Abstract
The Capiibary catchment, SE Paraguay, forms part of the Guarani Aquifer System and is intensively used for cashcrop agriculture (soy bean, wheat, maize), with two or more harvests per year. The aim of this study was to investigate the effects of no-till agriculture with frequent herbicide and fertilizer applications on groundwater quality under subtropical climate. Water samples taken from 81 wells showed rather low nutrient (e.g. nitrate) concentrations, probably due to the high humus content of the no-till soils and the prevalent climatic conditions which allow constant microbial recycling of nutrients. The denitrificaton potential of the aquifer is, however, small. Further analysis in seven wells showed no indication of pesticides in groundwater. This is probably attributable to a combination of the effects of no-till agriculture and the subtropical climate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Paraguay is a largely agrarian country. Agriculture contributes around 30 % to its gross domestic product, employs one-third of the labour force and contributes 80 % to its export revenues (Berry 2010). In the southeast of the country, its most fertile region, soil and climate conditions allow two or sometimes three harvests per year of cash crops such as soy beans, corn and wheat. Large-scale application of fertilizers and pesticides is common. This has raised concerns on potential negative impacts on groundwater quality, the main drinking water source. The situation in SE Paraguay, however, cannot be simply compared to the situation in temperate climates where such negative effects are well documented. Therefore, aspects of soil type, climate and agricultural practices need to be assessed. This was one of the tasks of the Paraguayan–German cooperation project PAS-PY (Sustainable management and protection of groundwater in Paraguay).
The catchment of the Capiibary river
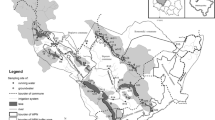
The catchment of the Capiibary river (971 km2), located in the Itapúa province, was chosen as representative study area for high-intensity agriculture under subtropical climate (Fig. 1). Climate, soil type and agriculture in the neighbouring Brazilian federal state Paraná and the Argentinean province Misiones are very similar to the situation in the Capiibary catchment. The findings presented here should therefore be representative for these regions as well. Combined, these regions are an important contributor to the international agricultural commodities markets, especially for soy bean, wheat and corn.
About 75 % of the Capiibary catchment are being used for agriculture, while 22 % are forested. The remaining 3 % are rivers, urban areas and roads. Water supply for the 30,000 people living in the catchment solely depends on groundwater. The average annual precipitation rate is around 1,800 mm/a, single rainfall events can yield up to 60 mm in 1 h. The climate is warm with an average annual temperature of 21 °C.
Hydrogeology: the Guarani Aquifer System
The southeast of Paraguay is part of the trans-boundary Guarani Aquifer System (GAS) which is shared by Brazil, Argentina, Paraguay and Uruguay (Araújo et al. 1999; Amore 2011; Schmidt and Vassolo 2011; Hirata et al. 2011). The main aquifer is an Aeolian sandstone of Triassic to Jurassic age (Scherer 2000). In Paraguay it is called Misiones Formation and Botucatu Formation in Brazil. Its porosity reaches up to 20 % (Hirata et al. 2011) and wells thus generally display good yields. The Misiones sandstone is commonly overlain by up to several hundreds of metres of tholeiitic flood basalts from the early Cretaceous, the Alto Paraná Formation (Comin-Chiaramonti et al. 1997, 1999, 2007). The basalts act as confining layer for the sandstone, but themselves form a fractured aquifer of lower yield. The underlying Independencia Formation and the crystalline basement are of low and very low hydraulic conductivity, respectively (Schmidt and Vassolo 2011).
In about 60 % of the Capiibary catchment, the Misiones sandstone is covered by tholeiitic basalt, while in the rest it is exposed (Fig. 2). The soils formed from the sandstones have both low field capacity and nutrient content. They are therefore mostly used as grazing pastures. The fine-grained soils, ultisols or oxisols, commonly called Terra Rossa (red earth) formed from the basalt, on the other hand, are considered to be amongst the most fertile in South America, due to their favourable soil structure, water retention capacity and (initial) nutrient content. Kubota et al. (2005) measured average saturated hydraulic conductivities of 1 × 10−4 cm/s for Paraguayan Terra Rossa soils after 10 years of no tillage (soy bean–wheat) and 1 × 10−3 cm/s for virgin forest soils. Clay contents range between 20 and 80 %, with a mean of around 50 % (Kubota et al. 2005). Soil erosion by wind and heavy rainfall is a problem for these fine-grained soils in the undulated catchment.
For a Brazilian part of the GAS, Gómez et al. (2010) derived a recharge rate of 1.3 and 8.1 % of annual precipitation for the flood basalt and the sandstone, respectively. This would correspond to 23.4 and 146 mm/a, respectively, at an annual rainfall rate of 1,800 mm. Also for a Brazilian part of the GAS, Rabelo and Wendland (2009) report recharge rates of 215 mm/a for basalt and 465 mm/a for sandstone (at 1,370 mm/a rainfall) which seem unreasonably high. According to Gómez et al. (2010), a small proportion of water from the basalt percolates to the sandstone.
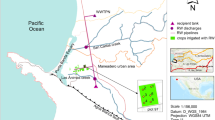
Groundwater recharge rates in the Capiibary catchment were obtained from river baseflow measurements. Therefore, the flow rate of the Capiibary river was tracked close to its outlet to the river Paraná. Additionally, the discharge of two tributaries was studied close to their confluence with the Capiibary river (Fig. 1). One of them, the Arroyo Cerro has a catchment comprised almost exclusively of basalt, the other, the Arroyo Capiibary Chico, predominantly of sandstone. We obtained recharge rates of 83 mm/a for the basalt, 120 mm/a for the sandstone and 110 mm/a for the entire catchment. Similar values were obtained by Schmidt and Vassolo (2011) from the calibration of a numerical model.
Agriculture in the Capiibary catchment
Historically, the local indigenous people lived as hunter-gatherers and used slash-and-burn agriculture. In the early 1700s, Jesuit missionaries founded large collective farms, the so-called Misiones which fell into decline after the expulsion of the Jesuits in 1767 (Livi-Bacci and Maeder 2004; Jackson 2008). European settlers reached the region around the year 1900. Initially, their agriculture was based on subsistence farming and perennial crops such as yerba mate (Ilex paraguariensis) and tung (Vernicia fordii). Large-scale cash crop farming began in the 1960s, resulting in rampant clearing of forests between 1970 and 2000 (Weisskoff 1992; Huang et al. 2006, 2009). The cleared forest soils experienced significant losses of organic matter and biological activity (Table 1). Additionally, conventional agriculture employing ploughs showed serious disadvantages under the subtropical climate. The high rainfall rates caused severe erosion, especially immediately after ploughing, when the soil was bare. To overcome these problems no-till farming was introduced to the Capiibary catchment in the mid 1980s. Today, it is applied in more than 90 % of all the farmed area (Derpsch et al. 2010).
No-till agriculture is based on the concept of avoiding soil disturbances induced by ploughing. The new crop is planted directly into the remains of the previous crop. The main advantages are:
-
less wind and rain erosion due to constant plant cover (Blanco-Canqui et al. 2009; Leys et al. 2010),
-
build-up of soil organic matter (Table 1) due to continuous incorporation of plant residues and lower mineralisation due to less exposure to atmospheric oxygen (Riezebos and Loertz 1998; Tiscareno Lopez et al. 2004; Carneiro Amado et al. 2006),
-
better retention of nutrients in the soil (Angle et al. 1989, 1993; Bundy et al. 2001; Kimmell et al. 2001; Daverede et al. 2003; Blanco-Canqui et al. 2009),
-
higher soil water retention due to less evaporation and lower soil temperature (Lal 1973; Derpsch et al. 2010),
-
less outwash of pesticides through surface run-off (Shipitalo and Owens 2006; Shipitalo et al. 2008),
-
lower cost for machinery (ploughs) and fuel.
The gradual compaction of soil is a possible disadvantage (Vazquez et al. 1989; Kubota et al. 2005). The most important disadvantage is, however, the lack of weed control through ploughing. Instead, herbicides have to be applied in regular intervals. The most important herbicide in the Capiibary catchment is glyphosate which was designed for application with genetically modified soy beans. In no-till plots, the burrows of ground-living animals are not destroyed and may act as preferential pathways for the infiltration of contaminants, especially pesticides (Shirmohammadi et al. 1989; Isensee et al. 1990; Steenhuis et al. 1990; Edwards et al. 1992, 1993; Levanon et al. 1993; Gish et al. 1995; Dao 1995; Shipitalo and Edwards 1996; Gaston and Locke 1996; Smith et al. 1996; Ogden et al. 1999; Gjettermann et al. 2004; Stoddard et al. 2005; Cullum 2009).
Today, soy beans, wheat, maize, sunflowers and sorghum are the most important crops (in this order) in the Capiibary catchment. Agriculture is fully mechanized and conducted on large fields. Fertilizer is usually applied twice per year (for each crop) at dosages ranging from 60 to 250 kg per hectare (Table 2). The most common fertilizer ingredient is phosphate which is used for all types of crops. Nitrogen is applied in all crops except soy beans, which can fix atmospheric nitrogen (Watanabe et al. 2006). Potassium is the third most important fertilizer component but is not always applied. Composition and dosages of fertilizers vary considerably and the cooperative Colonias Unidas could only provide a total annual application of around 10,000 tons in the catchment. We were not able to deduce the individual mass of phosphate, nitrogen and potassium from this figure.
Available data for pesticides were more comprehensive. About 450 tons of herbicide were used in the Capiibary catchment in 2008 (Fig. 3), of which 97 % was glyphosate [N-(phosphonomethyl)glycine]. It is usually applied two, sometimes three times per year, at dosages of 2–3 l per hectare. It is not used with sunflower. Atrazine is sometimes used in cases of emergency with corn and sorghum, at a dosis of 1.5 kg/ha. About 25,000 l of fungicides were also used in 2008, including priori extra (mixture of azoxystrobin and cyproconazole), carbendazim and lancer. This was complemented by about 15 tons of the organophosphate insecticides cypermethrin (synthetic pyrethroid) and acephate.
Methods
Sales figures for fertilizer and pesticides were provided by the local agricultural cooperative, Cooperativa Colonias Unidas, Hohenau, for the year 2008. As most of the local farmers are members and other local vendors are small in comparison, we can safely assume these figures to be representative. We did, though, discount agricultural areas outside the catchment served by the cooperative.
Water samples were taken from 81 active water wells between November 2008 and August 2010, in each case using the installed pump. Some of the wells take water from the sandstone, some from the basalt, many from both (Fig. 4). Well depths usually vary between 60 and 200 m. Some wells outside the catchment were included due to their identical geology. The parameters such as pH, temperature, oxidation–reduction potential, dissolved oxygen and electrical conductivity were measured in the field using a WTW 350i device (WTW GmbH) installed in a flow cell (UIT GmbH, Germany). Cation and trace element samples were filtered (0.45 µm) and stabilised in the field by the addition of ultrapure nitric acid (65 %, Merck). Cations were analysed using ICP-OES (Spectro Ciros CCD, Spectro, USA). Dissolved inorganic carbonate species were determined by automatic titration using a Schott Titroline alpha plus. Ion chromatography (Dionex ICS-3000, USA) was used to analyse the remaining anions. The analytical results for main ions and trace elements are presented in Electronic Supplementary Material 1.
It should be noted that all wells are open boreholes, with casing usually installed only in the upper 20–30 m. Groundwater samples may thus represent a mix from the entire uncased borehole length. A greater depth thus only indicates higher proportions of deeper groundwater in the sample, but does not exclude the presence of shallow groundwater. Many wells tap both the basalt and the sandstone, while the percentage coming from each formation is unknown. A distinction of the two formations in the diagrams 5–10 was thus not feasible.
Analysis of pesticides and metabolites in groundwater was performed by a specialised laboratory (SOFIA GmbH, Berlin, Germany), accredited according to ISO/IEC 17025:2005. A total of 598 components, covering all common herbicides, insecticides and fungicides (and their metabolites) actually in use in the catchment, were analysed. Additionally, many persistent pesticides commonly used in preceding decades (e.g. Lindane, DDT) were included. The list of analysed substances can be found in Electronic Supplementary Material 2. The sample preparation and analysis techniques are summarized in Table 3. Samples were taken in 2009 from seven wells during regular groundwater sampling. Additionally, three surface waters were sampled before and after sowing, in September and November 2009, at the locations of the discharge measurements (Fig. 4). Split samples were sealed into glass and plastic bottles, as for example Paraquat and Diquat tend to adsorb onto glass surfaces. Samples were kept cool (4 °C) and in the dark prior to analysis.
River discharge was measured monthly from 2008 to 2010 using an Ott ADC (Ott Hydrometrie GmbH) following EN ISO 748 (2007).
Results and discussion
Groundwater quality: natural background
Figure 5 shows that both pH and electrical conductivity (EC) increase with depth. The increase in EC must be attributed to water–rock interactions. This is evidenced by the concentrations of sodium, calcium and bicarbonate which show an increase with depth, while the concentration of the conservative anion chloride (ca. 0.9 mg/l) remains more or less constant (Figs. 6, 7). The parallel increase of calcium, magnesium and bicarbonate with depth (Figs. 6c, d, 7c), accompanied by an increase of pH (Fig. 5), strongly suggests the dissolution of carbonate phases to be the dominant reaction of water–rock interaction. The correlation coefficients of calcium (R 2 = 0.97), magnesium (R 2 = 0.73) and bicarbonate (R 2 = 0.82) concentrations with electrical conductivity are a clear indication of the relative importance of this reaction. Calcite dissolution was also postulated as one of the main reactions for the equivalent Brazilian Botucatu aquifer by Meng and Maynard (2001).
With the exception of the very deep samples, redox potentials are relatively high and dissolved concentrations of iron and manganese are generally low (Fig. 8), indicating oxic conditions throughout the water column. This, in turn, indicates the absence of reactive organic matter which could otherwise create a distinct redox zonation. The presence of high amounts of reactive organic matter in Aeolian sandstones and basalt is unlikely for both.
Dissolved silicon concentrations generally increase with depth and thus with rising pH and mineralisation (Fig. 9). This indicates the dissolution of a silicate phase. The most probable candidate is detrital alkali feldspar from the Misiones sandstones, as also postulated for the Brazilian Botucatu aquifer by Meng and Maynard (2001) and Hirata et al. (2011). The increase of sodium (Fig. 6b) with depth cannot be explained by dissolution of minerals from the tholeiitic Alto Paraná basalts, which contain mostly phases dominated rather by calcium than sodium, e.g. pyroxenes and calcium-rich feldspar. On the other hand, calcium liberated during such weathering is probably “lost” in the background of calcium from calcite dissolution, while sodium from albite dissolution remains visible. Potassium shows no significant correlation with depth (Fig. 6a) and mineralisation (R 2 = 0.52). Potassium feldspar dissolution is thus improbable although the signal might be blurred by potassium input from fertilizers.
The general hydrochemical characteristics of the Capiibary groundwater are very similar to the results presented by Meng and Maynard (2001); Sracek and Hirata (2002) and Gastmans et al. (2010a, b) for Brazilian parts of the GAS. Meng and Maynard (2001) and (Gastmans et al. 2010a, b) also invoke calcite and feldspar dissolution with increasing residence times to be the dominating processes. The sometimes elevated fluoride concentrations in deeper wells in Brazil (Meng and Maynard 2001), however, were not found in the Capiibary catchment. The residence times in our case were probably not sufficiently long to attain equilibrium with fluorite. Here, the mean and maximum fluoride concentrations are 0.1 and 0.4 mg/l, respectively. Deep groundwaters here and in the Brazilian part of the GAS show elevated pH (>9.0) and are of the NaHCO3-type, indicating a repetitive cycle of calcite dissolution, followed by removal of calcium and replacement by sodium through ion exchange (Sracek and Hirata 2002; Gastmans et al. 2010a, b).
The deep high pH waters display a relative decrease of dissolved silicon concentrations (Fig. 9, blue symbols), which indicates the precipitation of a solid silicate phase, possibly analcime, as described by Hirata et al. (2011). This is probably related to the higher concentrations of sodium in the deeper parts of the aquifer, released by cation exchange. According to Dove and Rimstidt (1993), elevated alkali concentrations can significantly accelerate silicate precipitation kinetics.
Agricultural impacts on groundwater quality: nutrients
Elevated concentrations of nitrate, potassium, phosphate and chloride in groundwater are common in areas of high-intensity agriculture, especially in temperate climates (e.g. Spalding and Exner 1993; Canter 1996; Houben et al. 2001; Burow et al. 2010; Puckett et al. 2011). The nitrogen concentrations in groundwater of the Capiibary catchment, however, are surprisingly low. Due to the oxidizing redox conditions, the bulk of the nitrogen is present as nitrate and ammonium and nitrite concentrations are small. 75 % of all samples have nitrate concentrations below 10 mg/l, and 95 % have below 20 mg/l (Fig. 10). Not a single sample exceeds the Paraguayan drinking water limit of 45 mg/l for nitrate which is even lower than the WHO limit of 50 mg/l. This can probably be attributed to agricultural practices, soil type and climatic conditions (Fig. 11). No-till agriculture accumulates organic matter in the topsoil and, at the same time, prevents mineralisation of humus. This prevents conversion of nitrogen bound in organic matter to nitrate (Savard et al. 2010). The warm climate allows year-round biological activity of plants and soil microorganisms which take up most available nitrogen. Therefore, little nitrate is available to be washed out into groundwater, even though the soy bean plots have positive nitrogen balances (Watanabe et al. 2006). Another fact worth mentioning is the absence of use of animal manure due to relatively low animal stocking.
Watanabe et al. (2006) analysed soil nitrate concentrations under very similar pedological and agricultural conditions in Eastern Paraguay. They found 0.8–2.5 mg/l nitrate in pasture plots and of 21.5 mg/l in soy bean–wheat plots, both 90 cm below surface. The corresponding ammonium concentrations were 0.08–0.22, and 0.01–0.89 mg/l, respectively. Assuming full oxidation of ammonium, the maximum nitrate concentration from a soy bean-wheat plot would be around 25 mg/l. It should be noted that these concentrations are not necessarily the net input concentrations to groundwater, as deep-rooting plants may extract water from this depth. Nevertheless, the maximum nitrate concentrations in near-surface groundwater in the Capiibary catchment are in the same range (Fig. 7d). The highest potassium and chloride concentrations also occur in the upper 100–150 m of the groundwater column (Figs. 6a, 7a).
The oxic redox conditions in the aquifer (Fig. 8), which indicate low contents of organic matter, indicate that the heterotrophic nitrate reduction potential of the aquifer is probably rather low. Sulphate concentrations are also very low throughout the water column, indicating that there is a low or no autotrophic nitrate reduction potential via pyrite oxidation (Houben et al. 2001). The denitrification capacity of the aquifer is thus generally low and small inputs may thus persist.
The concentration of phosphate, a component of almost all fertilizer applications in the catchment, has a mean of 0.3 mg/l (excluding 10 values below the detection limit of 0.03 mg/l). Phosphate mobility in systems rich in iron oxides, such as the Terra Rossa soils, is usually quite limited due to adsorption (e.g. Guzman et al. 1994). A few samples show somewhat elevated concentrations (>1.0 mg/l), but these were exclusively found in wells located in urban zones and thus most probably related to wastewater input from cesspits and phosphate-containing detergents. High prevalence of faecal bacteria in the same wells hint in the same direction.
Concentrations of the trace elements cadmium and uranium, commonly associated with phosphate fertilizers (e.g. Taylor 1997), were analysed in 48 samples. Concentrations of cadmium are all below 0.05 μg/l and of uranium all are below 2 μg/l. Compared to the drinking water limit of 10 μg/l, both are thus not of hygienic concern. The same applies to arsenic, all 48 samples show concentrations below 0.5 μg/l.
Concentrations of pesticides and their metabolites in groundwater
Groundwater from seven selected wells was sampled and analysed for pesticides and metabolites (Table 4, ESM 2). All of them were located in agricultural areas, six of them in soils derived from basalt. The results unanimously showed no traces of current and previously used pesticides and metabolites in groundwater (Table 4). The absence of the more persistent pesticides used in the past, may be attributed to absorption, degradation or complete outwash. The absence of the most common pesticide, glyphosate and its main metabolite AMPA, can be explained by its characteristic properties. It sorbs well to organic matter, iron and aluminium oxides (see references in Vereecken 2005; Borggaard and Gimsing 2008), all of which are highly abundant in the Terra Rossa soils. The continuous microbial activity of the subtropical no-till soil also aids in limiting its propagation as glyphosate is readily degraded microbially (Selim et al. 2003; Vereecken 2005; Borggaard and Gimsing 2008).
A study executed by the Paraguayan Ministry of Agriculture (MAG) and the German Corporation for Technical Cooperation (GTZ) 1996/97 in agricultural plots near Obligado, Capiibary catchment, came to similar findings (Jansen 1999). They analysed the concentration of glyphosate and 2,4-D in plant matter and soil material immediately after pesticide application (1 or 2 days later) on soy bean and wheat plots and, again, 5 months later, prior to the harvest. Significant pesticide concentrations (up to 1.89 mg/kg glyphosate and 0.85 mg/kg 2,4-D) were found immediately after application only in the plant litter (mulch). Minor concentrations of glyphosate (0.03 mg/kg) were found in one case in the top soil (0–10 cm). Deeper soil layers (20–30 cm) showed no detectable pesticide concentrations. Before the harvest, no traces of pesticides were found, neither in the soil nor in the plant matter. This underlines the importance of the soil for the retention and degradation of pesticides.
This absence of pesticides in groundwater does not, however, rule out possible mobilisations in the future. Transport towards groundwater would become more probable with glyphosate applications on prewetted soil, followed by heavy rainfall, leading to a fast outwash through preferential flow paths (Vereecken 2005).
Three surface waters were analysed for pesticides and metabolites in September and November 2009, that is, before and after sowing and herbicide application. In the first sampling campaign, the complete pesticide spectrum was analysed, in the second only glyphosate and AMPA were considered. Both campaigns showed no concentrations above detection limits. The limited surface run-off from no-till plots is probably one of the explanations for this (Shipitalo and Owens 2006; Shipitalo et al. 2008). The so-called “living screens”, plantations of sugar cane or elephant grass around agricultural plots, often several metres high, may have aided as well (Fig. 11). They are intended to diminish erosion of soil and transport of pesticide spray by wind. The cooperative has also installed several spring-fed water filling stations for spray equipment, all equipped with an absorbing filter bed for spills. They have helped to eliminate the practice of filling and washing equipment in the rivers. These technical measures rely on conscious farmers. In the Capiibary catchment this is ensured through training by the cooperative.
Conclusions
Despite the very intensive agriculture, the chemical groundwater quality in the Capiibary catchment is generally good. This is in stark contrast to temperate climates, where high fertilizer dosages are often reflected by elevated nitrate concentrations in groundwater. The subtropical climate of SE Paraguay is one key parameter to explain this difference: nutrients are continuously recycled due to soil temperatures which allow constant microbiological activity and prevent mineralisation of fixed organic to mobile inorganic nitrogen. Additionally, the accumulation of organic matter in no-till plots prevents the mobilisation of nitrate into groundwater. At least under favourable conditions, this also limits the outwash of pesticides towards rivers and groundwater.
While the hydrochemical buffering capacity of the soil zone is high, the denitrification potential of the aquifer, both heterotrophic and autotrophic, is quite low so that ultimately any nitrate outwash penetrating the root zone may penetrate far.
Since high-intensity agriculture in the Capiibary catchment started around 30 years ago, the concentrations of common agricultural contaminants in rivers and groundwater may still be on the rise and should be tracked in intervals of around 5 years for the whole catchment and annually for selected monitoring wells.
References
Amore L (2011) The Guarani aquifer: from knowledge to water management. Int J Water Res 27(3):463–476
Angle JS, Gross CM, McIntosh MS (1989) Nitrate concentrations in percolate and groundwater under conventional and no-till Zea mays watersheds. Agric Ecosyst Environ 25(4):279–286
Angle JS, Gross CM, Hill RL, McIntosh MS (1993) Soil nitrate concentrations under corn as affected by tillage, manure, and fertilizer applications. J Environ Qual 22:141–147
Araújo L, Franca A, Potter P (1999) Hydrogeology of the Mercosul aquifer system in the Paraná and Chaco-Paraná Basins, South America, and comparison with the Navajo-Nugget aquifer System, USA. Hydrogeol J 7:317–336
Berry A (2010) Losing ground in the employment challenge—the case of Paraguay. Transaction Publishers, New Brunswick p 340
Blanco-Canqui H, Stephenson RJ, Nelson NO, Presley DR (2009) Wheat and sorghum residue removal for expanded uses increases sediment and nutrient loss in runoff. J Environ Qual 38:2365–2372
Borggaard OK, Gimsing AL (2008) Fate of glyphosate in soil and the possibility of leaching to ground and surface waters: a review. Pest Manag Sci 64:441–456
Bundy LG, Andraski TW, Powell JM (2001) Management practice effects on phosphorus losses in runoff in corn production systems. J Environ Qual 30:1822–1828
Burow KR, Nolan BT, Rupert MG, Dubrovsky NM (2010) Nitrate in groundwater of the United States, 1991–2003. Environ Sci Technol 44(13):4988–4997
Canter LW (1996) Nitrates in groundwater. CRC Press, Boca Raton p 265
Carneiro Amado TJ, Bayer C, Conceição PC, Spagnollo E, Costa de Campos BH, da Veiga M (2006) Potential of carbon accumulation in no-till soils with intensive use and cover crops in Southern Brazil. J Environ Qual 35:1599–1607
Comin-Chiaramonti P, Cundari A, Piccirillo EM, Gomes CB, Castorina F, Censi P, De Min A, Marzoli A, Speziale S (1997) Potassic and sodic igneous rocks from eastern Paraguay: their origin from the lithospheric mantle and genetic relationships with the associated Parana flood tholeiites. J Petrol 38(4):495–528
Comin-Chiaramonti P, Cundari A, DeGraff JM, Gomes CB, Piccirillo EM (1999) Early cretaceous-tertiary magmatism in eastern Paraguay (western Paraná Basin): geological, geophysical and geochemical relationships. J Geodyn 28:375–391
Comin-Chiaramonti P, Marzoli A, de Barros Gomes C, Milan A, Riccomini C, Velazquez VF, Mantovani MMS, Renne P, Tassinari CCG, Vasconcelos PM (2007) The origin of post-Paleozoic magmatism in eastern Paraguay. Geol Soc Am Spec Pap 430:603–633
Cullum RF (2009) Macropore flow estimations under no-till and till systems. Catena 78(1):87–91
Dao TH (1995) Subsurface mobility of metribuzin as affected by crop residue placement and tillage method. J Environ Qual 24:1193–1198
Daverede IC, Kravchenko AN, Hoeft RG, Nafziger ED, Bullock DG, Warren JJ, Gonzini LC (2003) Phosphorus runoff: effect of tillage and soil phosphorus levels. J Environ Qual 32:1436–1444
Derpsch R, Friedrich T, Kassam A, Hongwen L (2010) Current status of adoption of no-till farming in the world and some of its main benefits. Int J Agric Biol Eng 3(1):1–25
DIN 38407-2 (1993) German standard methods for the determination of water, waste water and sludge; jointly determinable substances (group F); determination of low volatile halogenated hydrocarbons by gas chromatography (F 2)
DIN 38407-22 (2001) Determination of glyphosate and aminomethyl phosphic acid (AMPA) by high performance liquid chromatography (HPLC), post-column derivatization and fluorescence detection (F 22)
Doran JW (1980) Soil microbial and biochemical changes associated with reduced tillage. Soil Sci Soc Am J 44:765–771
Dove P, Rimstidt JD (1993) Silica–water interactions. Rev Miner 29:259–308
Edwards WM, Shipitalo MJ, Owens LB, Dick WA (1992) Rainfall intensity affects transport of water and chemicals through macropores in no-till soil. Soil Sci Soc Am J 56:52–58
Edwards WM, Shipitalo MJ, Owens LB, Dick WA (1993) Factors affecting preferential flow of water and atrazine through earthworm burrows under continuous no-till corn. J Environ Qual 22:453–457
EN ISO 10695 (2000) Water quality—determination of selected organic nitrogen and phosphorus compounds—gas chromatographic methods
EN ISO 748 (2007) Hydrometry—measurement of liquid flow in open channels using current-meters or floats
Gastmans D, Chang HK, Hutcheon I (2010a) Groundwater geochemical evolution in the northern portion of the Guarani Aquifer System (Brazil) and its relationship to diagenetic features. Appl Geochem 25:16–33
Gastmans D, Chang HK, Hutcheon I (2010b) Stable isotopes (2H, 18O and 13C) in groundwaters from the northwestern portion of the Guarani Aquifer System (Brazil). Hydrogeol J 18(6):1497–1513
Gaston LA, Locke MA (1996) Bentazon mobility through intact, unsaturated columns of conventional and no-till Dundee soil. J Environ Qual 25:1350–1356
Gish TJ, Shirmohammadi A, Vyravipillai R, Wienhold BJ (1995) Herbicide leaching under tilled and no-tillage fields. Soil Sci Soc Am J 59:895–901
Gjettermann B, Hansen HCB, Jensen HE, Hansen S (2004) Transport of phosphate through artificial macropores during film and pulse flow. J Environ Qual 33:2263–2271
Gómez AA, Rodríguez LB, Vives LS (2010) The Guarani Aquifer System: estimation of recharge along the Uruguay–Brazil border. Hydrogeol J 18(7):1667–1684
Guzman G, Alcantara E, Barron V, Torrent J (1994) Phytoavailability of phosphate adsorbed on ferrihydrite, hematite and goethite. Plant Soil 159:219–225
Hirata R, Gesicki A, Sracek O, Bertolo R, Giannini PC, Aravena R (2011) Relation between sedimentary framework and hydrogeology in the Guarani Aquifer System in São Paulo state, Brazil. J South Am Earth Sci 31:444–456
Houben GJ, Martiny A, Bäßler N, Langguth H-R, Plüger WL (2001) Assessing the reactive transport of inorganic pollutants in groundwater of the Bourtanger Moor area (NW Germany). Environ Geol 41(3/4):480–488
Huang C, Kim S, Altstatt A, Townshend JRG, Davis P, Song K, Tucker CJ, Rodas O, Yanosky A, Clay R, Musinsky J (2006) Rapid loss of Paraguay’s Atlantic forest and the status of protected areas—a Landsat assessment. Remote Sens Environ 106:460–466
Huang C, Kim S, Song K, Townshend JRG, Davis P, Altstatt A, Rodas O, Yanosky A, Clay R, Tucker CJ, Musinsky J (2009) Assessment of Paraguay’s forest cover change using Landsat observations. Glob Planet Change 67(1–2):1–12
Isensee AR, Nash RG, Helling CS (1990) Effect of conventional vs. no-tillage on pesticide leaching to shallow groundwater. J Environ Qual 19:434–440
ISO/IEC 17025:2005 General requirements for the competence of testing and calibration laboratories
Jackson RH (2008) The population and vital rates of the Jesuit Missions of Paraguay, 1700–1767. J Interdiscip Hist 38(3):401–431
Jansen A-E (1999) Impacto ambiental del uso de herbicidas en siembra directa [Environmental impacts of herbicide use in no-till agriculture]. Report Project Conservación de Suelos [Soil Conservation], Paraguayan Ministry of Agriculture/GTZ, p 43 [unpublished]
Kimmell RJ, Pierzynski GM, Janssen KA, Barnes PL (2001) Effects of tillage and phosphorus placement on phosphorus runoff losses in a grain sorghum–soybean rotation. J Environ Qual 30:1324–1330
Kubota A, Bordon J, Hoshiba K, Horita T, Ogawa K (2005) Change in physical properties of “Terra Rossa” soils in Paraguay under no-tillage. Soil Sci Soc Am J 69:1448–1454
Lal R (1973) No-tillage effects on soil conditions and maize production in western Nigeria. Plant Soil 40:321–331
Levanon D, Codling EE, Meisinger JJ, Starr JL (1993) Mobility of agro-chemicals through soil from two tillage systems. J Environ Qual 22:155–161
Leys A, Govers G, Gillijns K, Berckmoes E, Takken I (2010) Scale effects on runoff and erosion losses from arable land under conservation and conventional tillage: the role of residue cover. J Hydrol 390:143–154
Livi-Bacci M, Maeder EJ (2004) The missions of Paraguay: the demography of an experiment. J Interdiscip Hist 35(2):185–224
Meng SX, Maynard JB (2001) Use of statistical analysis to formulate conceptual models of geochemical behavior: water chemical data from the Botucatu aquifer in São Paulo state, Brazil. J Hydrol 250(1–4):78–97
Ogden CB, van Es HM, Wagenet RJ, Steenhuis TS (1999) Spatial-temporal variability of preferential flow in a clay soil under no-till and plow-till. J Environ Qual 28:1264–1273
Puckett LJ, Tesoriero AJ, Dubrovsky NM (2011) Nitrogen contamination of surficial aquifers—a growing legacy. Environ Sci Technol 45(3):839–844
Rabelo JL, Wendland E (2009) Assessment of groundwater recharge and water fluxes of the Guarani Aquifer System, Brazil. Hydrogeol J 17(7):1733–1748
Riezebos HT, Loerts AC (1998) Influence of land use change and tillage practice on soil organic matter in southern Brazil and eastern Paraguay. Soil Tillage Res 49(3):271–275
Römbke J, Förster B (1997) Untersuchung von Bodenproben zweier Standorte in Paraguay [Analysis of soil samples from two sites in Paraguay]. Report Project Conservación de Suelos [Soil Conservation], Paraguayan Ministry of Agriculture/GTZ, p 44 [unpublished]
Savard MM, Somers G, Smirnoff A, Paradis D, van Bochove E, Liao S (2010) Nitrate isotopes unveil distinct seasonal N-sources and the critical role of crop residues in groundwater contamination. J Hydrol 381:134–141
Scherer CMS (2000) Eolian dunes of the Botucatu Formation (Cretaceous) in southernmost Brazil: morphology and origin. Sedim Geol 137:63–84
Schmidt G, Vassolo S (2011) Untersuchungen zu einem der größten Grundwasservorkommen Südamerikas: Der Guaraní-Aquifer in Paraguay [Investigations of a key groundwater system in South America: The Guaraní Aquifer in Paraguay]. Grundwasser 16(3):187–194
Selim HM, Zhou L, Zhu H (2003) Herbicide retention in soil as affected by sugarcane mulch residue. J Environ Qual 32:1445–1454
Shipitalo MJ, Edwards WM (1996) Effects of initial water content on macropore/matrix flow and transport of surface-applied chemicals. J Environ Qual 25:662–670
Shipitalo MJ, Owens LB (2006) Tillage system, application rate, and extreme event effects on herbicide losses in surface runoff. J Environ Qual 35:2186–2194
Shipitalo MJ, Malone RW, Owens LB (2008) Impact of glyphosate-tolerant soybean and glufosinate-tolerant corn production on herbicide losses in surface runoff. J Environ Qual 37:401–408
Shirmohammadi A, Magette WL, Brinsfield RB, Staver K (1989) Ground water loading of pesticides in the Atlantic Coastal Plain. Ground Water Monit Remediat 9(4):141–146
Smith NJ, Martin RC, St Croix RG (1996) Levels of the herbicide glyphosate in well water. Bull Environ Contam Toxicol 57:759–765
Spalding RF, Exner ME (1993) Occurrence of nitrate in groundwater—a review. J Environ Qual 22:392–402
Sracek O, Hirata R (2002) Geochemical and stable isotopic evolution of the Guarani Aquifer System in the state of São Paulo, Brazil. Hydrogeol J 10(6):643–655
Steenhuis TS, Staubitz W, Andreini MS, Surface J, Richard TL, Paulsen R, Pickering NB, Hagerman JR, Geohring LD (1990) Preferential movement of pesticides and tracers in agricultural soils. J Irrig Drainage Eng 116(1):50–66
Stoddard CS, Grove JH, Coyne MS, Thom WO (2005) Fertilizer, tillage, and dairy manure contributions to nitrate and herbicide leaching. J Environ Qual 34:1354–1362
Taylor MD (1997) Accumulation of cadmium derived from fertilisers in New Zealand soils. Sci Total Environ 208:123–126
Tiscareño López M, Velásquez Valle M, Salinas Garcia J, Báez González AD (2004) Nitrogen and organic matter losses in no-till corn cropping systems. J Am Water Resour Assoc 40(2):401–408
Vazquez L, Myhre DL, Gallaher RN, Hanlon EA, Portier KM (1989) Soil compaction associated with tillage treatments. Soil Tillage Res 13:35–45
Vereecken H (2005) Mobility and leaching of glyphosate: a review. Pest Manag Sci 61:1139–1151
Watanabe T, Shimoda K, Hoshiba K, Horita T (2006) Effects of agro-pastoral systems on nitrogen balance in soil in Colonia Yguazu, Alto Parana, Paraguay. JIRCAS Working Rep 51:73–78
Weisskoff R (1992) The Paraguayan agro-export model of development. World Develop 20(10):1531–1540
Acknowledgments
We thank the Cooperativa Colonias Unidas, Hohenau for providing data and support. This study was funded by the German Federal Ministry of Economic Cooperation and Development (BMZ) through the Paraguayan–German Project 2004.2189 PAS-PY (Manejo sostenible y protección de aguas subterráneas), carried out jointly by the Secretaria del Medio Ambiente (SEAM), Paraguay and the Federal Institute of Geosciences and Natural Resources (BGR), Germany.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Houben, G.J., Eisenkölbl, A., Dose, E.J. et al. The impact of high-intensity no-till agriculture on groundwater quality in the subtropical Capiibary catchment, SE Paraguay. Environ Earth Sci 74, 479–491 (2015). https://doi.org/10.1007/s12665-015-4055-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-015-4055-x