Abstract
In this study, arsenic concentration of Haraz River water at 20 stations and relative risk and hazard levels regarding ingestion and dermal exposure routes are evaluated. Furthermore, the quantitative threat caused by the consumption of Rainbow trout muscle from the area is also analyzed. The concentration of arsenic increases from upstream areas towards the downstream estuarine zone with a substantial rise in the central part. Arsenic-containing drainage discharged from the Central Alborz coal mine, hot spring spas, as well as municipal (Amol city) and agricultural (numerous rice paddies) land uses that become denser towards downstream are considered as major pollution sources. The inhabitants are not exposed to a significant hazard or risk regarding dermal exposure. However, for the oral ingestion exposure route, all 20 samples present hazard quotient values greater than unity and risk values greater than one in ten thousand. The results show that if the river water is used for drinking, a high-risk status would be imposed on consumers. Finally, the concentration of arsenic in muscle tissues of ten Rainbow trout fish samples was found to range from 0.48 to 1.30 μg/kg of dry weight which is below the allowed daily intake. However, if we consider that lots of other constituents in the total daily intake within the study area contain arsenic, estimated values may be interpreted as a trigger for further health threats.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recent industrial and agricultural development has resulted in a remarkable increase in pollution loads imposed by toxic metals, which are a significant environmental hazard for invertebrates, fish, and humans (Uluturhan and Kucuksezgin 2007; Mzoughi and Chouba 2012; Kargar et al. 2012; Serbaji et al. 2012; Ogundiran et al. 2012). Having the potential to be strongly accumulated and bioconcentrated in sediments and aquatic food chains, toxic metals may easily result in sub-lethal effects or even deaths in local fauna populations (Megeer et al. 2000; Jones et al. 2001; Almeida et al. 2002 ; Xu et al. 2004). As conservative and inert pollutants, they always have the potential to enter water column in response to slight changes in water bodies and consequently to threat the related ecosystems (Wilcock 1999; Chow et al. 2005; Olivares-Rieumont et al. 2005; Hope 2006; Daniel and Prabhakara Rao 2012; Conceição et al. 2013; Bu-Olayan and Thomas 2013). One of the most important toxic elements that have been proved to be hazardous to humans, animals, and plants is arsenic. Both anthropogenic and geopogenic activities are responsible for arsenic dissipation within the environment. However, geopogenic resources, rather than anthropogenic ones, are responsible for the arsenic contamination of water bodies around the world (Burger et al. 2002; Öztürk et al. 2009; Andjelkovic et al. 2013; Singh et al. 2014). The major natural source of metals/metalloids including arsenic in aquatic systems is considered to be weathering of soils and rocks (Zeynali et al. 2009). Among different forms of arsenic, inorganic compounds are supposed to have the most adverse effects on freshwater aquatic species.
The more water-soluble compounds are usually more toxic and more likely to have systemic effects than the less-soluble compounds, which are more likely to cause chronic pulmonary effects if inhaled. Water-soluble inorganic arsenic compounds are absorbed through the gastrointestinal tract (>90 %) and lungs; distributed primarily to the liver, kidney, lung, spleen, aorta, and skin and are mainly excreted in the urine at rates as high as 80 % in 61 h following oral dosing (Crecelius 1977; US 1984; ATSDR 1989).
A great variety of media like food, water, air, and soil are involved in human exposure to arsenic. Regarding food media, fish and other seafood account for the majority of total arsenic exposure (Yi et al. 2011; Dsikowitzky et al. 2013). Arsenic compounds are bioaccumulated by different aquatic species and consequently transferred to humans within the food chain (Korn et al. 2010; Saei-Dehkordi and Fallah 2011). Due to higher trophic levels and also as a remarkable and common element of the human diet, fish are considered a suitable subject for investigation of arsenic bioaccumulation in aquatic bodies (Burger et al. 2002).
Rainbow trout (Oncorhynchus mykiss) is a Pacific trout species and belongs to the Salmonidae family (Fallah et al. 2011). They survive in cold, clear and well-oxygenated lakes, rivers, and streams within a temperature range of 0 °C to over 25 °C. However, the ideal temperature is estimated to be between 13 and 15.5 °C. Due to its rapid growth and high nutritional value, Rainbow trout is widely farmed in many countries around the world (Gall and Crandell 1992). Besides, it is the main freshwater fish species farmed in northern Iran. Its farming started in 1959 in Iran, and production has increased remarkably over recent decades (Fallah et al. 2011).
Quantitative risk and hazard analysis make it possible to have access to a spatial and temporal view on the severity of contaminants’ adverse effects on ecosystems. A lot of research has addressed the risk levels relevant to chronic exposure to arsenic-contaminated water and fish species (Donohue and Abernathy 1999; Koch et al. 2001; Liu et al. 2009; Phan et al. 2010; Muhammad et al. 2010).
In the current study, arsenic concentration in surface water in one of the most significant southern Caspian Sea basins (Haraz River) has been determined and the relative potential risk and hazard levels regarding ingestion and dermal exposure routes are evaluated. Furthermore, regarding the numerous Rainbow trout fish farms within the basin, a similar analysis is implemented through consumption of this fish by humans.
Study area
Being considered as one of the most significant southern Caspian Sea basins, the Haraz River basin stretches from the northern Alborz valleys to the southern coasts of the Caspian Sea in the Mazandaran province of Iran. The overall length of the main stream and drainage area of the basin are estimated to be 185 km and 5,100 km2, respectively. Namarestagh, Shirkola, Razan, and Chelorud are among the main tributaries that feed the river.
Generally, the central and southern parts of the study area comprise super giant Paleozoic and Mezozoic lime, dolomite, and shale deposits. According to seismology, geothermal and volcanic activity, and uplift rates, the asthenosphere must be shallower than normal in the central Iranian plateau region (Hassanzadeh 1994). The immediate basement in the Alborz Mountains is a folded and thrust-faulted passive-margin sedimentary sequence of carbonate, siliciclastic, and volcanic rocks that ranges in age from Cambrian to Eocene (Davidson et al. 2004). Jurassic passive margin deposits are represented by the clastic (sandstone-shale) Shemshak Formation, and the Lar and Delichi carbonates. The Eocene Karadj Formation comprises submarine tuffs and is suggestive of the onset of active continental margin magmatism (Dehghani and Makris 1984).
Coal-rich layers are observed to a large extent especially in central parts of the basin. This area has been well known as a rich source of minerals from time immemorial. Lots of mines (coal, limestone, sand, and gravel, etc.) have been excavated during recent decades among which the Central Alborz Coal Mine is internationally recognized. Magmatic activity through volcanic deposits has formed hydrothermal springs in central parts of the basin. Arsenic may easily be released into the water column through exposure of spoil and sulfide ores to atmospheric oxygen and moisture and carried downstream by river flow. Such a process is postulated where hydrothermal springs enter the river stream.
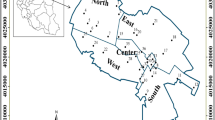
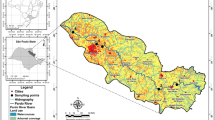
A variety of water uses like drinking, industrial, recreation, and agriculture are practised within the basin. However, fish farming along the main river, recreation through hot spring spas in central parts, and irrigation of rice paddies in downstream plains are considered as major water use options. The study area as well as the water and fish sampling stations are shown in Fig. 1.
Materials and methods
Sample collection
Surface water was collected using a 2-liter plastic water sampler. Samples were acidified using concentrated nitric acid (5 mL of HNO3/L of water sample), stored in polyethylene bottles, transported to the laboratory, and filtered through a 0.45-micron filter. The concentration of dissolved arsenic was determined in the filtered water. Rainbow trout fish were collected from ten selected fish farming stations (Fig. 1). The fish were caught using nylon fishing nets and preserved in an ice box during transportation to the laboratory. Similar sized male fish were chosen to minimize any bias. A pre-cleaned stainless scalpel was used for fish dissection. Fish muscles were rinsed with deionized water (Milli-Q, 18.2 MX/cm), frozen at −70 °C and freeze dried before being ground and subjected to metal analysis (Onsanit et al. 2010).
Metal analysis
Fish muscle digestion was conducted according to the method described by Onsanit et al. (2010). In short, 0.1 gram of fish muscle was digested in 3 mL of ultra-pure HNO3 at 80 °C for 48 h. In order to digest lipids, 0.5 mL HClO4 and 0.5 mL H2O2 were added to the mixture. Having been cooled and diluted to specified volumes with ultra-pure water, the solution was centrifuged to collect the supernatant for arsenic analysis. In order to control the accuracy of analysis, International certified standard reference material of muscle tissue (SRM 2976, National Institute of Standards and Technology NIST) was digested in a similar way as the fish muscle. The results showed a recovery rate of 85–110 % for arsenic concentration. Arsenic concentration in water samples was analyzed for by inductively coupled plasma mass spectrometry (ICP-MS, PerkinElmer, Elan 9000). Furthermore, some samples were analyzed in triplicate and a reference solution of arsenic was also checked using Standard Methods to avoid measurement errors.
Risk assessment
Chronic daily intakes (CDI) through ingestion exposure route for non-carcinogenic cases for adults and children are calculated through Eqs. 1 and 2, respectively,
On the other hand, Eqs. 3 and 4 estimate the age-adjusted chronic daily intakes for carcinogenic approach:
For the dermal exposure route, carcinogenic and non-carcinogenic CDIs are calculated using Eq. 5:
In order to evaluate the quantitative risk and hazard threats caused by fish consumption, Eq. 6 is taken into consideration:
And finally, Eqs. 7 and 8 estimate the excess lifetime cancer risk (ELCR) and the relevant hazard quotient (HQ), respectively,
Input parameters for exposure assessment, risk and hazard analysis are indicated in Table 1.
Results
The arsenic concentrations in water samples collected from 20 stations along the river is shown in Fig. 2. The lowest concentration (29 μg/l) was found at Station two, the highest concentration was recorded at Station eighteen while the mean value is 110 μg/l. Reported values may be considered extremely high when compared to the maximum concentration of arsenic in the Kampong Cham watershed of Cambodia of 2.37 μg/l (Phan et al. 2010) or in Lake Awassa and Koka of Ethiopia of 3 μg/l (Dsikowitzky et al. 2013).
As seen the mean concentration of arsenic in the central and northern parts of the basin is somewhat higher than that in the southern areas. This may be due to northward transport and enhanced sorption/desorption of arsenic-containing suspended sediments in northern areas where heavier loads of local pollution are discharged into the river.
In order to run exposure assessments, the arsenic concentration in water samples was investigated. Oral and dermal ingestion were considered as possible exposure routes for water media while ingestion was selected as the sole route for rainbow trout (a nutritional medium). For carcinogenic and non-carcinogenic effects of arsenic, exposure analysis was carried out separately to estimate both potential risks and hazards. Accordingly, chronic daily intakes for both cases were calculated (Eqs. 1 and 3).The excess lifetime cancer risks (Eq. 7) and relative hazard quotients (Eq. 8) through oral ingestion of water were estimated. For hazard quotient analysis, three different scenarios (child, adult, and adjusted) were included to cover a more detailed scope. The results obtained are listed in Table 2.
Generally, an increasing pattern is observed in HQ and ELCR values from southern to northern stations. In order to have a more detailed view of critical areas, the spatial distribution of hazard and risk values are shown in Figs. 3 and 4, respectively.
For threats caused by arsenic, the dermal exposure route is not at all as important as oral ingestion. However, because of the abundance of rice paddies flooded by river water and also hot spring spas with international touristic interests, the dermal exposure route is also taken into consideration. To assess the dermal exposure route in contact with water as the medium, the relative chronic daily intakes for non-carcinogenic (Eq. 2) and carcinogenic (Eq. 5) cases and the respective hazard quotients (Eq. 7) and Excess lifetime cancer risks (Eq. 8) were calculated and are shown in Table 3. In a similar manner to the oral ingestion route, the three different cases of child, adult, and adjusted are considered here.
As seen in Table 3, the hazard quotient values in all three different cases are below unity and indicate no significant hazard. However, ELCR values change from 3.27E−06 to 1.40E−05.
The spatial distribution of arsenic risk caused by dermal exposure within the study area is shown in Fig. 5. Furthermore, colonies of rice paddies and hot spring spas are also considered.
Ten fish farms were selected for the determination of arsenic in muscle tissues of farmed rainbow trout (Fig. 1). The mean concentrations of arsenic were used for human health risk assessment. For default values for average body weight of target persons, the daily consumption rate of fish, exposure frequency, and duration as indicated in Table 1, the relevant chronic daily intakes were calculated (Table 4).
Allowed daily intake (ADI) of inorganic arsenic, calculated from the provisional tolerance weekly intake (PTWI) set by the Joint Food and Agriculture Organization/World Health Organization Expert Committee on Food Additives is considered to be 2.14 (μg/kg bw/day) (FAO 2008). In comparison to the ADI, chronic daily intakes in all ten samples show lower values. Accordingly, no health risk would threaten the people consuming such fish from designated farms. However, fish sampled from downstream farms show CDI values around three times higher than those sampled from upstream farms.
The arsenic concentrations in the Rainbow trout muscle tissues of this study are compared to some similar ones in the literature in Table 5.
Data obtained from a literature survey showed that arsenic concentrations in the above fish muscles varied widely depending on the site where the animals were caught. With an average As concentration of 1.2 mg/kg dry weight, the fish sampled in this study manifest the highest concentrations after the cases studied by Svobodova et al. (2002) and Harkabusová et al. (2009).
Conclusions
The concentration of arsenic in Haraz River water and the attendant excess lifetime cancer risk and hazard levels are evaluated. Furthermore, the threats caused by consumption of Rainbow trout from the area are also taken into consideration. The concentration of arsenic showed an increase from upstream mountainous areas towards the downstream estuarine zone. A similar distribution pattern for organic (Nasrabadi et al. 2011) and other inorganic pollutants (Nasrabadi et al. 2010a, b; Mohseni-Bandpei and Yousefi 2013; Karbassi et al. 2008) has been observed in the study area. Such a pattern seems to be reasonable if we consider the activities of the Central Alborz coal mine and hot spring spas for tens of years in the central and lower areas of the basin.
In comparison to 10 ppb which is indicated as the guideline value for arsenic in drinking water by the World Health Organization (World Health Organization 2008), US EPA and Institute of Standards and Industrial Research of Iran (ISIRI 1053), all water samples are evaluated to be risky for drinking. The highest arsenic concentration and consequently risk and hazard values occur at Stations 12, 17, and 20. Long-term operation of the Central Alborz coal mine as well as the services offered by tourist hot spring spas in central parts of the basin make up the arsenic-rich drainage discharged to the river stream. Such phenomena may be addressed as the major reasons to select Station 12 as a critical case. On the other hand, centralization of municipal (Amol city) and agricultural (numerous rice paddies) land uses in northern parts of the basin compared to southern zones explains the higher risk levels in the four stations 17–20.
HQ values greater than unity are considered as hazardous cases for human health. All HQs by the dermal exposure route show values far below unity. However, for the oral ingestion route, the reverse is the case; the present values for all 20 samples are greater than 1 and around 20 percent of the cases show values even greater than ten. In comparison to similar studies (Kavcar et al. 2009; Muhammad et al. 2010), the severity of non-carcinogenic effects that threaten human health is highly significant.
Risk values higher than one in a million [10−6] are generally regarded to be unacceptable by the US EPA. However, according to less strict guidelines the acceptable level is one in ten thousand [10−4] World Health Organization (2008). For the oral ingestion route, around 65 percent of exposed individuals have experienced Excess lifetime cancer risks greater than 10−3, while the minimum risk value among the remainder of 35 % is evaluated to be 6.07E−04. The results show that if the river water is used for drinking purposes, a high-risk status would be imposed on consumers. Dermal exposure to such water, however, seems not to be equally dangerous; only 35 % of the total cases show risk values slightly greater than 10−5 and the lowest estimated value is 3.27E−06.
Finally, the ten Rainbow trout fish samples obtained from fishing farms along the river which were analyzed for arsenic in their muscle tissues show values from 0.48 to 1.30 μg/kg of dry weight. Although the estimated chronic daily intakes of arsenic through consumption of such farmed fish show values less than the ADI, if we bear in mind that lots of other media are present in the total daily intake of arsenic in the study area, such values may be interpreted as a trigger for further health threats.
References
Almeida JA, Diniz YS, Marques SFG, Faine IA, Ribas BO, Burneiko RC, Novelli EIB (2002) The use of oxidative stress responses as biomarkers in Nile Tilapia (oreochromis niloticus) exposed to in vivo cadmium contamination. Environ Int 27:673–679
Andjelkovic I, Manojlovic D, Skrivanj S, Pavlovic BM, Amaizah NR, Roglic G (2013) As(III) and As(V) sorption on MnO2 synthesized by mechano-chemical reaction from aqueous phase. Int J Environ Res 7(2):395–402
ATSDR (Agency for Toxic Substances and Disease Registry) (1989) Toxicological profile for arsenic. agency for toxic substances and disease registry, US Public Health Service, Atlanta, GA. ATSDR/TP, 88/02
Bu-Olayan AH, Thomas BV (2013) Effect of trace metals levels in wastewater discharges, sediment and euchelus asper in kuwait marine environment. Int J Environ Res 7(3):779–784
Burger J, Gaines KF, Boring CS, Stephens WL, Snodgrass J, Dixon C (2002) Metal levels in fish from the Savannah River: potential hazards to fish and other receptors. Environ Res 89:85–97
Chow TE, Gaines KF, Hodgson ME, Wilson MD (2005) Habitat and exposure modeling for ecological risk assessment: a case study for the raccoon on the Savanah River Site. Ecol Model 189:151–167
Conceição FT, Navarro GRB, Silva AM (2013) Anthropogenic influences on Cd, Cr, Cu, Ni, Pb and Zn concentrations in soils and sediments in a watershed with sugar cane crops at São Paulo State, Brazil. Int J Environ Res 7(3):551–560
Crecelius EA (1977) Changes in the chemical speciation of arsenic following ingestion by man. Environ Health Perspect 19:147–150 (Cited in US EPA, 1984)
Daniel R, Prabhakara Rao AVS (2012) An efficient removal of arsenic from industrial effluents using electro-coagulation as clean technology option. Int J Environ Res 6(3):711–718
Davidson J, Hassanzadeh J, Berzins R, Stockli DF, Bashukooh B, Turrin B, Pandamouz A (2004) The geology of Damavand volcano, Alborz Mountains, Northern Iran. Geol Soc Am Bull 116:16–29
Dehghani GA, Makris J (1984) The gravity field and crustal structure of Iran. Neues Jahrbuch fur Geologie und Palaontologie Abhandlungen 168:215–229
Donohue JM, Abernathy CO (1999) Exposure to inorganic arsenic from fish and shellfish. In: Chappell WR, Abernathy CO, Calderon RL (eds) Arsenic exposure and health effects. Elsevier, Oxford, pp 89–98
Dsikowitzky L, Mengesha M, Dadebo E, Eduardo C, Carvalho V, Sindern S (2013) Assessment of heavy metals in water samples and tissues of edible fish species from Awassa and Koka Rift Valley Lakes, Ethiopia. Environ Monit Assess 185:3117–3131
Fallah AA, Saei-Dehkordi S, Nematollahi A, Jafari T (2011) Comparative study of heavy metal and trace element accumulation in edible tissues of farmed and wild rainbow trout (Oncorhynchus mykiss) using ICP-OES technique. Microchem J 98:275–279
FAO (2008) Food security statistics: food consumption. Statistics division. Food and agriculture organization of the United Nations. http://www.fao.org/es/ESS/faostat/foodsecurity/index_en.htm
Gall GAE, Crandell PA (1992) The rainbow trout. Aquaculture 100:1–10
Harkabusová V, Mach aráčková B, Čelech ovs ká O, Vitoulová E (2009) Determination of arsenic in the rainbow trout muscle and rice samples, Czech. J Food Sci 27:404–406
Hassanzadeh J (1994) Consequence of the Zagros continental collision on the evolution of the central Iranian plateau. J Earth Space Phys 21:27–38
Hope BK (2006) An examination of ecological risk assessment and management practices. Environ Int 32(8):983–995
Jones I, Kille P, Sweeney G (2001) Cadmiun delays grouth hormone expression during rainbow trout development. J Fish Biol 59:1015–1022
Karbassi AR, Nouri J, Mehrdadi N, Ayaz GO (2008) Flocculation of heavy metals during mixing of freshwater with Caspian Sea water. Environ Geol 53:1811–1816
Kargar M, Khorasani NA, Karami M, Rafiee GH, Naseh R (2012) An investigation on As, Cd, Mo and Cu contents of soils surrounding the Meyduk tailings dam. Int J Environ Res 6(1):173–184
Kavcar P, Sofuoglu A, Sofuoglu S (2009) A health risk assessment for exposure to trace metals via drinking water ingestion pathway. Int J Hyg Environ Health 212:216–227
Koch I, Reimer KJ, Beach A, Cullen WR, Gosden A, Lai VWM (2001) Arsenic speciation in fresh-water fish and bivalves. In: Chappell WR, Abernathy CO, Calderon RL (eds) Arsenic exposure and health effects IV. Elsevier, Oxford, pp 115–123
Korn MGA, Dos Santos GL, Rosa SM, Teixeira LSG, De Oliveira PV (2010) Determination of cadmium and lead in cetacean Dolphinidae tissue from the coast of Bahia state in Brazil by GFAAS. Microchem J 96:12–16
Liu Y, Zheng B, Fu Q, Meng W, Wang Y (2009) Risk assessment and management of arsenic in source water in China. J Hazard Mater 170:729–734
Megeer JC, Szebedinszky C, McDonald DG, Wood CM (2000) Effect of chronic sublethal exposure to waterborne Cu, Cd, or Zn in rainbow trout 1: ionoregulatory disturbance and metabolic costs. Aquat Toxicol 50(3):231–243
Mohseni-Bandpei A, Yousefi Z (2013) Status of water quality parameters along Haraz River. Int J Environ Res 7(4):1029–1038
Muhammad S, Shah MT, Khan S (2010) Arsenic health risk assessment in drinking water and source apportionment using multivariate statistical techniques in Kohistan region, Northern Pakistan. Food Chem Toxicol 48:2855–2864
Mzoughi N, Chouba L (2012) Heavy metals and PAH assessment based on mussel Caging in the North Coast of Tunisia (Mediterranean Sea). Int J Environ Res 6(1):109–118
Nasrabadi T, Nabi Bidhendi GR, Karbassi AR, Mehrdadi N (2010a) Partitioning of metals in sediments of the Haraz River (Southern Caspian Sea basin). Environ Earth Sci 59:1111–1117
Nasrabadi T, Nabi Bidhendi GR, Karbassi AR, Mehrdadi N (2010b) Evaluating the efficiency of sediment metal pollution indices in interpreting the pollution of Haraz River sediments, southern Caspian Sea basin. Environ Monit Assess 171(1–4):395–410
Nasrabadi T, Nabi Bidhendi GR, Karbassi AR, Grathwohl P, Mehrdadi N (2011) Impact of major organophosphate pesticides used in agriculture to surface water and sediment quality (Southern Caspian Sea basin, Haraz River). Environ Earth Sci 63:873–883
Ogundiran MB, Ogundele DT, Afolayan PG, Osibanjo O (2012) Heavy metals levels in forage grasses, leachate and lactating cows reared around lead slag dumpsites in Nigeria. Int J Environ Res 6(3):695–702
Olivares-Rieumont S, de la Rosa D, Lima L, Graham DW, D’Alessandro K, Borroto J, Martínez F, Sánchez J (2005) Assessment of heavy metal levels in Almendares River sediments-Havana City, Cuba. Water Res 39:3945–3953
Onsanit S, Ke C, Wang X, Wang KJ, Wang WX (2010) Trace elements in two marine fish cultured in fish cages in Fujian province, China. Environ Pollut 158(5):1334–1342
Öztürk M, Özözen G, Minareci O, Minareci E (2009) Determination of heavy metals infish, water and sediments of Avsar Dam Lake in Turkey. Iran J Environ Health, Sci Eng 6:73–80
Phan K, Sthiannopkao S, Kim KW, Hung Wong M, Sao V, Hashim JH, Mohamed Yasin MS, Aljunid SM (2010) Health risk assessment of inorganic arsenic intake of Cambodia residents through groundwater drinking pathway. Water Res 44:5777–5788
Risk Assessment Information System (RAIS) (2009) USEPA (Electronic data base). http://www.rais.ornl.gov/
Saei-Dehkordi SS, Fallah AA (2011) Determination of copper, lead, cadmium and zinc content in commercially valuable fish species from the Persian Gulf using derivative potentiometric stripping analysis. Microchem J 98:156–162
Serbaji MM, Azri C, Medhioub K (2012) Anthropogenic contributions to heavy metal distributions in the surface and sub-surface sediments of the northern coast of Sfax, Tunisia. Int J Environ Res 6(3):613–626
Singh SK, Ghosh AK, Kumar A, Kislay K, Kumar C, Tiwari RR, Parwez R, Kumar N, Imam MD (2014) Groundwater arsenic contamination and associated health risks in Bihar, India. Int J Environ Res 8(1):49–60
Svobodova Z, Elechovska O, Machova J, Randak T (2002) Content of arsenic in market-ready rainbow Trout (Oncorhynchus mykiss). Acta Vet BRNO 71:361–367
Uluturhan E, Kucuksezgin F (2007) Heavy metal contaminants in Red Pandora (Pagellus erythrinus) tissues from the Eastern Aegean Sea, Turkey. Water Res 41:1185–1192
US EPA (1984) Health assessment document for arsenic. Office of health and environmental assessment, environmental criteria and assessment office, Research Triangle Park, NC. EPA, 600/8-32-021F
Wilcock DN (1999) River and inland water environments. In: Nath B, Hens L, Compton P, Devuyst D (eds) Environmental management in practice, 3, p 328
World Health Organization (2008) Guidelines for drinkingwater quality. 3rd edn, Incorporating the first and second addenda, vol 1, Recommendations, p 515
Xu YJ, Liu XZ, Ma AJ (2004) Current research on toxicity effect and molecular mechanism of heavy metals on fish. Marine Sci 28(10):67–70
Yi Y, Yang Z, Zhang S (2011) Ecological risk assessment of heavy metals in sediment and human health risk assessment of heavy metals in fishes in the middle and lower reaches of the Yangtze River basin. Environ Pollut 159:2575–2585
Zeynali F, Tajik H, Asri-Rezaei S, Meshkini S, Fallah AA, Rahnama M (2009) Determination of copper, zinc and iron levels in edible muscle of three commercial fish species from Iranian coastal waters of the Caspian Sea. J Anim Vet Adv 8:1285–1288
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nasrabadi, T., Abbasi Maedeh, P., Sirdari, Z.Z. et al. Analyzing the quantitative risk and hazard of different waterborne arsenic exposures: case study of Haraz River, Iran. Environ Earth Sci 74, 521–532 (2015). https://doi.org/10.1007/s12665-015-4058-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-015-4058-7