediment sample was treated with sodium hypochlorite (NaOCl) to remove organic matter. Grain size was analyzed using a Malvern Mastersizer 2,000 laser diffractometer capable of analyzing particle sizes between 0.02 and 2,000 µm (RSD < 2.5 %). The percentages of samples in each of the following three grain-size groups were determined: <4 µm (clay), 4–63 µm (silt), and >63 µm (sand).
The separation and determination of AVS, CRS, and ES were conducted following the cold diffusion procedure described by Hsieh and Shieh (1997). Briefly, AVS, CRS, and ES were separated sequentially by 6 M HCl (18 h), acidic Cr(II) (48 h), and Cr(II) plus N, N-dimethylformamide (24 h), respectively, under a pure N2 atmosphere, at ambient temperature. The liberated H2S was passively trapped in an alkaline Zn solution (20 % ZnOAc). The quantity of S for each solid-phase RIS species trapped in ZnS was determined by iodometric titration (RSD < 8 %). Sediment samples of known weight (~2 g) were loaded into centrifuge tubes containing 50 mL 1 N HCl for extraction of reactive iron under stirring. After centrifugation (3,000 rpm) of the suspensions, the resultant supernatants were filtrated for analysis of Fe2+ and total reactive Fe separately. The reactive iron was determined using the ferrozine method (Wallmann et al. 1993). Fe3+ was calculated from the difference between total Fe and Fe2+ (RSD < 6 %). All treatments were undertaken in N2 glove box to avoid oxidation of Fe2+ and sulfide during sample handling.
Results
Grain-size distribution
As shown in Fig. 2, the grain sizes of marine surface sediments were mostly dominated by silt, with the average value of 57 %. The grain sizes of sediments in most rivers were dominated by sand, with the average value of 49 %, and there was a fine to coarse transition in the rivers from northern/western ones to the eastern/southern ones (Fig. 1).
Concentrations of TDOC, TDN, and salinity in marine and fluvial water bodies
The concentrations of TDOC, TDN, and salinity in the water samples are presented for the whole Laizhou Bay marine region and 14 major tidal rivers in Table 1. In the rivers, the concentrations of TDOC and TDN ranged from 2.4 to 71.9 mg L−1 and 2.4 to 12.4 mg L−1, respectively. In the marine area, the concentrations of TDOC and TDN were much lower, ranging from 1.89 to 5.27 mg L−1 and 0.27 to 0.68 mg L−1, respectively. In the coastal sites of E4-6 and F1, TDOC and TDN were high, suggesting pollution by terrestrial sources. The Xiaoqing River (XQH), Yuhe River (YH), and Dihe River (DH) all receive municipal and industrial wastewater, resulting in high TDOC and TDN concentrations at site F1 (Pan et al. 2011). The Weihe River (WH) and Jiaolaihe River (JLH) also receive municipal wastewater, resulting in high TDOC and TDN at sites E4-6. These results indicate that the discharge of these heavily polluted rivers is a major source of pollutant carbon and nitrogen to the coastal zone of Laizhou Bay. The salinity ranged from 26.7 to 31.1 psu in the marine region. Most of salinities are similar, the lowest value being at site A1, close to the Yellow River estuary and hence diluted by the freshwater discharge (Fig. 1). For the rivers, salinities are variable but most of them are higher than average fresh water values, ranging from 0.5 psu (Yellow River, YHH-1, 2) to 31.5 psu (Bailanghe estuary, WFG, higher than seawater). Because Laizhou Bay is an important salt production area, with many plants producing salt by solar evaporation of saline groundwater, residual bitterns are discharged into local rivers directly, resulting in high salinities in some areas such as the Bailanghe River (BLH), Yuhe River (YH), and Dajiawa River (DJW).
Distributions of TOC, TN, and TS in surface sediment
In the marine sediments, TOC contents varied between 0.65 and 1.97 % dry weight, with an average of 1.27 % (Table 2), higher than that previously reported at a nearby site (0.87 %) (Zhu et al. 2012). TN content ranged from 0.01 to 0.07 % dry weight, with an average of 0.04 %. TS content ranged from 0.01 to 0.09 % dry weight, with an average of 0.03 %. Higher TOC and TN contents were all recorded at fluvial sampling stations. For all the rivers, TOC, TN, and TS contents showed a clear decreasing trend seaward from the mainland to the estuary (Table 2). It appears that, in general, industrial and domestic wastewater discharges inland resulted in organic matter enrichment (anthropogenic inputs), causing increasing TOC, TN, and TS. The highest TOC (7.04 %), TN (0.53 %), and TS (0.42 %) were found in the upstream part of the Xiaoqing River (XQH-2). This river drains the cities (including some special industrial parks) of Jinan, Zibo, and Weifang in Shandong province, and hence the water quality is heavily impacted by pollution discharge, leading to the high TOC, TN, and TS in the surface sediments.
Distribution of AVS, CRS, and ES in sediment
Detailed concentrations and proportions of S in the sediments of Laizhou Bay are shown in Table 2. In most marine sites, CRS was the dominant fraction of total RIS except for site F2 (Fig. 1). However, in most rivers, AVS was the dominant RIS, with the highest concentration of 6,832 μg g−1 in the Dihe River (DH-2). However, for the upstream of Xiaoqing River (XQH-2), Yuhe River (YH-3), and Jiaolaihe River (JLH-2), CRS was the dominant RIS. In the marine area, AVS concentrations fall in a wide range from 1.17 to 371 μg g−1 (Table 2). The higher end of this range is consistent with AVS concentrations found in northern Yellow Sea coastal surface sediments (202–344 μg g−1) (Sheng et al. 2013a), but 89 % (24 of 27) of our samples fall below the lower end of their range, consistent with the high TOC/TS ratios found in these sediments (Table 3). In the rivers, the AVS levels (6.56–6,832 μg g−1) are consistent with many natural estuaries (e.g., 2,880 μg g−1, Morse and Cornwell 1987) and Shijing River (1,043 μg g−1) located in the Pearl River delta (Sheng et al. 2011).
Discussion
Grain-size characteristics
The grain sizes of marine surface sediments of Laizhou Bay were mostly dominated by silt (57 %), which are consistent with a previous report (Qiao et al. 2010). There is an anti-clockwise circulation that dominates in Laizhou Bay and the residual current near the Yellow River mouth is mainly southward, so the transport of suspended sediment (silt) off the river mouth will influence the distribution of marine sediment grain sizes directly. However, in the marine sediments, there was a definite change in grain size across the bay, with the eastern side (sites C1, D4-6, E4-6) more coarse grained, which may be influenced by the coarse sediment input of local rivers. The Weihe River (WH) and Jiaolaihe River (JLH) are two major rivers in the eastern side of Laizhou Bay and they have coarse sediment, which matches with that side of the bay. The grain sizes of sediments in most rivers were dominated by sand (49 %), which may relate to the geological composition of riverbed (i.e., sandy local soil). Furthermore, the main function of rivers is draining floods, so the fine particles would be flushed and driven into the estuary by strong current during flooding in the rainy season, resulting in sand dominating the grain-size distribution.
Relationships of TOC, TN, and TS in surface sediment
The ratio between TOC and TN (C/N) is frequently used to discriminate between organic matter of terrestrial and marine origin in estuarine sediments (Hedge et al. 1997). Marine algae typically have C/N ratios of 4–10 due to an abundance of protein, whereas land plants have C/N ratios of 20 or higher due to high cellulose content (Meyers 1994). It is therefore reasonable for a mixture of both organic end-member sources to yield sediment C/N ratios between 10 and 20. In this study, except for site A5 (11.94), all ratio values are higher than 20 in the coastal area (mean value 37.4) (Table 3), which suggests that the dominant source of organic matter to Laizhou Bay sediments is terrestrial material. Because site A5 is close to Longkou Port (the largest port of foreign trade in China, with a throughput of 66 million tons in 2012), the low value of C/N may be attributed to pollutant discharge and sedimentation from boats (discharge or spill) and port, although this detailed explanation needs further study. For the rivers, the average value of C/N for rivers is 28.5 (Table 3), which is consistent with the ratio (C/N 34) of the Amazon River (Hedges et al. 1994). Because some rivers receive a combination of rainwater, residual brines from salt production, municipal and industrial wastewater, and other kind pollutants, huge variations (10.7–67.3) in C/N ratios were observed between different rivers.
TOC/TS ratios (C/S) can be a useful tool for determining palaeodepositional environments in ancient sedimentary rocks. Berner (1984) found that C/S mass ratios are 1.88–3.75 in marine sediments and 18.75–93.75 in freshwater sediments (calculated from corresponding S/C molar ratios). In this study, the average C/S mass ratio is 55.91 in the marine area and 35.08 in rivers (Table 3). The highest ratio in the marine area (187.76) was for site A1, close to the Yellow River Estuary. For rivers, the highest ratio (179.14) was at site HH3 within the Yellow River (200 km from the estuary), and the second highest (92.23) was at site HH1, within the estuary. All the highest ratios were in the Yellow River, suggesting that the high C/S ratios in the marine area are related to the input of Yellow River sediment (Qiao et al. 2010). Interestingly, all these ratios are much higher than those in sediments off other major deltas such as the Mississippi River Delta (~1.05) and Amazon River Delta (~2.63) (Aller et al. 2004). In this study, although C/S ratios in the fluvial sediment (mean 35.08) are consistent with freshwater sediment (Berner 1984: 18.75–93.75), the marine sediment values are exceptionally high (more than an order of magnitude above typical values quoted by Berner 1982). This phenomenon can be explained by the following reasons: 1) high C/S ratios in sediments are associated with high TOC (Morse and Emeis 1990); 2) the dominant source of organic matter to Laizhou Bay sediments is from terrestrial discharge. The burial of large amounts of terrestrial organic C (i.e., lignin, tannin, suberin and cutin) is more resistant to mineralization than organic C from typical marine sources, leaving high residual terrestrial organic C, and resulting in high C/S and C/N ratios; and 3) influence of the input of Yellow River sediment. There is a positive correlation between TOC and TS in the fluvial sediments (R 2 of TS-TOC is 0.54). This indicates that the sulfate reduction in the fluvial sediment was controlled by TOC. In contrast, there is no correlation between TOC and TS in the marine sediments (R 2 0.05). The lack of a correlation in the marine environment along with the high C/S shows that there is no overprinted pattern of early diagenetic sulfate reduction from marine sediments in Laizhou Bay.
AVS, CRS and ES in sediment
In the rivers and estuaries, AVS and CRS were the dominant RIS. These results are similar to those reported by Morgan et al. (2012) who showed that high organic carbon content in estuarine sediments can lead to the stabilization of a significant fraction of the RIS pool as AVS. Furthermore, the relationships of TOC-AVS, TOC-CRS, and TOC-ES for fluvial sediment exhibit statistically significant positive correlations (P < 0.001), suggesting that TOC controls sulfate reduction and formation of sulfides (Fig. 3). Southern Laizhou Bay is one of the important salt production areas of China, with many plants producing salt by solar evaporation of saline groundwater. Residual bitterns are discharged into local rivers directly, so there might be high sulfate concentrations in river water and sediments (Xue et al. 2000: average concentration of sulfate in salt and brine wells is 8.18 g L−1 in South coast of Laizhou Bay, 2.82 g L−1 in Laizhou Bay sea water, and 0.04 g L−1 in freshwater in this area). Thode-Andersen and Jørgensen (1989) reported that ES may be the most abundant short-term sulfate reduction product in near-surface sediments, as a result of incomplete oxidation of pore-water sulfide by O2, Fe3+, and Mn4+ species (Morse and Cornwell 1987). However, in this study, ES was the lowest portion of RIS. The low ES concentrations in these sediments perhaps indicate that the ES had been transferred to AVS or CRS through rapid geochemical conversion.
Relationships between RIS and Fe in sediment
Gerritse (1999) showed that total Fe and TS show significant covariation in marine sediments, while in fluvial sediments they do not. However, in this study, the opposite is true, with distinct positive correlation between different RIS pools and reactive Fe in fluvial sediment (R 2 > 0.65, Fig. 3), but not in marine sediment (R 2 ~ 0.04). Furthermore, the relationships of Fe2+-AVS, Fe2+-CRS, and Fe2+-ES for fluvial sediment exhibit statistically significant positive correlations (P < 0.001), clearly indicating that reactive Fe (Fe2+) controls the formation of RIS pools in fluvial sediment in the region.
As shown in Fig. 2, Fe(III) dominated the total reactive Fe of the sediments, indicating that iron oxide is the dominant fraction of Fe in Laizhou Bay. In the fluvial sediments, the average concentration of reactive Fe is 14,137 μg g−1, and the average molar ratio of reactive Fe/S is 19.96 (Table 3). This ratio is consistent with the work of Gerritse (1999), which was based on sites in Australia. As was the case for C/S, the highest Fe/S ratio in the marine area (189.46) was for site A1, close to the Yellow River Estuary. The highest fluvial ratio (178.29) was at site HH3 within the Yellow River. Furthermore, there is no correlation among three sites (HH1, HH2, and HH3) in Yellow River for total reactive Fe and TS (R 2 0.1). Therefore, high ratios of Fe/S in Laizhou Bay are related to the input of Yellow River sediment in recent years. In the fluvial sediment, the formation of RIS was controlled by TOC and reactive iron synchronously, but this was not the case in the marine sediments.
Conclusions
The concentrations, accumulation, and composition of several different species of inorganic sulfur have been determined in surface sediments in rivers and the coastal zone of Laizhou Bay. In fluvial surface sediments, CRS and AVS dominate RIS and concentrations of different RIS correlate with TOC and Fe. These results indicate that sulfate reduction and formation of RIS were controlled by TOC and reactive iron synchronously in the river sediments. In the marine sediments, there is no correlation between TOC and TS, but the high C/N indicates that the source of organic matter delivered to Laizhou Bay sediments is terrestrial. The high ratios of C/S and Fe/S indicate that diagenetic processes in Laizhou Bay were affected by rapid deposition of sediment from the Yellow River in recent decades.
References
Aller RC, Heilbrun C, Panzeca C, Zhu Z, Baltzer F (2004) Coupling between sedimentary dynamics, early diagenetic processes, and biogeochemical cycling in the Amazon-Guianas mobile mud belt: coastal French Guiana. Mar Geol 208:331–360
Anthony S, William D, Zhang H (2010) Formation of iron sulfide at faecal pellets and other microniches within suboxic surface sediment. Geochim Cosmochim Acta 74:2665–2676
Berner RA (1982) Burial of organic carbon and pyrite sulfur in the moden ocean: its geochemical and environmental significance. Am J Sci 282:451–473
Berner RA (1984) Sedimentary pyrite formation: an update. Geochim Cosmochim Acta 48:605–615
Berner RA, Raiswell R (1983) Burial of organic carbon and pyrite sulfur in sediments over phanerozoic time: a new theory. Geochim Cosmochim Acta 47:855–862
Bottrell SH, Hatfield D, Bartlett R, Mortimer RJG (2010) Concentration, sulfur isotopic composition and origin of organo-sulfur compounds in pore-waters of a highly polluted raised peatland. Org Geochem 41:55–62
Burton ED, Phillips IR, Hawker DW (2005) Reactive sulfide relationships with trace metal extractability in sediments from southern Moreton Bay, Australia. Mar Pollut Bull 50:589–608
Canfield DE, Kristensen E, Thamdrup B (2005) The Sulfur Cycle. Adv Mar Biol 48:313–381
Coulson JP, Bottrell SH, Lee JA (2005) Recreating atmospheric sulphur deposition histories from peat stratigraphy: diagenetic conditions required for signal preservation and reconstruction of past sulphur deposition in the Derbyshire Peak District, UK. Chem Geol 218:223–248
Gerritse RG (1999) Sulphur, organic carbon and iron relationships in estuarine and freshwater sediments: effects of sedimentation rate. Appl Geochem 14:41–52
Hedge JI, Keil RG, Benner R (1997) What happens to terrestrial organic matter in the ocean? Org Geochem 27:195–212
Hedges JI, Ertel JR, Richey JE, Quay PD, Benner R, Strom M, Forsberg B (1994) Origin and processing of organic matter in the Amazon River as indicated by carbohydrates and amino acids. Limnol Oceanogr 39:743–761
Hsieh YP, Shieh YN (1997) Analysis of reduced inorganic sulfur by diffusion methods: improved apparatus and evaluation for sulfur isotopic studies. Chem Geol 137:255–261
Johnston SG, Burton ED, Aaso T, Tuckerman G (2014) Sulfur, iron and carbon cycling following hydrological restoration of acidic freshwater wetlands. Chem Geol 371:9–26
Meyers PA (1994) Preservation of elemental and isotopic source identification of sedimentary organic matter. Chem Geol 114:289–302
Morgan B, Burton ED, Rate AW (2012) Iron monosulfide enrichment and the presence of organosulfur in eutrophic estuarine sediments. Chem Geol 296–297:119–130
Morse JW, Berner RA (1995) Water determines sedimentary C/S ratios? Geochim Cosmochim Acta 59:1073–1077
Morse JW, Cornwell JC (1987) Analysis and distribution of iron sulfide minerals in recent anoxic marine sediments. Mar Chem 22:55–69
Morse JW, Emeis KC (1990) Controls on C/S ratios in hemipelagic sediments. Am J Sci 290:1117–1135
Morse JW, Rickard D (2004) Chemical dynamics of sedimentary acid volatile sulfide. Environ Sci Technol 38:131A–136A
Mortimer RJG, Galsworthy AMJ, Bottrell SH, Willmot LE, Newton RJ (2011) Experimental evidence for rapid biotic and abiotic reduction of Fe(III) at low temperatures in salt marsh sediments: a possible mechanism for formation of modern sedimentary siderite concretions. Sedimentology 58:1514–1529
Pan X, Tang J, Chen Y, Li J, Zhang G (2011) Polychlorinated naphthalenes (PCNs) in riverine and marine sediments of the Laizhou Bay area, North China. Environ Pollut 159:3515–3521
Qiao S, Shi X, Zhu A, Liu Y, Bi N, Fang X, Yang G (2010) Distribution and transport of suspended sediments off the Yellow River (Huanghe) mouth and the nearby Bohai Sea. Estuar Coast Shelf S 86:337–344
Sheng Y, Fu G, Chen F, Chen J (2011) Geochemical characteristics of inorganic sulfur in Shijing River, South China. J Environ Monitor 13:807–812
Sheng Y, Sun Q, Bottrell SH, Mortimer RJG, Shi W (2013a) Anthropogenic impacts on reduced inorganic sulfur and heavy metals in coastal surface sediments, north Yellow Sea. Environ Earth Sci 68:1367–1374
Sheng Y, Qu Y, Ding C, Sun Q, Mortimer RJG (2013b) A combined application of different engineering and biological techniques to remediate a heavily polluted river. Ecol Eng 57:1–7
Sullivan LA, Bush RT, Fyfe D (2002) Acid sulfate soil drain ooze: distribution, behaviour and implications for acidification and deoxygenation of waterways. In: Lin C, Melville MD, Sullivan LA (eds) Acid sulfate soils in Australia and China. Science Press, Beijing, pp 91–99
Thode-Andersen S, Jørgensen BB (1989) Sulfate reduction and the formation of 35S-labelled FeS, FeS2 and S° in coastal marine sediments. Limnol Oceanogr 34:793–806
Wallmann K, Hennies K, Klnig I (1993) A new procedure for determining reactive Fe(II) and Fe(III) minerals in sediments. Limnol Oceanogr 38:1803–1812
Wang C, Wang X (2007) Spatial distribution of dissolved Pb, Hg, Cd, Cu and As in the Bohai Sea. J Environ Sci-China 19:1061–1066
Xue Y, Wu J, Ye S, Zhang Y (2000) Hydrogeological and hydrogeochemical studies for salt water intrusion on the South Coast of Laizhou Bay, China. Groundwater 38:38–45
Zhang R, Zhang G, Zheng Q, Tang J, Chen Y, Xu W, Zou Y, Chen X (2012) Occurrence and risks of antibiotics in the Laizhou Bay, China: impacts of river discharge. Ecotox Environ Safe 80:208–215
Zhao Z, Tang J, Xie Z, Chen Y, Pan X, Zhong G, Sturm R, Zhang G, Ebinghaus R (2013) Perfluoroalkyl acids (PFAAs) in riverine and coastal sediments of Laizhou Bay, North China. Sci Total Environ 447:415–423
Zhong G, Tang J, Zhao Z, Pan X, Chen Y, Li J, Zhang G (2011) Organochlorine pesticides in sediments of Laizhou Bay and its adjacent rivers, North China. Mar Pollut Bull 62:2543–2547
Zhu M, Liu J, Yang G, Li T, Yang R (2012) Reactive iron and its buffering capacity towards dissolved sulfide in sediments of Jiaozhou Bay, China. Mar Environ Res 80:46–55
Zhu M, Chen L, Yang G, Fan C, Li T (2014) Kinetic characterization on reductive reactivity of iron(III) oxides in surface sediments of the East China Sea and the influence of repeated redox cycles: implications for microbial iron reduction. Appl Geochem 42:16–26
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Grant No.: 41373100 and 40906045).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sheng, Y., Sun, Q., Shi, W. et al. Geochemistry of reduced inorganic sulfur, reactive iron, and organic carbon in fluvial and marine surface sediment in the Laizhou Bay region, China. Environ Earth Sci 74, 1151–1160 (2015). https://doi.org/10.1007/s12665-015-4101-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-015-4101-8