Abstract
Nitrogen is one of the primary elements that causes eutrophication. In recent years, more stringent environmental standards have come into effect, and nitritation processes have been promoted as a way to remove the high nitrogen content of recycle water and piggery wastewater, which have extremely high ammonium nitrogen concentrations. In this study, the effects of different influent ammonium nitrogen concentrations were evaluated with respect to the rate of nitritation. The influent ammonium nitrogen concentrations ranged from 286 to 2,620 mg/L, so the nitritation reactor was operating under fluctuating ammonium nitrogen concentrations during the experimental period. In the experiment, stable nitritation rates were observed under varying ammonium nitrogen concentrations. Methods to control the influent ammonium nitrogen concentrations were assessed for wastewater with fluctuating ammonium nitrogen concentrations. Nitritation was found to be useful process for removing nitrogen from wastewater with high nitrogen content; additionally, nitritation provides an economic advantage over nitrification.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As living standards improve along with economic development, people tend to take a greater interest in environmental issues such as potable water quality and safe water resources. The government of South Korea has set very stringent regulation for the discharge of effluents from municipal wastewater treatment plants (MWTPs), which went into effect in 2012. Conventional secondary treatment processes, including the removal of organic compounds and suspended solids, are not capable of remove enough nitrogen to comply with these new regulations. To address this, newly built MWTPs, as well as many existing ones, have begun to implement a nitrogen removal process that would help to prevent the exhaustion of dissolved oxygen, which causes increased nitrogen concentrations, eventually leading to eutrophication (Seo et al. 2006; Wei et al. 2010; Wu et al. 2010).
The removal of high nitrogen concentrations in recycle water and piggery wastewater will greatly relieve eutrophication problems related to nitrogen (Han et al. 1998). To help retrofit the nitrogen removal processes of existing plants, nitrogen removal processes were tested using recycled water in MWTPs and piggery wastewater (Gil 2006; Jung et al. 2006). Recycle water and piggery wastewater are both very high in ammonium nitrogen (NH4-N). Sludge thickener supernatant, anaerobic digester supernatant, and decanted water in MWTPs are collectively referred to as recycle water. Recycle water returns to the main stream at the MWTP and causes a change in the C/N (carbon/nitrogen) ratio; the high content of nitrogen of recycle water therefore has an adverse effect on the operation of the MWTP (Choi et al. 2003; Gil et al. 2012; Im and Gil 2011b; Li et al. 2004). Piggery wastewater is increasing in quantity and is generally treated at a piggery wastewater treatment plant or a MWTP. As the quantities of produced piggery wastewater increase, the regulatory standards become stricter. Because of the increased volume of water treated at MWTPs and more stringent standards, it is increasingly difficult to adequately treat water with high concentrations of nitrogen.
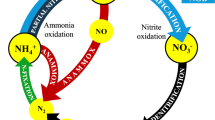
A commonly used method to remove high nitrogen concentrations from wastewater is the biological nutrient removal (BNR) process. Nitrogen is mainly removed through nitrification–denitrification. Nitrification–denitrification converts ammonium nitrogen to nitrate (NO3 −-N) and nitrogen is removed in the form of a gas (N2). This process has been used for many years at a number of MWTPs across the world. Recent studies on nitritation–denitritation have shown that this process is economically beneficial and it is compatible with efficient post processes (Choi et al. 2001; Im and Gil 2011a). Nitritation–denitritation converts ammonium nitrogen to nitrite (NO2 −-N), rather than nitrate, allowing oxygen to be saved during the process. The carbon source is also saved during denitritation. With nitritation, anaerobic ammonium oxidation (ANAMMOX) can be utilized as a post process through which nitrite and ammonium nitrogen are converted to nitrogen gas under anaerobic conditions. This process has been widely regarded as innovative and has been studied by many researchers (Cui et al. 2005; Dircks et al. 1999; Gali et al. 2008; Hellinga et al. 1998; Joss et al. 2011; Li et al. 2010; Wu et al. 2010). Such an advantage process, if applied properly, will be effective in improving the MWTP nitrogen treatment process.
To evaluate the applicability of nitritation to actual plants with recycle water and piggery wastewater, a laboratory-scale reactor was operated in this study. The purpose of this study was to kinetically examine the effects of influent ammonium nitrogen concentrations for optimization of the nitritation process.
Materials and methods
Materials
The reactor used in this study was operated with an anaerobic digester supernatant from the ‘J’ MWTP in Seoul, Korea, piggery wastewater from the ‘Y’ piggery wastewater treatment plant in Yongin, Korea, and effluent from an anaerobic digester at the ‘H’ piggery wastewater treatment plant in Hongsung, Korea. Actual wastewater was fed into the reactor as the influent. To maintain alkalinity, sodium bicarbonate (NaHCO3), which is a component of the influent, was added so that the alkalinity/NH4-N ratio would remain above 7.14. The pH of the influent wastewater was between 7.2 and 8.7. Concentrations of ammonium nitrogen and chemical oxygen demand (COD) were between 186 and 2,620 mg/L, and between 2,830 and 16,840 mg/L, respectively. Of the three influent wastewaters, the highest ammonium nitrogen concentration and COD were highest from the Y piggery wastewater treatment plant (median value 15,955 mg/L), and the lowest was from the anaerobic digester supernatant from the J MWTP (median value 4,920 mg/L). Table 1 shows the characteristics of the influent wastewater used in this study.
Methods
As shown in Fig. 1, the laboratory-scale reactor was designed with a cylindrical acrylic container with a volume of 8 L. To analyze the effects of the influent ammonium nitrogen concentrations on the nitritation process, anaerobic digester supernatant, piggery wastewater and anaerobic digester from piggery wastewater were used. These wastewaters are known to contain high concentration of ammonium nitrogen. And these wastewaters cause negative problems in MWTP. The ammonium nitrogen concentrations of the influent wastewater samples varied. The sludge retention time also varied in accordance with the concentrations of the influent ammonium nitrogen for stable nitritation. A water jacket was installed outside the reactor to maintain a reactor temperature of 35 °C. Air was provided continuously to the reactor to maintain a specific DO concentration for optimal nitritation to occur. A mixer was used in the reactor for complete mixing of the system so that the concentration of the effluent from the reactor would be representative of the concentration inside the reactor. Ammonium nitrogen concentration in the influent and the effluent of the reactor were measured with a DR2800 (HACH) by Nessler’s method. Nitrite and nitrate concentrations were analyzed using ion chromatography (Dionex ICS-1000, Dionex Corp., Sunnyvale, CA, USA), and the pH and DO levels were assessed with a pH meter (Accumet-AB15, Fisher Scientific, Waltham, MA, USA) and a DO meter (YSI-550A, YSI Inc., Yellow Springs, OH, USA). Alkalinity was measured using the titration method (Standard Methods 2320 B), and COD was measured with a closed reflux colorimetry (Standard Methods 5220 D) of the Standard Methods (APHA 1998).
Results and discussion
Operation results
Figure 2 shows the influent ammonium nitrogen, effluent ammonium nitrogen, nitrite, and nitrate concentrations of the reactor. In Fig. 3, the ammonia removal rate (removed ammonium nitrogen/ammonium nitrogen in influent) and nitrite conversion rate (nitrite in effluent/removed ammonium nitrogen) are shown. Through Figs. 2 and 3, overall operation results were confirmed according to change of operation conditions (SRT condition). The periods of reactor operation were divided into stages C1–C5, according to the different ammonium nitrogen concentrations. C1–C5 had ammonium nitrogen concentrations of 2,210–2,620, 1,790–1,930, 880–940, 610–720, and 186–280 mg/L, respectively. In Table 2, the solid retention time (SRT) ranges of influent ammonium nitrogen concentrations, ammonia removal rates, and nitrite conversion rates are summarized for each operational period. To obtain a stable nitritation rate, the reactor was operated at a relatively longer SRT because of the high influent ammonium nitrogen concentrations. Different SRTs were applied to different influent ammonium nitrogen concentrations. In Figs. 2 and 3, the operational results obtained after process stabilization under optimum operational conditions are presented. The highest concentration of ammonium nitrogen was observed in the C1 period; in this period nitrite concentrations in the effluent were higher than those of the other periods. However, this period had the lowest ammonia removal rate.
After the C1 period, nitrite concentrations in the effluent were observed to decrease (C1 median value 919 mg/L to C2 median value 818 mg/L), while the nitrite conversion rate was observed to increase (47.8–52.7 %). This phenomenon was a result of the difference between the ammonium nitrogen concentrations in the influent wastewater for the different time periods, and the high influent ammonium nitrogen concentrations, despite leading to low nitrite conversion rates, caused the nitrite concentration to increase in the effluent. The ammonia removal rate was stable during the entire operational period, and the nitrite conversion rate tended to increase as the operation progressed from C1 to C5. The laboratory nitritation reactor was operated below conditions during the operation periods: temperature 35 °C, DO 2–3 mg/L, pH in laboratory nitritation reactor 7–8, and reactor type CSTR. These results mean that the influent ammonium nitrogen concentration had an effect on the nitrite conversion rate. And optimum SRT conditions for nitritation were shown different values according to influent ammonium nitrogen concentration.
Ammonia removal rates and nitrite conversion rates
Figure 4 shows the ammonia removal rate and nitrite conversion rate with the variation of influent ammonium nitrogen concentrations from 186 to 2,620 mg/L. The ammonia removal rate was stable during the entire operational period. The nitrite conversion rate was found to increase from 40 to 60 % in C1, and continued to increase to over 60 % between periods C2 through C5, reaching 80 % in periods C3 through C5. The nitrite conversion rate tended to increase as the reaction proceeded, reaching a maximum in C5. However, the nitrite conversion rate was observed to vary greatly between C4 and C5, even though the influent ammonium nitrogen concentration was relatively low. These results indicate that stable nitritation requires proper controls to maintain constant operational conditions, such as the control on the ammonium nitrogen concentration of influent wastewater, which will result in highly (about 80 %) efficient nitritation.
Results of the influent ammonium nitrogen concentration
Figures 5 and 6 show the experimental results with different influent ammonium nitrogen concentrations, operational SRTs, ammonia removal rates and nitrite conversion rates. As shown in Fig. 5, high influent ammonium nitrogen concentrations required a longer SRT. As the influent ammonium nitrogen concentration continued to decrease, the required SRT decreased. It was determined that the influent ammonium nitrogen concentration is related to the SRT and the ammonia removal rate. In Fig. 6, the nitrite conversion rate changed for each operational period. The maximum nitrite conversion rate was 78.9 % during the period C5. During the period C1, the minimum nitrite conversion rate of 47.8 % was obtained and the laboratory reactor was operated with the longest SRT and the highest influent ammonium nitrogen concentration. Overall, the nitrite conversion rate was observed to be low, unlike the ammonia removal rate, and it fluctuated even under the same conditions. Nitritation was affected more by the influent ammonium nitrogen concentration than was the ammonia removal. In particular, the SRT required for stable nitritation varied with the fluctuating influent ammonium nitrogen concentrations, which finally led to variable ammonia removal rates and nitrite conversion rates.
Conclusion
-
1.
The nitrite conversion rate was influenced by the influent ammonium nitrogen concentrations more than the ammonia removal rate was.
-
2.
Under constant conditions, the nitrite conversion rate undergoes greater fluctuation than the ammonia removal rate. When removing wastewater with high nitrogen concentrations, nitritation should be conducted under optimum operational conditions to control the high ammonium nitrogen content.
-
3.
The SRT required for stable nitritation varied with the influent ammonium nitrogen concentrations. It is necessary to determine the proper SRT by controlling the ammonium nitrogen concentration of the influent wastewater for stable nitritation.
-
4.
For the useful nitritation, control of influent ammonium nitrogen concentration is required. SRT for the nitritation in high ammonium nitrogen concentration condition is longer than low ammonium nitrogen concentration condition. Therefore, the control of the influent ammonium nitrogen concentration is suggested as one of the methods to reduce the biological reactor size and to increase the treatment efficiency in MWTP. Thus, when nitritation installed in MWTP, control of influent ammonium nitrogen concentration was considered because of high nitrite conversion rate and economical advantage through to reduce biological reactor size.
References
APHA (American Public Health Association) (1998) Standard methods for the examination of water and waste water, 20th edn. American Public Health Association, Washington, DC
Choi JH, Lee YW, Yoon ZH (2001) Removal of concentrated nitrogen from the recycle water by biological nitritation-denitritation. J Korean Soc Environ Eng 23(3):371–381
Choi YG, Kim KD, Kim HJ, Kim YJ, Jung TH (2003) Respirometry for COD fractionation of wastewater. J Korean Soc Water Wastewater 17(4):503–509
Cui YW, Peng YZ, Wang YY, Gan XQ, Ye L (2005) Achieving and maintaining biological nitrogen removal via nitrite under normal conditions. J Environ Sci 17(5):794–797
Dircks K, Pind PF, Mosb KH, Henze M (1999) Yield determination by respirometry—the possible influence of storage under aerobic conditions in activated sludge. Water SA 25(1):69–74
Gali A, Dosta J, Lopez-palau S, Mata-Alvarez J (2008) SBR technology for high ammonium loading rates. Water Sci Technol 58(2):467–472
Gil KI (2006) Nitritation of anaerobic digester supernatant from sludge processing in MWTP. J Korean Soc Water Qual 22(3):540–545
Gil KI, Rho HY, Kim DI, Michael KS (2012) Comparison of bio-P module and the modified bio-P module in the step-feed biological nutrient removal process. Environ Earth Sci 65:929–936
Han DJ, Kang SW, Rim JM (1998) Factors influencing nitrite build-up in nitrification of high strength ammonia wastewater. Korean J Sanit 13(2):128–138
Hellinga C, Schellen AAJC, Mulder JW, van Loosdrecht MCM, Heijnen JJ (1998) The Sharon process : An innovative method for nitrogen removal from ammonium-rich waste water. Water Sci Tech 37(9):135–142
Im JY, Gil KI (2011a) Evaluation of nitritation of high strength ammonia with variation of SRT and temperature using piggery wastewater. J Korean Soc Water Qual 27(5):563–571
Im JY, Gil KI (2011b) Effect of anaerobic digestion on the high rate of nitritation treating piggery wastewater. J Environ Sci 23(11):1787–1793
Joss A, Derlon N, Cyprien C, Burger S, Szivak I, Traber J, Siegrist H, Morgenstern E (2011) Combined nitration-Anammox: advances in understanding process stability. Environ Sci Technol 45(22):9735–9742
Jung JW, Kim SW, Tsuno H (2006) Development of biological filtration process for effective nitrogen removal and its control strategies in tertiary treatment of sewage. J Korean Soc Water Qual 22(2):230–237
Li XM, Yang Q, Zeng GM, Cornelius A, Rosenwinkel KH, Kunst S, Weichgrebe D (2004) Model-based evaluation on the conversion ratio of ammonium to nitrite in a nitritation process for ammonium-rich wastewater treatment. J Environ sci (China) 16(6):1005–1010
Li N, Wang P, Liy Q, Cao H (2010) Microwave enhanced chemical reduction process for nitrite containing wastewater treatment using sulfaminic acid. J Environ Sci 22(1):55–61
Seo IS, Kim HS, Kim YK, Kim JY (2006) Nitrogen and phosphorus removal in domestic wastewater using SBR process with flow changing continuous feed and cyclic draw. J Korean Soc Water Qual 22(2):203–208
Wei YX, Li YF, Ye ZF (2010) Enhancement of removal efficiency of ammonia nitrogen in sequencing batch reactor using natural zeolite. Environ Earth Sci 60:1407–1413
Wu L, Peng C, Zhang S, Peng YZ (2010) Nitrogen removal via nitrite form municipal landfill leachate. J Environ Sci 21(11):1480–1485
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2013R1A2A2A01068579).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Im, J., Gil, K. Effects of the influent ammonium nitrogen concentration on nitrite accumulation in a biological nitritation process. Environ Earth Sci 73, 4399–4404 (2015). https://doi.org/10.1007/s12665-014-3724-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-014-3724-5