Abstract
The sorption of phosphorus (P) was measured in calcareous paddy soils of Iran at room temperature as a function of pH and soil/solution ratio using batch experiments. Seven soils of different properties and five soil/solution ratios, 1:10, 1:25, 1:50, 1:100, 1:150, and eight pHs, 2, 3, 4, 5, 6, 7, 8, 9 were employed for this study. The pH value of these seven soil samples was adjusted to cover a range of 2–9 by addition of 1 M HCl or 1 M NaOH. The sorption of P was affected by the soil solution pH; at low pH the sorption of P was high. The soil/solution ratio was found to have significant effect on the sorption of P. A larger soil/solution ratio, i.e., more soil for a constant mass of solution, resulted in a smaller maximum P sorption value. This result also held true for Freundlich distribution coefficient at most studied pHs. There were significant correlations between P sorption and Freundlich distribution coefficient and pH and soil/solution ratio. The equations obtained can be used to predict the solubility of P and Freundlich distribution coefficient as a function of pH and soil/solution ratio in calcareous paddy soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phosphorus (P) is one of the essential nutrients required for the growth of plants and animals, but long-term applications of organic and inorganic fertilizers have resulted in increase in the P status of soil. Many soils are now considered to be a potential diffuse source of P to surface waters. The high P concentration in water affects plants and can lead to eutrophication (Zhou et al. 2005). The major P sorbents are Fe (hydr) oxides and clay minerals (Hinsinger 2001; Hiemstra and Van Riemsdijk 1999; Gustafsson 2001). In addition, P sorption is influenced by the chemistry of soil solutions, such as pH, ionic strength, concentration of organic ligands and redox potential (e.g., Sah and Mikkelsen 1986; Hinsinger 2001; Kirk 2002; Spiteri et al. 2008). Gustafsson et al. (2012), Jehangir et al. (2012) and Zhou and Zhu (2003), recently showed that the pH and soil/solution ratio can affect P sorption and indicated that the pH is one of the most important factors affecting sorption of P in soils. Aqueous P speciation changes with pH, affecting P sorption on soil surfaces. Gustafsson et al. (2012) stated that the pH value may vary with time and agricultural practices. In addition, sorption of P may be varied during irrigation/drainage and floods (Zhou and Zhu 2003).
In Iran long-term applications of P fertilizers and animal manures have resulted in accumulation of P in the soils (Jalali 2007), increasing the risk of P losses to aquatic ecosystems (Jalali 2009). There have been fewer studies on the P sorption in the paddy soils of Isfahan Province in central Iran. This is an important rice production area, covering 46,000 km2 and soils are calcareous type (i.e., more than 50 % of the soils have a content of total carbonate >10 %) derived from dolomite parent materials. In recent years, the application of P fertilizers to paddy soils in Isfahan has been increasing in order to enhance the per unit area yield of rice. The increasing input of P into agricultural soils in this area has become an important environmental problem.
While many published papers have studied acidification of paddy soils (e.g., Zhang et al. 2013; Wen et al. 2013), accumulation and mobilization of trace elements (Tu et al. 2013; Hundal et al. 2013; Li et al. 2013; Zhong et al. 2012; Rogan et al. 2010; Wu and Zhang 2010), there is limited information on the behavior and availability of applied P in calcareous paddy soils especially when pH and soil/solution ratio changes occur (von Wandruszka 2006; Jalali 2007; Ige et al. 2008; Curtin and Syers 2001; McDowell and Sharpley 2001; McDowell et al. 2003; Zhou et al. 2005; Devau et al. 2011). Understanding the sorption of P in calcareous paddy soils receiving continuous different P forms is essential in order to develop appropriate P management strategies for sustainable agricultural production and environmental protection. At present, only a few studies have incorporated the effect of pH and soil/solution ratio on P sorption predictions. Thus, the objectives of our study were to determine P sorption under different pHs and soil/solution ratios.
Materials and methods
Soil sampling
Twenty-eight surface paddy soil samples were collected from three different localities widely distributed in the Isfahan Province in central Iran (between longitudes 51°20′0.5″−51°30′45.3″E and latitudes 32°21′24.0″−32°32′57.6″N). Information on the study area and soil sampling were provided by Jalali and Hemati (2013) and Jalali and Hemati Matin (2014). The major soil types are Aridisols belonging to great groups Calciargids, Haplocambids, Haplogypsids and Haplosalids (Pirzadeh et al. 2010). From these soil samples, seven soils with different physical and chemical properties were selected (Table 1). Oxalate-extractable P, Fe and Al were determined by 0.5 g of soil after its pH reached 5.5 after shaking with 30 ml of 1 M ammonium acetate (pH 5.5) for 1 h. In some samples, shaking with ammonium acetate was repeated several times until the solution was reached the referred pH. Then the solution was decanted and the soils were dried. After drying, the samples were shaken with 30 ml of 0.175 M ammonium oxalate (pH 3) for 2 h (in darkness). Iron and aluminum concentration were measured with atomic adsorption spectrophotometer (model Varian spectra 220), UV–vis spectrophotometer (model Varian Cary 100), respectively (Mehra and Jackson 1960). Concentration of magnesium in soil solution was measured by titration method (Bower and Wilcox 1965). A P saturation index (PSI) was calculated by dividing the molar concentration of oxalate-extractable P by the sum of the molar concentrations of oxalate-extractable Fe and Al, and multiplying by 100 to convert to percent.
Batch sorption experiments
Batch sorption experiments were performed at room temperature to determine soil sorption capacity under different pHs and soil/solution ratios. In order to obtain the soil/solution ratios of 1:10, 1:25, 1:50, 1:100 and 1:150, a 10 mM CaCl2 solution (25 ml) containing 5, 10, 15, 20 and 30 mg l−1 of P were inserted into a series of 50-ml centrifuge tubes with 2.5, 1, 0.5, 0.25 and 0.16 g of soil. After that the sorption mixtures were agitated for a pre-determined time (24 h) period using horizontal shaker. The 24-h equilibration period was selected based on batch extraction studies by Zhou and Zhu (2003). They found that batch equilibrium was attained at 10−12 h for various extracting solutions. The influence of the aqueous phase pH on P sorption was studied by adjusting the reaction mixture to different initial pH values (2, 3, 4, 5, 6, 7, 8 and 9) and analyzed for residual P after equilibrium contact time. We acidified or alkalized the aqueous phase by adding either acid (1 M HCl) or alkali (1M NaOH). This resulted in a matrix of 7 soils × 5 soil/solution ratios × 8 pH values for 280 unique conditions. There were two samples for each of these conditions resulting in a total of 560 samples. After equilibration, soils were separated from solution by centrifugation followed by filtration. The residual P in the aqueous phase was analyzed colorimetrically using ascorbic acid method of Murphey and Riley (1962). Both Langmuir and Freundlich models were used. While the Freundlich model was very well fitted to the sorption data, the Langmuir model was not fitted well with all data (data not shown). The computer program Visual MINTEQ version 2.30 (Allison et al. 1991) was used to predict saturation indices (SI) and P species in the equilibrium solution concentration when 5 and 30 mg P l−1 under varying pH solution and two soil/solution ratio were applied in the sorption experiments.
Results and discussion
Soil characteristics
Table 1 shows the chemical and physical properties of the soil samples. The average CaCO3 content is about 25 %, while the pH values varied from 7.6 to 9.3. The CEC values from 11.0 to 23.7 cmolc kg−1. Soil organic matter varied from 0.6 to 2.2 %. Based on the texture, soils can be classified as loam, silty clay, silty clay loam and sandy loam.
Influence of pH
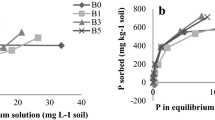
The effect of pH on the sorption of P under different soil/solution ratio is depicted in Fig. 1. In general, the extent of P sorption on the soil showed a decrease as the pH of the aqueous solution increased from 2.0 to 9.0. The sorption of P when 30 mg P l−1 was applied to the soil was used to calculate maximum P sorption (Q 30) and the effect of pH on the mean maximum sorption (mean of seven soils) of P under different soil/solution ratio is indicated in Fig. 2. The average Q30 decreased with increasing pH and reached a maximum in a pH range of 6.0−7.0 and then decreased with further increases in pH under all different soil/solution ratios (Fig. 2). The effect of pH on the mean Freundlich distribution coefficient under different soil/solution ratio is indicated in Fig. 3. Table 2 presents the results of regression equations. In general Kf decreased with increasing pH and less significant correlation was found in comparison with Q30. This could be attributed to the anionic sorption. Németh et al. (1998) stated that the P sorption on soil is determined by the surface charge and the protonation state of P in the bulk solution. As pH increases and surface charge becomes more negative, the sorption of anions like P decreases.
The metal Fe and Al content has been considered to be the main factor that determines sorption capacity, because of the high specific surface of the iron/aluminum (hydr) oxides. In addition, P in calcareous soils would be expected to not only adsorb on minerals, but also interact with the dissolved Ca to form phosphate minerals. Freeman and Rowell (1981) indicated that in calcareous soils, P sorption is greatly controlled by the adsorption and precipitation reactions on Ca-carbonate surfaces. However, the effect of increasing pH on soluble P is dependent on soil mineralogy as well as initial and final soil pH (Erich et al. 2002). In addition to adsorption/desorption processes, solubility and dissolution of P is controlled by mineral equilibria, including the aging and transformation of P minerals over long periods (McDowell et al. 2003; Spiteri et al. 2007).
Soil/solution ratio effect on pH-dependent P sorption
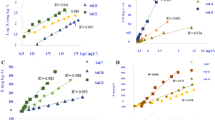
The relationship between the mean Q30 and the soil/solution ratio at different pHs is shown in Fig. 4. The change of the Q30 as a function of soil/solution ratio was similar for the all soils and Fig. 5 indicate the maximum P sorption at different pHs and soil/solution ratios. As it can be seen at each pH, Q 30 decreased with an increase in soil/solution ratios, which is in agreement with recent findings by Zhou and Zhu (2003) and Cucarella and Renman (2009). It has been indicated that P sorption varies with soil/solution ratios (Hope and Syers 1976; Nair et al. 1984). The effect of soil/solution ratio on the mean Freundlich distribution coefficient under different pH is indicated in Fig. 6. In general Kf increased with decreasing soil/solution ratio in all studied pHs except at pHs 4. Several investigators have studied the relationship between Kf and soil/solution ratio, their results were similar to our observations (Puls et al. 1991; Chang et al. 2002). It was indicated when the soil sorption capacity is high in relation to the amount of sorbate present, increasing soil/solution ratio (higher soil concentration), cannot increase the amount of sorption (Harter and Naidu 2001). Correlation coefficients along with parameters of fitted equations were presented in Table 2.
Cucarella and Renman (2009) stated that a variation in the soil/solution ratio may alter either the equilibrium constant or the complex concentration by reaching a new equilibrium. Smaller ratios may lead to higher concentrations of P sorbed to the material, that is, a higher percentage of P removal. Some authors indicated that the smaller the ratio, the longer the time needed to reach equilibrium (Søvik and Kløve 2005; Ádám et al. 2007). Cucarella and Renman (2009) stated that in the field more soil is in contact with the solution and using small soil/solution ratio may cause a big difference between batch tests and field conditions.
Zhou and Zhu (2003) concluded that agricultural irrigation/drainage and floods are disadvantageous to the sorption of P on the soil because it can result in the release of soil P into water. Yin et al. (2002) concluded that due to the effect of soil/solution ratio, higher soil/solution ratios which are approximately close to the field conditions should be used.
Saturation indices and P speciation
Figures 7 and 8 show variation of SI with pH calculated for two soils (only soil 1 and soil 5 were chosen because these soils had maximum differences in physical and chemical properties among the studied soils) at low and high added P and low and high soil/solution ratio. The geochemical speciation indicated that in general all solution samples in two soils were supersaturated with respect to hydroxyapatite (HA, Ca5(PO4)3OH), octacalcium phosphate (OCP, Ca4H(PO4)3·3H2O), β-tricalcium phosphate (TCP, Ca3(PO4)2), Ca3(PO4)2(am2), vivianite (Fe3(PO4)2·8H2O) and undersaturated with respect to dicalcium phosphate dehydrate (DCPD, CaHPO4·2H2O), dicalcium phosphate (DCP, CaHPO4), and magnesium phosphates (MgHPO4·3H2O and Mg3(PO4)2). In general, SI increased with increasing pH and at high added P and low soil/solution ratio, more P minerals were saturated. Thus, the main mechanisms that control adsorption of P in these soils are precipitation of calcium and iron P minerals.
The contribution of different forms of P to the total P concentration in the soil solution differed between the various soil/solution ratio and soil solution pH (see supporting information figures S1 and S2). In general, at low pH most P was present in its H2PO4 − form; however, at higher pH most of the total P was present as HPO4 2− and CaHPO4 (aq). However, in soil 5 at low P added and low soil/solution ratio, most P was present as H2PO4 −, while for soil 1 CaHPO4 (aq) and HPO4 2− were the dominant species. The pH is one of the most important factors affecting the solubility of P in soils. The results indicated that aqueous P speciation changes with pH and soil/solution ratio which affect P sorption soils.
Most previous studies were conducted under conventional experimental conditions and did not consider variation in pH and soil/solution ratio. Characterization of P sorption under varying conditions in calcareous paddy soils provides information about mechanism of P retention and release into the overlying water. The results of present experiments can be used to predict soil P behavior.
Conclusion
The sorption behavior of P on seven paddy soils was investigated at varying soil/solution ratios (1:10, 1:25, 1:50, 1:100, 1:150) and different pH from 2 to 9 using a batch equilibration experiment. The results indicated that the sorption of P was influenced by the pH and soil/solution ratio. The sorption of P on calcareous paddy soils decreased with increasing pH. At low pH values (2−3), the sorption of P increased, while it was decreased with increasing pH. The P sorption was also influenced by soil/solution ratio and more P was sorbed as more water was available. Release and retention of P in paddy soil may affect the water quality and nutrient status of soils. Chemical equilibria with P-containing minerals can control the dissolved P concentration in soil solution and groundwater.
References
Ádám K, Krogstad T, Vråle L, Søvik AK, Jenssen PD (2007) Phosphorus retention in the filter materials shell sand and filtralite P batch and column experiment with synthetic P solution and secondary wastewater. Ecol Eng 29:200–208
Allison J, Brown D, Novo-Gradac DS KJ (1991) MINTEQA2/PRODEFA2, A geochemical assessment model for environmental systems: version 3.0, users manual. US Environmental Protection Agency, Athens, (EPA/600/3-91/021)
Bower CA, Wilcox LV (1965) Soluble salts. In: Black CA (ed) Methods of soil analysis part 2, chemical and microbiological properties. ASA, SSSA, Madison, USA
Chang TW, Wang MK, Lin C (2002) Adsorption of copper in the different sorption/water ratios of soil systems. Water Air Soil Poll 138:199–209
Cucarella V, Renman G (2009) Phosphorus sorption capacity of filter materials used for on-site wastewater treatment determined in batch experiments—A comparative study. J Environ Qual 38:381–392
Curtin D, Syers JK (2001) Lime-induced changes in indices of soil phosphate availability. Soil Sci Soc Am J 65:147–152
Devau N, Hinsinger P, Le Cadre E, Colomb B, Gérard F (2011) Fertilization and pH effects on processes and mechanisms controlling dissolved inorganic phosphorus in soils. Geochim Cosmochim Acta 75:2980–2996
Erich MS, Fitzgerald CB, Porter GA (2002) The effect of organic amendments on phosphorus chemistry in a potato cropping system. Agric Eco Environ 88:79–88
Freeman JS, Rowell DL (1981) The adsorption and precipitation of phosphate onto calcite. J Soil Sci 32:75–84
Gustafsson J (2001) Modelling competitive anion adsorption on oxide minerals and an allophone-containing soil. Eur J Soil Sci 52:639–653
Gustafsson J, Mwamila LB, Kergoat K (2012) The pH dependence of phosphate sorption and desorption in Swedish agricultural soils. Geoderma 189–190:304–311
Harter RD, Naidu R (2001) An assessment of environmental and solution parameter impact on trace-metal sorption by soils. Soil Sci Soc Am J 65:597–612
Hiemstra T, Van Riemsdijk WH (1999) Surface structural ion adsorption modelling of competitive binding of oxyanions by metal (hydr) oxides. J Colloid Interf Sci 210:182–193
Hinsinger P (2001) Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil 237:173–195
Hope GD, Syers JK (1976) Effects of solution: soil ratio on phosphate sorption by soils. J Soil Sci 27:301–306
Hundal HS, Singh K, Singh D, Kumar R (2013) Arsenic mobilization in alluvial soils of Punjab, North-West India under flood irrigation practices. Environ Earth Sci 69(5):1637–1648
Ige DV, Akinremi OO, Flaten D (2008) Evaluation of phosphorus retention equations for Manitoba soils. Can J Soil Sci 88:327–335
Jalali M (2007) Phosphorous status and sorption characteristics of some calcareous soils of Hamadan, western Iran. Environ Geol 53:365–374
Jalali M (2009) Phosphorous concentration, solubility and species in the groundwater in a semi-arid basin, southern Malayer, western Iran. Environ Geol 57:1011–1020
Jalali M, Hemati N (2013) Chemical fractionation of seven heavy metals (Cd, Cu, Fe, Mn, Ni, Pb, and Zn) in selected paddy soils of Iran. Paddy Water Environ 11:299–309
Jalali M, Hemati Matin N (2014) Soil phosphorus forms and their variations in selected paddy soils of Iran. Environ Monit Assess 85:8557–8565
Jehangir H, Bhadha S, Daroub H, Timothy AL (2012) Effect of kinetic control, soil:solution ratio, electrolyte cation, and others, on equilibrium phosphorus concentration. Geoderma 173–174:209–214
Li TQ, Jiang H, Yang X, He ZL (2013) Competitive sorption and desorption of cadmium and lead in paddy soils of eastern China. Environ Earth Sci 68(6):1599–1607
McDowell RW, Sharpley AN (2001) Approximating phosphorus release from soils to surface runoff and subsurface drainage. J Environ Qual 30:508–520
McDowell R, Mahieu N, Brookes P, Poulton P (2003) Mechanisms of phosphorus solubilisation in a limed soil as function of pH. Chemosphere 51:685–692
Mehra OP, Jackson ML (1960) Iron oxide removal from soils and clays by dithionite-citrate systems buffered with sodium bicarbonate. Clay Clay Miner 7:317–327
Murphey J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Nair PS, Logan TJ, Sharpley AN, Sommers LE, Tabatabai MA, Yuan TL (1984) Inter laboratory comparison of a standard phosphorus adsorption procedure. J Environ Qual 13:591–595
Németh Z, Gáncs L, Gémes G, Kolics A (1998) pH dependence of phosphate sorption on aluminum. Corros Sci 40:2023–2027
Pirzadeh M, Afyuni M, Khoshgoftarmanesh A, Schulin R (2010) Micronutrient status of calcareous paddy soils and rice products: implication for human health. Biol Fert Soils 46:317–322
Puls RW, Powell RM, Clark D, Eldred CJ (1991) Effects of pH, solid/solution ratio, ionic strength, and organic acids on Pb and Cd sorption on kaolinite. Water Air Soil Poll 57–58:423–430
Rogan N, Dolenec T, Serafimovski T, Tasev G, Dolenec M (2010) Distribution and mobility of heavy metals in paddy soils of the Koani field in Macedonia. Environ Earth Sci 61(5):899–907
Sah RN, Mikkelsen DS (1986) Sorption and bioavailability of phosphorus during the drainage period of flooded-drained soils. Plant Soil 92:265–278
Søvik AK, Kløve B (2005) Phosphorus retention processes in shell sand filter systems treating municipal wastewater. Ecol Eng 25(2):168–182
Spiteri C, Slomp CP, Regnier P, Meile C, Van Cappellen P (2007) Modelling the geochemical fate and transport of wastewater-derived phosphorus in contrasting groundwater systems. J Contam Hydrol 92:87–108
Spiteri C, Van Cappellen P, Regnier P (2008) Surface complexation effects on phosphate adsorption to ferric iron oxyhydroxides along pH and salinity gradients in estuaries and coastal aquifers. Geochim Cosmochim Acta 72:3431–3445
Tu CL, He TB, Lu XH, Lang YC, Li LB (2013) Accumulation of trace elements in paddy topsoil of the Wudang County, Southwest China: parent materials and anthropogenic controls. Environ Earth Sci 70(1):131–137
von Wandruszka R (2006) Phosphorus retention in calcareous soils and the effect of organic matter on its mobility. Geochem Trans 7:1–8
Wen XJ, Duan CQ, Zhang DC (2013) Effect of simulated acid rain on soil acidification and rare earth elements leaching loss in soils of rare earth mining area in southern Jiangxi Province of China. Environ Earth Sci 69(3):843–853
Wu CF, Zhang LM (2010) Heavy metal concentrations and their possible sources in paddy soils of a modern agricultural zone, southeastern China. Environ Earth Sci 60(1):45–56
Yin Y, Impellitteri CA, You SJ, Allen HE (2002) The importance of organic matter distribution and extract soil:solution ratio on the desorption of heavy metals from soils. Sci Total Environ 15(287):107–119
Zhang CP, Wu P, Tang CY, Tao XZ, Han ZW, Sun J, Liu H (2013) The study of soil acidification of paddy field influenced by acid mine drainage. Environ Earth Sci 70(7):2931–2940
Zhong LY, Liu LM, Yang JW (2012) Characterization of heavy metal pollution in the paddy soils of Xiangyin County, Dongting lake drainage basin, central south China. Environ Earth Sci 67(8):2261–2268
Zhou Q, Zhu Y (2003) Potential pollution and recommended critical levels of phosphorus in paddy soils of the southern Lake Tai area, China. Geoderma 115:45–54
Zhou A, Tang H, Wang D (2005) Phosphorus adsorption on natural sediments: modeling and effects of pH and sediment composition. Water Res 39:1245–1254
Acknowledgments
Four anonymous reviewers made valuable comments on the manuscript. The authors gratefully express their gratitude for their thoughtful and thorough reviews.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jalali, M., Hemati Matin, N. Sorption of phosphorus in calcareous paddy soils of Iran: effects of soil/solution ratio and pH. Environ Earth Sci 73, 2047–2059 (2015). https://doi.org/10.1007/s12665-014-3555-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-014-3555-4