Abstract
Temporal changes in pore-water salinity and metal concentrations were investigated in soils from two sites (residential and wetland areas) located in the Old Bridge Township, NJ, after Hurricane Sandy hit the Northeast of the United States of America. Core and surface soil samples were collected in both the residential and wetland sites and then analyzed by field portable X-ray fluorescence (NITON XL3t-600 series FP-XRF). Pore-water salinity was determined from continuous measurements (every 10 min) of conductivity in a single sampling well installed in the wetland site. One month after Hurricane Sandy, pore-salinity was as high as 27 g/L, but gradually decreased to 15 g/L in approximately 3 months. Then, it increased gradually to 26 g/L 3 months later. High metal concentrations (lead, arsenic, copper, chromium, and iron) were measured in the surface and top 2-cm soil layer in both residential and wetland sites, often exceeding background levels within weeks of Hurricane Sandy. These metal contaminations were interpreted as being associated with storm surge from Hurricane Sandy that caused substantial flooding of the coastal areas by large amounts of seawater, loaded with dissolved metal and adsorbed metals to suspended sediments from the Raritan Bay Slag Superfund site. The changes in salinity in wetland areas indicated the intrusion of seawater, thus providing evidence for metal-contaminated seawater altering the wetland’s geochemistry. The transport and deposition of metal contaminants in the coastal areas by Hurricane Sandy increased the risk of human exposure to these contaminants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hurricane Sandy made landfall on October 29, 2012 in southern New Jersey (NJ). As part of the efforts to investigate the effects of the hurricane, a team was dispatched to conduct measurements in the quadrant area of Fig. 1, which is located in Laurence Harbor, NJ. In the proximity of the measurement locations exists the Raritan Bay Slag Site, located in the Laurence Harbor section of Old Bridge and in Sayreville, Middlesex County, New Jersey. The site is a major source of metal contamination including lead (Pb), arsenic (As), copper (Cu), chromium (Cr), and antimony (Sb) in site sediment, surface water and groundwater, and the site was included in the federal Superfund site list in 2009 (EPA 2012). The potential release and transport of metal contaminants, and subsequent exposure to them by potential receptors, have raised concerns regarding their impacts to the environment and human health. The risk to human health concerned particularly residents in the vicinity of the site, recreational users of the beaches and construction and utility workers. It was also important to measure the variation of the salinity with time as the change in salinity affects ecosystems (Hart et al. 1990; Jolly et al. 2008), especially when systems change from methanogenesis to sulfate reduction (Wang et al. 1996).
Two sites (a residential and a wetland area) in the coastal areas of Old Bridge Town, NJ, were elected for monitoring the potential impact of Hurricane Sandy. Flooding of these areas occurred traditionally from inland flows, bringing freshwater to the low land. However, in October 2012, the storm surge from Hurricane Sandy caused substantial flooding of the NJ coastal areas with large amounts of seawater whose salinity is ~32 g/L. This anomaly of a large volume of water of different chemistry has undoubtedly stressed the coastal ecosystems, and could conceivably lead to substantial alterations in their geochemistry and ecology. It is important to evaluate whether these ecosystem alterations are reversible with time. The rate of these system transformations is equally important as a slow return to a pre-hurricane state could be tantamount to no recovery. This study was directed at (1) monitoring temporal changes in pore-water salinity in the wetland area and metal concentrations in soil samples from wetland and residential areas, and (2) identifying potential trend in coastal ecosystem resilience and recovery. The results indicate that the coastal ecosystems were negatively impacted as a result of metal accumulation and an increase in pore- water salinity due to seawater intrusion. Temporal changes in metal concentrations suggest a partial recovery of the studied sites.
Site description
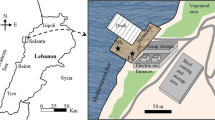
The study took place at Laurence Harbor, Old Bridge Township, NJ (Fig. 1). The studied area has a topographic level of 1.83 m, while the measured water level exceeded 3.35 m during Hurricane Sandy (USGS 2013). As a result, it was completely inundated by large amounts of seawater from the storm surge. The selected area has a particular importance as it lies within the Raritan Bay Superfund Slag Site (EPA 2012). The latter spans approximately 2.10 km in length and consists of the water front area between Marquis’ Creek and the area just beyond the western jetty at the Cheesequake Creek Inlet (Fig. 1). The major stream in the vicinity of the site is the Cheesequake Creek which is a tidal creek that drains tidal wetlands located southwest of the creek outlet and discharges to Raritan Bay between two jetties referred to as the Cheesequake Creek western and eastern jetties (Fig. 1). The contaminants at the site include lead, arsenic, copper, chromium, iron and antimony that are found in site soils, surface water and groundwater.
Materials and methods
Determination of pore-water salinity
Water salinity was measured using a stainless steel single port sampling well (1 m below ground) installed in the wetland area (Fig. 1). A hand auger was first used to drill a hole and then the well was set up in the hole. The well was made of 0.46 × 10.97 m galvanized drive point (J48-12-HomeDepot). It was extended to the surface using galvanized steel pipes and couplings. The drive point was perforated to allow water passing through it. A self-logging Schlumberger CTD-diver was placed at the bottom of the well to record the pressure and electrical conductivity of water at 10-min intervals. The measured electrical conductivity was converted to salinity as described by Fofonoff (1985).
Soil sample collection, preparation and measurements
Soil samples were collected in both residential and wetland areas. A stainless steel core sampler was driven into the soil using a sliding hammer; then, a plastic liner was used to collect each core sample. The core samples were kept on ice in coolers, which were transported to the laboratory at NJIT within 2–3 h. The samples were then placed into fridges and kept at 0 °C. until analysis by field portable X-ray fluorescence (FP-XRF). Prior to XRF measurements, the samples were air-dried and loaded into the sample holders. The measurements were performed using the NITON XL3t-600 series FP-XRF following EPA Method 6200 (EPA 1998) operating either in soil mode (metal concentrations <2 wt%) or mining mode (metal concentrations ≥2 wt%). Field portable XRF is reported as an effective tool for rapid, quantitative assessment of soil metal contamination and for monitoring the efficacy of remediation strategies (Kilbride et al. 2006; Markey et al. 2008; Radu and Diamond 2009). In this study, FP-XRF is applied to determinate the metal concentrations in the surface and subsurface soil. Each element in the periodic table has a unique characteristic X-ray spectrum. Basically, high-energy X-rays or gamma rays (primary source) are used to excite metals in the samples, and their emission characteristics are detected or measured by the instrument (e.g., NITON XRF- XL3t 600 series).
Soil samples in both residential and wetlands were tested for organic matter content based on the weight loss-on-ignition method (e.g., Schulte and Hoskins 2009). The samples were dried for 2 h at 105 °C, and further heated at 550 °C for 1.5 h. Then, the percent weight of soil organic matter loss-on-ignition (SOM-LOI) was calculated. The rationale beyond this test is that the soil organic matter content influences the adsorption/desorption processes of metals.
Results
Temporal changes in pore-water salinity
The pore-water salinity of samples collected in the wetland area after Hurricane Sandy is reported in Fig. 2. This concentration gradually decreased from 27 to ~15 g/L in approximately 3 months. Then, the salinity increased again to 26 g/L 3 months later (Fig. 2). Measurements performed during a week after 15 months of Hurricane Sandy (data not shown for brevity) indicated a gradual decrease again from 22 to 20 g/L, confirming temporal increase and decrease in salinity.
Temporal changes in metal concentrations
The temporal changes in selected metal (lead, arsenic, copper, chromium, iron, and antimony) concentrations are plotted in Figs. 3, 4, 5, 6 and 7, for both the residential and wetland areas investigated during this study. Error bars shown on the figures represent instrumental errors in measurements. Within a week of Hurricane Sandy, the concentration of lead (Pb) in both residential (Fig. 3a) and wetland (Fig. 3b) areas was elevated (~150–300 ppm) in surface sediments (SS) and the top 2-cm soil layer (TL), while remaining ~100 ppm in the middle layer (ML: 14–16 cm) and ~40 ppm in the bottom layer (BL: 28–30 cm). In the residential area (Fig. 3a), the observed concentrations in the SS and TL dramatically dropped to ~30 ppm within days and remained <50 ppm for the last measurement performed a year later. Lead concentration returned rapidly (approximately a month) to values relatively comparable to background concentrations (Fig. 3a) (EPA 2012). However, in the wetland area (Fig. 3b), the concentrations in the SS and TL remained elevated 3 months after the hurricane. Figure 3b also shows that the lead concentration increased with time in the ML and BL, reaching values as high as 335 ppm within a month. After 1 year, the concentrations in the TL, ML and BL were comparable to the upper limit of measured background concentration (193 ppm) (EPA 2012), while the concentration in the SS remained substantially higher (~300 ppm).
Temporal variation in lead (Pb) concentrations at different depths (from surface to 30 cm below ground): a Residential area, and b wetland area. Solid red lines represent reported background concentrations for residential sediment (1.1–6.9 ppm) and wetland sediment (71.6–193 ppm) (EPA 2012). Error bars represent instrumental errors in measurements
Temporal variation in arsenic (As) concentrations at different depths (from surface to 30 cm below ground): a residential area, and b wetland area. Solid red lines represent reported background concentrations for residential resident (0.3–3.1 ppm) and wetland sediment (13.7–49.5 ppm) (EPA 2012). Error bars represent instrumental errors in measurements
Temporal variation in copper (Cu) concentrations at different depths (from surface to 30 cm below ground): a residential area, and b wetland area. Solid red lines represent reported background concentrations for residential sediment (0.4–2.4 ppm) and wetland sediment (42.5–176.0 ppm) (EPA 2012). Error bars represent instrumental errors in measurements
Temporal variation in chromium (Cr) concentrations at different depths (from surface to 30 cm below ground): a residential area, and b wetland area. Solid red lines represent reported background concentrations for residential sediment (1.1–7.8 ppm) and wetland sediment (30.1–84.2 ppm) (EPA 2012). Error bars represent instrumental errors in measurements
Temporal variation in iron (Fe) concentrations at different depths (from surface to 30 cm below ground): a residential area, and b wetland area. Solid red lines represent reported background concentrations for residential sediment (0.1 %) and wetland sediment (1.6–4.1 %) (EPA 2012). Error bars represent instrumental errors in measurements
Arsenic (As) concentrations were much higher in the wetland area (0–45 ppm) (Fig. 4b) than in the residential area (0–24 ppm) (Fig. 4a) despite the observed large variability in the measurements as shown by the instrumental error bars. These concentrations were ~20–45 ppm in all layers within a week of Hurricane Sandy. In the residential area, As concentrations rapidly decreased in all layers within a 3-week period (Fig. 4a). An increase (~10 ppm) was observed in the SS and ML after ~3 months of the hurricane, but the concentrations were low (<3.6 ppm) after 1 year. These values were comparable to reported background concentrations of 0.3–3.1 ppm (EPA 2012) (Fig. 4a). In the wetland areas, As concentrations were higher (>30 ppm) in the SS and TL compared with 12.4 ppm and 20.7 ppm in the ML and BL approximately a week of Hurricane Sandy, respectively. A temporal decrease from 32.6 to 22.7 ppm was observed in the SS. The TL decreased dramatically from 44.6 to 12.2 ppm approximately a week of Hurricane Sandy, then increased to 36.8 ppm within a month, followed a dramatic drop to 11.26 ppm 1 year later. In the ML and BL, As concentrations showed a dramatic drop to 0 ppm a week of Hurricane Sandy, but the values relatively increased with time. Although the large variability and temporal changes in measurements, As concentrations overall returned to values comparable with reported background concentrations (13.7–49.5 ppm) approximately a year of Hurricane Sandy (Fig. 3b).
Copper (Cu) showed concentrations between 0 and ~90 ppm in both residential (Fig. 5a) and wetland (Fig. 5b) areas. In the residential area, the concentrations of Cu were 88.4 ppm in the SS, 54.6 ppm in the TL, 62.6 ppm in the ML, and 0 ppm in the BL within a week of Hurricane Sandy. Then, the concentration dropped to 0 ppm in the SS, TL and ML within 2 weeks despite some variability in the measurements as shown by the instrumental error bars. Except for the ML that showed an increase to 24.1 ppm approximately a month later followed by a decrease, Cu concentrations remained in general between 0 and ~12 pm including the last measurement 1 year later. Copper concentrations at approximately a year of Hurricane Sandy were comparable to reported background concentrations (0.4–2.4 ppm) (EPA 2012). In the wetland area (Fig. 5b), Cu concentrations in the SS decreased from 90.1 ppm a week after Hurricane Sandy to 64.0 ppm 2 months later. It seems to have remained relatively constant as the measurement after 1 year gave 62.4 ppm. The Cu concentration in the TL was 76.9 ppm a week after the hurricane, and decreased to 22.9 ppm within 3 days. It then rebounded to a concentration of 76.5 ppm a month later. After 1 year, the observed concentration was 38.1 ppm in the TL i.e. lower than the measured values at SS. Copper concentration was at 0 ppm in the ML a week after Hurricane Sandy, but a rapid increase occurred within a month to 62.3 ppm, followed by a decrease to 50.5 ppm a year later. In the BL, Cu showed a concentration of 24.2 ppm a week after Hurricane Sandy. The concentration decreased to 0 ppm in 1 month, and increased again to 41.5 ppm a year later. Overall, the concentrations of Cu varied substantially with time in the wetland area, but measurements at approximately a year of Hurricane Sandy indicated that the values were comparable with the lower background concentration (42.5 ppm) (EPA 2012).
Chromium (Cr) concentrations varied between 0 and ~100 ppm in both residential (Fig. 6a) and wetland (Fig. 6b) areas. Some variability was observed in the measurements as shown by the instrumental error bars. In the residential area, the Cr concentrations were as high as 63.4 and 101.7 ppm after a week of Hurricane Sandy in the SS and TL, respectively. These concentrations decreased rapidly to 0 and 36.7 ppm in the SS and TL, respectively. In the ML, the concentration of Cr (26.4 ppm after a week of Hurricane Sandy) increased to 40.8 ppm within a month; then decreased to 0 ppm 1 year later. In the BL, the concentration was at 0 ppm after a month of Hurricane Sandy, but increased at 32.6 ppm approximately a year later. About a year of Hurricane Sandy, the concentration in the TL and ML fell within the range of reported background concentrations (1.1–7.8 ppm). However, the observed values in the SS and BL slightly exceeded the background concentrations. In the wetland area (Fig. 6b), the temporal variations in concentrations were small (37.4 < Cr < 45.3 ppm) in the SS, while significant variations were observed in the other layers. The TL remained at 0 ppm within a month of Hurricane Sandy, but increased to 43.64 ppm approximately a year later. The ML showed a concentration of 36.0 ppm within a week of Hurricane Sandy, but increased to 100.2 ppm within a month followed by a decrease to 40.9 ppm approximately a year later. In the BL, the concentration showed a sequence of abrupt increase and decrease with time, but remained between 0 and 52.2 ppm. About a year of Hurricane Sandy, the measured concentrations in all layers were comparable (~42 ppm) and fell within the range of reported background concentrations (30.1–84.2 ppm) (EPA 2012).
Iron (Fe) concentrations varied from 0 to ~6 % and 0 to ~9 % in the residential (Fig. 7a) and wetland (Fig. 7b) areas, respectively. In the residential area, Fe concentrations were substantially higher in the SS (6.23 %) than the other layers (<4 %) within a week of Hurricane Sandy. In the SS, the concentration abruptly decreased to 0.95 % in a month, but increased at 1.52 % approximately a year later. The TL showed a decrease from 2.97 to 1.91 % a week of Hurricane Sandy, followed by increase to 2.31 % in a month and then remained at 1.89 % approximately a year later. In the ML, temporal changes in Fe concentrations were small as the values remained between 2.68 and 3.67 % within approximately a year of Hurricane Sandy. The concentration in the BL was at 2.43 % a week of Hurricane Sandy, decreased to 1.61 % a month later, then rebounded at 3.04 % 2 months later. A year later, the concentration remained at 3.09 %. Overall, Fe concentrations in the residential area were mostly in the range of 1.52–3.09 %, thus largely exceeding the background concentration of 0.1 (EPA 2012). In the wetland area (Fig. 7b), higher Fe concentrations (~9 %) were measured in the SS and TL compared with ~4.3 % in the ML and BL within a week of Hurricane Sandy. Iron concentrations in the SS decreased from 8.51 % to 6.41 % within a month, but returned to 8.00 % approximately a year later. A dramatic drop from 9.11 to 2.04 % was observed in the TL within a week of Hurricane Sandy, but Fe concentrations increased again to 5.62 % in a month, and remained at 4.70 % approximately a year later. In the ML, Fe concentrations remained between 2.69 and 4.29 % within approximately a year of Hurricane Sandy. In the BL, temporal changes were characterized by an abrupt drop from 4.31 ppm a week of Hurricane Sandy to 1.15 % a month later, followed by a dramatic increase to 5.09 % approximately a year later. In the wetland area, except for the ML, the concentration in all layers approximately a year of Hurricane Sandy exceeded the range of background concentrations (1.6–4.1 %) (EPA 2012).
Antimony (Sb) concentrations remained at 0 ppm for all samples analyzed in this study, except for two samples collected in a month of Hurricane Sandy: a sample from the BL with 18.8 ppm and a sample from SS with 27.2 ppm. These two values seem to be outliers as their corresponding replicate measurements were 0 ppm. For comparison, background concentrations were 0.2 ppm for site-specific soils (concentrations detected only in two samples) and varied from 0.8 to 2 ppm in wetlands, respectively (EPA 2012).
Discussion
The pore-water salinity in the wetland was characterized by an alternating increase and decrease in salinity over several months (Fig. 2). The temporal increase in salinity could be plausibly explained by inundation of the wetland with high salinity water from the Raritan Bay (20–25 g/L) and the Stump Creek (20–22 g/L) during extreme events such as Hurricane Sandy or at a given high tide (mixture of Stump Creek-Bay Shore water). As the high salinity water slowly infiltrated in the ground, the pore-water salinity increased. The temporal decrease in salinity most likely resulted from precipitations (snow and rainfall) which accumulated important amounts of freshwater in the wetland. As freshwater from rainfall and melting snow slowly infiltrated into the ground, the salinity gradually decreased. Several episodes of precipitations and subsequent freshwater filtration in the ground would likely cause a steady decrease in the pore-water salinity until an extreme event causing again the flooding of the wetland by high salinity water. Other complex hydrodynamic interactions involving surface and groundwater could play a role in the observed temporal changes in salinity. For instance, earlier studies have reported the extensive interaction between groundwater and surface water in the site (EPA 2012). In this context, an extensive geochemical monitoring coupled with hydrogeological investigations is needed to quantify and better understand the dynamics of potential ground and surface water interactions, potential intrusion of seawater and subsequent changes in the wetland.
A striking observation is that elevated metal concentrations occurred in both residential and wetland sites studied during this investigation within a week of Hurricane Sandy. These metal concentrations could conceivably be associated with (a) a redistribution of existing metals in the soil profiles and/or (b) an accumulation of metals that were transported from the Raritan Bay Slag Superfund site as a primary source of contaminants. Seawater incursion into the studied sites could potentially induce a rapid mobilization of trace metals from mackinawite-rich sediments (benthic sediments with high concentrations of acid volatile sulfide) as a result of increased ionic strength of seawater-sediment suspensions and highly soluble metal-chloride complexes (Wong et al. 2013). Subsequently, mobilized metals could be redistributed and resorbed along the soil profiles. However, it does not seem that this was the dominant process although acid sulfate soils could be a significant source of metal contamination as they are widely distributed along the US Mid-Atlantic region including New Jersey coastline (Fanning et al. 2010). The effects of seawater on mackinawite sediments in the benthic zone would unlikely result rapidly in highest metal concentrations in surface sediments and the top soil layer as observed in this current study. That the surface sediments and the top 2-cm soil layer showed the highest metal concentrations within a week of Hurricane Sandy rather suggested that sediments loaded with high metal concentration were recently transported and deposited. This increase in metal concentration in the studied sites was interpreted as being mostly resulted from metal-rich water and sediments from run-off and inundations associated with Hurricane Sandy. Based on aerial photographs and chemical data, previous studies have demonstrated the potential release of metals from the Raritan Bay Slag Superfund site into the environment as a result of two mechanisms: erosion of particulates and leaching of metals (USACE 2010; EPA 2012). For instance, toxicity characteristic leaching procedure (TCLP) tests revealed that Pb and selected As values at the site exceeded the criteria to classify material as hazardous waste under the Resource Conservation and Recovery ACT (RCRA). Leachability tests, using a simulated seawater solution to verify the behavior of the slag from potential exposure to seawater, also revealed considerably high levels of leachable Pb (EPA 2012). Thus, existing leaching and chemical data in soils and surface waters support the interpretations that the slag heap could be the primary source causing the metal contamination in the studied residential and wetlands areas. The measured water-level rise at Laurence Harbor, Old Bridge Town, NJ, exceeded 3.35 m during Hurricane Sandy due to a combination of storm surge and astronomical tide (USGS 2013) compared with the site topographic level of 1.83 m (Fig. 8). The studied sites were completely inundated, and the measurements of pore-water salinity (27 g/L) in the wetlands a month after the hurricane confirm seawater intruding landwards. The Raritan Bay Slag Superfund site, located in the vicinity of the studied sites, is highly contaminated with metals (as high as 198,000, 2,400, 4,630, and 3,120 ppm for Pb, As, Cu, and Sb, respectively) (EPA 2012) that could plausibly be transported, as dissolved species or slag particles incorporated in sediments, to the studied sites. The potential migration pathways of metals from the source to the studied sites are illustrated in Fig. 8. The dominant wind and wave direction during the storm was from the northeast. The strong wave action eroded the contaminated sediments close to the seawall slag and displaced them. The transport of the sediments was offshore and in the western direction, following the long-shore current. The contaminated sediments accumulated in a depositional area close to the Cheesequake Creek inlet western jetty that interrupted the long-shore current. As the storm surge water moved inland from the Raritan Bay, it started transporting the suspended sediments into the Cheesequake Creek. The latter started to fill up with water and its capacity to contain the flow was reduced until the water eventually overtopped the creek banks. Due to site topography, the contaminated water moved to the adjacent wetland site while crossing the studied residential site. The wetland acted as a detention basin and stocked large amounts of the overtopped volume of water containing dissolved metals along with the contaminated sediments.
Illustration of potential metal migration pathways from the Raritan Bay Slag Superfund site until reaching our investigated sites (modified from EPA 2012). Solid contour lines (in black) and corresponding number (in red) represent the surface elevation (m)
Another interesting observation is the discrepancy in temporal and spatial changes in metal concentrations between the residential and wetland sites. In the residential site, metal concentrations appeared to rapidly decrease and mostly reached comparable values for all investigated depth (SS, TL, ML, and BL). However, in the wetland site, changes in metal concentrations were more complex: either a decrease or increase with time at investigated depths. A plausible explanation for the observed difference in the residential and wetland sites could be (a) their difference in soil composition/structures and (b) the behavior and dynamics of individual and group metal adsorption/desorption on soil particles. Specific metal forms and soil characteristics largely determine soil–metal interactions (Dermont et al. 2008). For instance, metal adsorption is strongly dependent upon the presence or clay minerals and organic matter (Walker and Hurl 2002). Their large surface area and variable charges on the surface offer greater potential for selective reactions or ion exchanges with metals. In general, wetland soils tend to accumulate more organic matter than upland soils. Wetland soils commonly comprise organic-rich surface layers, and the organic layer can extend in depth to the greatest portion of the soil zone (Reddy et al. 2000). The measured weight of soil organic matter loss-on-ignition (SOM-LOI) was an order of magnitude higher in the wetland (5.076 ± 3.648 %) than the residential (0.649 ± 0.372 %). This indicates that the wetland has significantly higher SOM as a positive linear correlation can be established between SOM and SOM-LOI (Wang et al. 2011). The higher SOM in the wetland site could favor more long-term metal adsorption in comparison with the residential site. In the latter, the elevated metal concentration in soil samples was transitory, suggesting that the metals were weakly adsorbed and rapidly washed out. Thus, their concentrations rapidly decreased within weeks and remained thereafter more comparable at all depths. Temporal changes in other abiotic factors (e.g., salinity and pH) could also influence the observed temporal and spatial fluctuations in metal concentrations in the wetland site. As seawater (7.5 < pH < 8.4, salinity ~32 g/L) intruded the wetland, both salinity and pH could increase, thus promoting metal sorption. It has been demonstrated that a negative correlation exists between soil pH and heavy metal (e.g., Pb, Cd, Cr, Cu) mobility (Sukreeyapongse et al. 2002; Antoniadis et al. 2008; Zeng et al. 2011).
Conclusions
Elevated metal concentrations were observed in the studied residential and wetland sites within a week of Hurricane Sandy. The contamination source appeared to be the Raritan Bay Superfund site, a heavily metal-contaminated site. A combination of storm surge, astronomical tide, and wind direction associated with Hurricane Sandy presumably transported the contaminants to the residential and wetland sites. Unlike the residential site where the elevated metal concentrations rapidly decreased, the wetland site showed more complex temporal and spatial changes. This discrepancy between the two sites could be related with their difference in soil composition and the complexity and dynamics of adsorption/desorption processes for each metal species. The wetland ecosystem contains more organic matter to support more long-term and dynamic metal–soil adsorption/desorption processes. The trend in pore-water salinity suggests temporary supply of the wetland with seawater from the Raritan Bay and freshwater from precipitations. While the residential site suggests a recovery from the metal accumulation, the wetland ecosystem has shown no evidence of such recovery. The wetland apparently served as a depository for the metal contaminants that came from the Raritan Bay Slag Superfund during the Hurricane Sandy. Therefore, continuous monitoring of the wetland ecosystem and its proper management is important for reducing potential risk to people and the environment.
References
Antoniadis V, Robinson J, Alloway B (2008) Effects of short-term pH fluctuations on cadmium, nickel, lead, and zinc availability to ryegrass in a sewage sludge-amended field. Chemosphere 71:759–764
Dermont G, Bergeron M, Mercier G, Richer-Laflèche M (2008) Metal-contaminated soils: remediation practices and treatment technologies. Pract Period Hazard Toxic Radioact Waste Manag 12:188–209
EPA (United States Environmental Protection Agency) (1998) US EPA Method 6200, field portable X-ray fluorescence spectrometry for the determination of elemental concentrations in soil and sediment
EPA (United States Environmental Protection Agency) (2012) Revised final feasibility study report: Raritan Bay Slag Superfund Site, Old Bridge and Sayreville, New Jersey. CDM Smith, Sept 25, 2012. USACE Contract No. W912DQ‐11‐D‐3004
Fanning D, Rabenhorst MC, Balduff D, Wagner D, Orr R, Zurheide P (2010) An acid sulfate perspective on landscape/seascape soil mineralogy in the US Mid-Atlantic region. Geoderma 154:457–464
Fofonoff N (1985) Physical properties of seawater: A new salinity scale and equation of state for seawater. J Geophys Res: Oceans (1978–2012) 90:3332–3342
Hart BT, Bailey P, Edwards R, Hortle K, James K, McMahon A, Meredith C, Swadling K (1990) Effects of salinity on river, stream and wetland ecosystems in Victoria, Australia. Water Res 24:1103–1117
Jolly ID, McEwan KL, Holland KL (2008) A review of groundwater–surface water interactions in arid/semi-arid wetlands and the consequences of salinity for wetland ecology. Ecohydrology 1:43–58
Kilbride C, Poole J, Hutchings T (2006) A comparison of Cu, Pb, As, Cd, Zn, Fe, Ni and Mn determined by acid extraction/ICP–OES and ex situ field portable X-ray fluorescence analyses. Environ Pollut 143:16–23
Markey AM, Clark CS, Succop PA, Roda S (2008) Determination of the feasibility of using a portable X-ray fluorescence (XRF) analyzer in the field for measurement of lead content of sieved soil. J Environ Health 70:24–29 (quiz 55–6)
Radu T, Diamond D (2009) Comparison of soil pollution concentrations determined using AAS and portable XRF techniques. J Hazard Mater 171:1168–1171
Reddy KR, D’Angelo EM, Harris WG (2000) Biogeochemistry of wetland. In: Summer ME (ed) Handbook of soil science. CRC Press, Boca Raton, pp G89–G119
Schulte EE, Hoskins B (2009) Recommended soil organic matter tests. In: Recommended soil testing procedures for the northeastern United States, 3rd edn. Northeastern Regional Publication, The Northeast Coordinating Committee for Soil Testing (NEC-1012) (revised July 2011)
Sukreeyapongse O, Holm PE, Strobel BW, Panichsakpatana S, Magid J, Hansen HCB (2002) pH-dependent release of cadmium, copper, and lead from natural and sludge-amended soils. J Environ Qual 31:1901–1909
USACE (United Stated Army Corps of Engineers) (2010) Final (revised) data gap evaluation technical memorandum. Remedial investigation/feasibility study Raritan Bay Slag Superfund Site, Old Bridge and Sayreville, New Jersey. CDM Smith, June 2010. USACE Contract No. W912DQ-08-D-0018, Task Order No. 018
USGS (United States Geological Survey) (2013) Monitoring storm tide and flooding from Hurricane Sandy along the Atlantic Coast of the United States, October 2012. In: McCallum BE, Wicklein SM, Reiser RG, Busciolano R, Morrison J, Verdi RJ, Painter JA, Frantz ER, Gotvald AJ (eds) Prepared under amission assignment with the Federal Emergency Management Agency. Geological Survey Open-File Report 2013-1043. http://pubs.usgs.gov/of/2013/1043/
Walker DJ, Hurl S (2002) The reduction of heavy metals in a stormwater wetland. Ecol Eng 18:407–414
Wang Z, Zeng D, Patrick WH Jr (1996) Methane emissions from natural wetlands. Environ Monit Assess 42:143–161
Wang Q, Li Y, Wang Y (2011) Optimizing the weight loss-on-ignition methodology to quantify organic and carbonate carbon of sediments from diverse sources. Environ Monit Assess 174:241–257
Wong VN, Johnston SG, Burton ED, Bush RT, Sullivan LA, Slavich PG (2013) Seawater-induced mobilization of trace metals from mackinawite-rich estuarine sediments. Water Res 47:821–832
Zeng F, Ali S, Zhang H, Ouyang Y, Qiu B, Wu F, Zhang G (2011) The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environ Pollut 159:84–91
Acknowledgments
The inputs of Dr. Lisa B. Axe of New Jersey Institute of Technology (NJIT) are well appreciated. Graduate students Christopher D’Ambrose, Chakravarthy Amoghranganath and Xuanming Zhang helped with field and laboratory work. This research was partially supported by NSF RAPID funding to the Center for Natural Resources Development and Protection under Grant Number (CBET-1313185). The comments provided by the editor and the anonymous reviewers helped improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Personna, Y.R., Geng, X., Saleh, F. et al. Monitoring changes in salinity and metal concentrations in New Jersey (USA) coastal ecosystems Post-Hurricane Sandy. Environ Earth Sci 73, 1169–1177 (2015). https://doi.org/10.1007/s12665-014-3539-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-014-3539-4