Abstract
Knowledge of chemical and isotopic composition of precipitation is an important tool for climatological, meteorological and hydrological applications. Rain and snow samples collected during June 2009 to November 2013 in Shahrood, northeastern Iran, have been analyzed for stable isotopes and physico-chemical constituents. Local Meteoric Water Line is characterized by a slope of 5.47 and the isotopic composition of precipitation is generally controlled by air temperature and the amount of precipitation. The precipitation electrical conductivity (EC), ranging from 10 to 1,970 μS/cm (mean: 201 μS/cm, SD: ±275), largely depends on the amount of precipitation and the number of dry days preceding each event. Snow samples have lower ECs. Volumes weighted mean (VWM) pH of the samples is 7.35 (mean: 7.53, SD: ±0.37) with only few acidic samples. Sulfate and calcium are the major ions in the precipitation, a consequence of widespread exposure of carbonates and urban and agricultural inputs. High nitrate concentration (VWM: 3.36 mg/L) reflects the impact of extensive fertilizer application in the region. Compared to years 2000–2001, the VWM EC of the precipitation has risen by 51 % from 41.7 to 63.1 μS/cm; 38 % of the rise is due to reduction in precipitation and 13 % is attributed to increased atmospheric pollution. Chemically, the main rise is in SO −24 , followed by Na+ and NO3 −. Dramatic rise in the number of vehicles in the country from 4 million to 17 million and 77 % increase in petroleum products consumption, are considered as the causes of salinization of atmospheric precipitation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The chemical and isotopic composition of wet atmospheric precipitation is a domain of active research due to the vital insight it provides concerning atmospheric pollution and processes (e.g., Das et al. 2005; Yang et al. 2011; Achotegui-Castells et al. 2013). Such studies are also fundamental in evaluating groundwater chemistry, surface water pollution and soil resources salinity (Blackburn and McLeod 1983; Moller 1990; Seinfeld and Pandis 2006). It is well documented that precipitation reduces the air pollution problem because it is the most effective process for the removal of particulate matter and gaseous pollutants dissolved in the atmosphere (Teixeira et al. 2008). Therefore, chemical composition of water resources can be adversely influenced by the quality of atmospheric precipitation. The temporal concentration of some elements (NO3 − for example) in the wet precipitation is increasing in some cities in China (Huang et al. 2013) and is decreasing in large areas in Europe and North America (Skjelkvåle and de Wit 2011). The acidity of precipitation is also increasing in some parts of the world. For instance, at an urban site in Guangzhou, south China, average pH of the atmospheric precipitation in 2006 was 4.49 (Cao et al. 2009). Hence, in a deteriorating environmental condition which humanity faces today, the temporal trend in the quality and isotopic content of wet atmospheric precipitation must be identified and quantified if one is to deal with, and mitigate, the environmental consequences of industrialization and urbanization. Atmospheric particles and constituents are supplied by a number of sources including marine, terrestrial and anthropogenic origins. In developing countries, records of the quantity of wet atmospheric precipitation are sufficiently available to enable the relevant authorities to interpret the trend and meet the data requirements of hydrological and climatological schemes. However, isotopic and chemical composition records are either rare or none exist. In Iran, with the exception of conference proceedings, there are very limited published reports. Farahmandkia et al. (2011) showed that the ranges of concentration of Pb, Zn, Cr and Cd in rain water in Zanjan, northwest Iran, are 5.8–22.2, 29.26–70, 1.4–4 and 0.25–1.2 (µg/L), respectively. They neither measured EC nor pH of the rainwater samples to allow comparison with Shahrood samples. Farahmandkia et al. (2011) concluded that road traffic and industrial activities are the main causes of high concentration of heavy metals in Zanjan’s precipitation. Also, Khosropour et al. (2011) measured the concentration of Cd and Pb in throughfall in Cupressus arizonica plantation in a forest park in the vicinity of Tehran as well as in precipitation in an adjacent open area. The concentration of Pb in the throughfall and open area ranged from 50 to 27 µg/L, respectively, and for Cd ranges from 10.75 to 8.55 µg/L, respectively. The practical finding of this research was the point that C. arizonica can absorb both air Pb and Cd. Concentration of a number of metals and the pH of Tehran atmospheric precipitation samples were determined by Kamani et al. (2013). They found that the concentration of elements decreases in the order such that Cd < Cr < Ni < Cu < Pb < Zn < Fe < Al, while pH ranges from 4.2 to 7.1 with an average of 5.1. They also demonstrated that some contaminants such as Al, Fe and Cr originate from natural sources while those such as Ni, Cd, Zn, Pb and Cu have their root in the anthropogenic sources.
Here, we report on the results of a long-term atmospheric monitoring program in Shahrood, a medium size city in northeastern Iran. The objective is to construct the Local Meteoric Water Line (LMWL) for hydrological purposes and to investigate the chemical composition, governing factors and the causes of saline wet atmospheric precipitation. This report can provide a base line for the future evaluation of temporal changes in the quality of atmospheric precipitation. Also, one immediate intended application of the LMWL is to identify the signature of urban recharge in the local aquifer underlying Shahrood, which has been recently touched upon by Kazemi (2011). Evaluation of the environmental impact of the booming car industry in Iran on the local atmospheric chemistry is one other application of this research.
Description of the study area
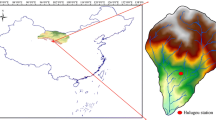
Shahrood (latitude 36°25′N, longitude 55°01′E), with a population of approximately 150,000 inhabitants lies 400 km to the east of Tehran, in northeastern Iran (Fig. 1). It is separated from the Caspian Sea, to the north, by the Alborz Mountain ranges. Shahrood receives an average annual rainfall of 153 mm in the form of snow and rain; its annual average air temperature is 14.8 °C (Kazemi 2013). Shahrood is a regional agricultural hub where extensive vineyards extend right into the city’s suburbs. Cherry, apricot and a variety of other agricultural crops are also abundant. All these are irrigated by groundwater. A number of small scale industries such as sugar mill factory and brake factory are also active in the region. However, the only large industry is the cement plant located some 20 km to the north of the city. Raw materials for this plant are extracted from the limestone queries of the Alborz mountains. The cement plant, agricultural activities and road traffic are the three main anthropological contributors to air pollution.
Sampling
From June 2009 to November 2013, a total of 124 wet atmospheric precipitation samples were collected on an event basis in the private villa of the first author in the outskirts of Shahrood. These included 99 rain, 15 snow and 10 mixture (snow and rain) samples. The ease of access to the sampling site has been an advantage because full control on the quality of the sampling procedure was possible (e.g., prevention of evaporation and avoiding dust contamination). From 2010 onward, virtually all precipitation events were sampled. In 2009, however, some events were missed. To collect enough volume of water and to sample low precipitation events, a voluminous hard plastic collector with an upper and lower diameter of 50 and 40 cm, respectively, was used. Immediately after each rainfall event, samples were transferred to room and analyzed for EC and pH after equilibrating to room temperature. In the next step, samples were filtered and poured into glass sample bottles. The collector was then cleaned, dried and re-installed for the next sampling event. It was possible to sample precipitation event of even less than 0.1 mm. In addition to collecting Shahrood rain and snow samples, in 26 February 2010, two samples were collected from the snow packs of the aforesaid Alborz mountains approximately 40 km to the north of Shahrood (Fig. 1). Snow pack samples were collected by removing the upper few centimeters of the snow pack to avoid the dust. For comparison purposes, in 9 April 2010, two rain samples were collected from Gorgan, a larger city located 100 km to the north of Shahrood (See Fig. 1). Therefore, altogether 128 samples including 102 rain, 16 snow and 10 mixture samples were collected and analyzed. Air temperature data were extracted from the website of the Meteorological Organization of Iran.
A similar monitoring program, the predecessor of the current research, was conducted by Kazemi and Mehdizadeh (2001) which included rain and snow sampling in the same mentioned private villa. Fifteen samples were collected from September 2000 till March 2001. Those samples were all analyzed for EC but only three of them were subjected to detailed chemical analysis. Kazemi and Mehdizadeh (2001) findings are here used for comparison purposes and identifying temporal trends. Furthermore, from March to May 2003, eight samples were collected by the first author and were analyzed for pH and EC. These, too, are used in this study for comparison.
Analytical technique and quality control
Samples were first measured for volume to calculate the amount (depth) of rain and snow fall. Samples were analyzed in the room temperature for EC and pH. Electrical conductivity was measured using a TwinCond EC meter (Horriba™, Japan) which requires only one drop of water. pH was measured using a pH meter manufactured by Horriba™. Anions, cations and stable isotopes of only 41 samples (37 Shahrood samples from June 2009 till February 2011, two snow pack samples and two Gorgan samples) were measured at the Isotope Hydrology Laboratory, Kumamoto University, Japan. The rest of the samples have not been analyzed yet. Anions and cations were analyzed using an ion chromatograph Metrohm™ model 761 Compact IC with Model 813 Compact auto sampler. For all samples, HCO3 − was calculated by difference. Deuterium and oxygen-18 were measured following equilibrium method using a Finnigan-Mat Delta-S Mass spectrometer. The precision limit for 2H measurements is 1 per mil and for 18O measurements is 0.1 per mil. The values of 2H and 18O and other specifications of the collected samples are presented in Table 1. To increase the accuracy of the analysis, five key steps were put into practice as follows.
-
Five laboratory standards with different isotopic compositions were measured in every batch of analysis.
-
Duplicates analysis were run on 10 % of the samples.
-
After analysis of each batch of samples, samples with doubtful results were identified and re-tested. For this task, three check points were applied including
(a) EC/TSS ratio of the samples,
(b) the relationship between amount of precipitation and EC of the respective sample and
(c) the correlation between temperature/season and isotope composition.
-
Due to the availability of enough volume of sample and also because of the greater importance, precipitation events of greater than 4 mm were divided into either two or three subsamples. By doing this, 12 samples were divided into two subsamples each, while three samples were divided into three subsamples each. Consequently, 15 samples were divided into 33 subsamples and analyzed separately. The results were then averaged out.
-
It is explained in the following sections that five samples have been deleted from the record after detailed statistical analysis of the data and detection of inconsistency.
Results
Local meteoric water line
The first LMWL for Shahrood has been constructed (Fig. 2). The slope of this line is 5.47 which is a typical of arid and semi-arid regions. Snow pack samples are isotopically lighter than Shahrood samples, as expected. Average 18O and 2H of Shahrood samples is −1.4 and −12.9 ‰, while that of snow pack is −11.73 and −77.55 ‰, respectively. A combination of factors including higher altitude, greater amount and (reverse) continental effects leads to lighter isotopes in snow packs. In contrast to snow pack samples, Gorgan samples with average 18O of −0.4 ‰ and 2H of −4.9 ‰, are isotopically heavier (see Table 1 and Fig. 1). This is because Gorgan is warmer, and its altitude is much lower, than Shahrood. Shahrood average temperature is 14.8 °C while that of Gorgan is 18.2 °C. Gorgan elevation is just 155 m above sea level, while Shahrood lies 1,380 m above sea level (Wikipedia). However, Gorgan’s average annual rainfall (600 mm) is higher than Shahrood (Kazemi 2011) because of proximity of Gorgan to Caspian Sea and the orographic effect of Alborz mountain ranges. It should be noted that the specific Shahrood sample which corresponds to the same date of Gorgan samples (i.e., 9.4.2010) is characterized by 18O of −1.8 ‰ and 2H of −15.6 ‰. This sample is slightly lighter than average Shahrood value, and considerably lighter than Gorgan samples (see Table 1).
There is a good correlation between local air temperature and the amount of rain and snow fall and 2H and 18O (Fig. 3). 18O values slightly better correlated with both temperature and precipitation amount than 2H. This is probably due to the aridity of the region because higher temperatures favor isotopic separation of oxygen isotopes. Compared to 18O, fractionation of 2H in higher temperatures is less efficient (Clark and Fritz 1997). Of these two, the local air temperature is the main controlling factor (average R 2 = 0.56) and precipitation amount plays a less significant role (average R 2 = 0.4). This is because the impact of the air temperature on the isotope concentration is more profound when compared with all other factors including elevation, continental and precipitation amount effects. Temperature effect is 0.7 ‰/1 °C for 18O and 5.6 ‰/1 °C for 2H (Mazor 2004).
As Fig. 3 shows, the correlation coefficient for both air temperature and amount of precipitation is substantially lower than 1. To obtain a higher R 2, we have combined both variables in the form of temperature/amount of precipitation ratio. The rationale is that higher air temperatures lead to isotopically heavier rainfalls, while lower precipitation amounts are isotopically heavier. Therefore, higher values of the ratio of temperature/precipitation should result in isotopically heavier precipitation. This is to say that low precipitation events in hot weathers should be isotopically very heavy. However, this was not supported by the data probably because in reality the air temperature and the amount of precipitation are independent variables. In other words, there is no assurance that high precipitation event happens in cold weathers or low precipitation events happen in hot weathers. Similarly, the plot of isotopic content against EC of the samples did not yield a meaningful correlation. This is due to the complexity and numerousness of the processes that control these two variables. A good positive correlation between the EC and the stable isotopes content of a group of water samples is an indication of evaporative enrichment. In spite of these, Fig. 4 suggests that the deuterium content of the precipitation is profoundly impacted by the seasonal effect. The time span of the monitoring program and especially the isotope analysis is not long enough to deduce the temporal changes in precipitation isotope characteristics. Only three samples were collected in 2009 and three samples in 2011, with the majority of the samples collected in 2010. However, as mentioned it seems that isotopes content of the precipitation changes seasonally.
Precipitation salinity
Electrical conductivity of any water sample is a good indication of its salinity. The EC of the collected samples ranges widely from 10 to 1,970 μS/cm (arithmetic mean: 201 μS/cm, standard deviation: ±275). The large SD value shows that the samples are highly heterogeneous. With the discard of five samples (explained in the next sections), the VWM EC of the samples is 72.4 μS/cm. There is not much published data to compare the EC of Shahrood precipitation to other locations or cities in Iran. However, for Tehran, based on Saeedi and Pajooheshfar (2012) research, the average EC of precipitation is 71.7 μS/cm, which is almost identical to Shahrood precipitation. One expects to see much higher ECs for Tehran. A few reasons can be provided to justify the similarity. The first reason is higher average annual rainfall of 233 mm for Tehran (note that for Shahrood, it is 153 mm). Second and more important is the above average rainfall of 302 mm for the study period (2008–2009) for Tehran and below average rainfall of only 117 mm for Shahrood (2010–2013). The third cause is the shorter study period for Tehran, i.e., 2 years against 4 years for Shahrood. The fourth major cause could be the point that Saeedi and Pajooheshfar (2012) likely did not analyze very low precipitation events such as we did in this study.
In comparison to other countries in the World, Shahrood’s precipitation EC is slightly higher than 66.5 μS/cm reported for urban Beijing, China (Yang et al. 2011). Beijing enjoys higher rainfall (441 mm) and accommodates much greater population than Shahrood. In addition, World Bank (2008) statistics show that car/person ratio for China (37/1,000) is considerably lower than that of Iran (128/1,000). Compared to Europe, Shahrood’s precipitation is up to five times more saline. This is due to much lower rainfall and poor vegetation cover in Iran which intensify dust blowing. Compared to southern Brazil with an average annual rainfall of 1,800 mm and an EC of 8 μS/cm (Teixeira et al. 2008), Shahrood’s precipitation is approximately nine times more saline.
VWM EC of snow samples is 57.8 μS/cm, considerably lower than 82.5 μS/cm for rain samples. Similar to Shahrood, Tehran’s snow samples’ EC is 49.8 μS/cm, substantially lower than rain samples’ EC of 78.5 μS/cm (Saeedi and Pajooheshfar 2012). Snow has little material dissolved in the ice crystal lattice but it sorbs and traps pollution on its surface. Mixed samples (snow + rain) exhibit even lower EC, 40.67 μS/cm. The lowest recorded EC, i.e., 10 μS/cm is for a mixed sample. It should be noted that snow and mixed samples are also fewer in number, so such comparison might not be quite correct.
Atmospheric solutes dissolved in precipitation are from marine, terrestrial or anthropogenic sources. In an attempt to find the factors which determine the salinity of rain and snow fall in Shahrood, the EC of the samples has been plotted against the amount of precipitation (Fig. 5). There is a clear decrease in the EC with the increase in the amount of precipitation. As it is seen in Fig 5, two samples with the EC of 990 and 1,610 μS/cm do not follow the trend possibly because of dust contamination brought about by windy condition. These two samples have been discarded, and deleted from further data analysis.
The number of dry days preceding each precipitation event relates to the EC of the samples. With the increase in the number of dry days (precipitation interval), the EC of the precipitation increases too. Dusts and the pollutants accumulated gradually upwind during the dry days are washed out from the atmosphere by the precipitation. This is shown in Fig. 6 where a good correlation is found between the ratio of the number of dry days preceding each precipitation event/amount of precipitation and the EC of the samples (R 2 = 0.76). Note that in this plot, three outlying samples have been deleted from the data as mentioned earlier. [In three similar plots, snow, rain and mixed samples were separately plotted against the ratio of number of previous dry days/amount of precipitation. Higher correlation coefficients were achieved. These have not been included here to avoid excessive figures]. This finding is important because it shows that long-term droughts are potentially capable of salinizing snow and rain since they increase the precipitation interval while reducing the precipitation amount. There is no correlation between the EC of the samples and the local air temperature; an indication that the samples were not subjected to evaporative enrichment.
Precipitation acidity
Table 1 shows that samples’ pH ranges from 6.6 to 8.5 with a VWM of 7.35 (arithmetic mean: 7.53, SD: ±0.37). Rain samples’ pH is 7.44, snow samples 7.05 and mixed samples 7.14. In sharp contrast to Tehran precipitation whose average pH is 5.1 (Kamani et al. 2013), only 11.8 % of the samples have pH values below 7. This is expectable because of large population density, and anthropogenic air pollution, difference between Shahrood and Tehran. All acidic samples are diluted and exhibit low EC. This shows that in the absence of soluble atmospheric particles, rain and snow are slightly acidic which is due to the production of carbonic acid. Also, there is a general increase in the pH with increase in the EC (Fig. 7), and by analog a reduction in pH with increase in the precipitation amount. This illustrates that the dissolution of the aerosol particles (which are predominantly Ca-bearing because of the extensive local carbonate outcrops) by the raindrops, supersedes the acidifying role of H2CO3. Alkaline pH of the samples is also an indication that the terrestrial sources of atmospheric particles overpower acidifying role of anthropogenic inputs. Acid rain is not expected in Shahrood in the near future. It should be pointed out that as it can be seen in Table 3, the average annual pH values for 2003 (7.39), 2009–2010 (7.33), 2010–2011 (7.16), 2011–2012 (7.17) and 2012–2013 (7.43) show insignificant temporal changes possibly because of the dominant role of the atmospheric dusts in controlling the pH of the precipitation.
Precipitation chemistry
Table 2 presents the results of the chemical analysis of the water samples and some other specifications. If bicarbonate is excluded, the major ions include Ca+2 and SO −24 followed by Na+ and NO3 −. High concentration of Ca is due to the extensive exposure of Cretaceous limestones which build dense limestone mountains overlooking Shahrood (Kazemi 2004, 2011). The average meq/L ratio of Na/Cl of the samples is 2.57, three times higher than seawater ratio of 0.86. This shows that there is a substantial terrestrial/anthropogenic source for Na+ because Na/Cl ratio higher than 1.5 is an indication that marine sources of such ions are insignificant (Thimonier et al. 2008). This confirms the results of enrichment factors (EF) calculations in the following paragraph. However, it is also likely that the particle-bound chloride (Cl−) has been converted to gas-phase Cl2 (Roberts et al. 2008). If this occurs, the concentration of chloride in precipitation is reduced while Na/Cl ratio is raised. It is also shown in the next sections that the source of SO −24 and NO3 − is mainly anthropogenic. Agricultural fertilizers are probably a major source of nitrate containing dusts in the atmosphere as Shahrood is a regional agricultural hub with extensive orchards and shallow rooted vegetation. However, for precise determination of the source of SO −24 , sulfur isotopes must be studied as was done in Bologna, Italy by Panettiere et al. (2000).
To further constrain the sources of various ions, EF has been calculated using the following formula. It should be, however, emphasized that in such a dry climate with annual average rainfall of only 150 mm, EF calculation could be an incorrect technique to employ (because of large amount of atmospheric dusts which are not washed away).
It should be noted that for calculating EF, some researchers take Na as the reference ion (e.g., Das et al. 2005; Teixeira et al. 2008) and some others take Cl− (e.g., Blackburn and McLeod 1983). Here, we have adopted both approaches, (Cl− and Na+ as the reference, each separately) and averaged out the results. Na+-based EFs’ are generally lower because as mentioned the concentration of Na in the samples is comparatively higher than seawater Na+. The average EF values reveal that with the exception of Cl−, all ions have major terrestrial/anthropogenic origins. Again, the highest EF of Ca+2 points to the influence of local Cretaceous limestones as discussed above. Magnesium shows the lowest EF value reflecting its marine source.
It is a routine practice to statistically analyze the precipitation chemistry data and present the results in the form of tables. These analyses usually include calculation of correlation coefficient between the concentrations of various cations and anions in the samples. The objective of such exercise is to uncover the source of various ions. In this research, there is a good correlation coefficient (R 2 = 0.7–0.95) between related pairs of ions, for instance Na+ and Cl−. The relevant analyses are not presented here in the text first because the raw data of the samples are given in Table 2. Second, because enrichment factor values can usually provide the same information. Similar to isotopic characteristics, the time span of the monitoring program is too short to allow deduction of temporal changes in chemical composition of Shahrood precipitation.
Trend in precipitation salinity
Among the scientific community, it is generally accepted that the worldwide environmental pollution shows an increasing trend, though it is more severe in developing countries. A rise in the salinity of atmospheric precipitation is therefore expected. In this research, the published atmospheric chemistry data for Shahrood (Kazemi and Mehdizadeh 2001) as well as samples from year 2003 have been used to investigate the trend of the precipitation salinity. These data are presented in Table 3. Kazemi and Mehdizadeh (2001) samples are for September 2000–March 2001 corresponding to the first 6 months of the Iranian Water Year when most of the rainfall occurs. [Note that Water Year in Iran is separated by calendar year by 6 months]. Therefore, for the last 4 years (2009–2013), the data for exactly the same period i.e., September of the previous year to March of the subsequent year, have been separated in Table 3. This table and Fig. 8a show that there is a near unity correlation (R 2 = 0.97) between VWM EC and the amount of precipitation in different years. The EC first rose from 41.7 in 2000–2001 to 47.9 in 2003 and then to 69.5 µS/cm in 2012–2013. This is a rise of 67 % in 12 years. However, the average EC of the last 4 years i.e., 2009–2013 (63.1 µS/cm) has been compared to EC during 2000–2001 assuming that it better represents the current situation. On this basis, the rise in EC in 12 years (2001–2013) is 51 %. For the same period, the precipitation amount has decreased by 24 %.
The increase in the precipitation salinity cannot be solely driven by the decrease in the precipitation amount. If this was the case, the EC of year 2011–2012 must have been less than that of 2000–2001. Therefore, other factors must also be involved in the enrichment of the EC of the precipitation. To compute the share of increased atmospheric pollution in the increment of EC, a novel technique has been applied. This approach is based on the rational that 0.03 deviation from unity regression (1–0.97) in Fig. 8a is due to increased anthropogenic inputs to the atmosphere. If reduction in precipitation was the sole cause, a regression coefficient of one should have been obtained for Fig. 8a. To calculate the share of the atmospheric pollution, the EC values have been altered to increase the regression coefficient to 1. These modified ECs are referred to as corrected ECs, i.e., EC of the precipitation if the sole cause of temporal increase in the EC is the reduction in the precipitation amount. To obtain the corrected ECs, first the EC of the last year (2012–2013) was corrected using the principle of inverse proportion. That is, 41.7 µS/cm × 104.7 mm = X × 75.4 mm, where X is the corrected EC value for year 2012–2013. In this case, X equals 57.94 µS/cm. Then, the first year (2000–2001) values (41.7 µS/cm, 104.7 mm) and the last year (2012–2013) values (57.94 µS/cm, 75.4 mm) were plotted on a scatter plot and the relevant line’s equation was derived (Fig. 8b). By applying the equation of this line to the values of the rest of the years, the corrected ECs for all years were obtained. These are shown in Table 3 and in Fig. 8c. As it is seen in Table 3, the average rise in the EC provoked by atmospheric pollution is 13 %. If subtracted from 51 % total rise in the EC, 38 % remains to be associated with reduction in the precipitation amount.
Compared to 2003 records, pH shows an insignificant decrease of about 2 %. In term of ions, the chief increase is in SO −24 followed by Na+ and NO3 −. Inferred increase in chloride is not reliable because the value in 2000–2001 is probably too low. Calcium has shown insignificant increase. However, for both pH and ions, inferred temporal trend can be erroneous because the number of analysis is too low. With regard to EC, however, comparison is valid because data points are sufficient. Salinization of precipitation is therefore clearly demonstrated.
The main cause of atmospheric pollution is the dramatic increase in the number of cars in the country. In the beginning of year 2001, the number of cars in Iran was estimated at 4 million. This rose dramatically to 14 million in 2011 (Road Police chief report 2011) and currently to about 17 million. Farahmandkia et al. (2011) have also found that road traffic and industrial activities are the main sources of heavy metals in the rain and snow water in Zanjan, northwest Iran. The other possible cause of salinity enrichment is the change in the rain/snow ratio. If rain/snow ratio increases, it can lead to salinization because rain drops, in comparison to snow, can dissolve more atmospheric particles.
The ions which show considerable increase i.e., SO −24 and NO3 − points to vehicle boom as the main cause of salinity increase. This is demonstrated in Fig. 9 where there is a near unity correlation coefficient between EC and SO −24 and a substantial correlation between EC and NO3 − despite complex geochemistry of the atmospheric nitrate. Elevated concentrations of sulfate are particularly a trade mark of urban atmospheric environments (Madhavi Latha and Badarinath 2004). Same is true for nitrogen (Galloway et al. 2003). Similar to Shahrood, NO3 −–N has shown an increasing trend during a 20 years period from 1986 to 2006 in Shenzhen City, China (Huang et al. 2013). However, increase in Na+ in Shahrood precipitation is difficult to justify. It should be pointed out that although car numbers increased by 3.5 times, the salinity of precipitation has not increased by the same pace. This is due to the following reasons:
-
Rationing of subsidized fuel since 2007 which has led to a decrease in per capita fuel consumption. On the basis of National Iranian Oil Products Distribution Company (NIOPDC) database, consumption of all types of petroleum products during 2000–2010 rose by 77 % from 2,271 × 103 to 4,027 × 103 barrels/day (Aqayan 2011).
-
Production of more efficient cars in terms of fuel consumption
-
Reduction in car usage by people due to heavy road traffic and stricter police control
-
The fact that the other pollutive industries have not expanded at the same pace of car industry.
Conclusions
This research offers the following conclusions:
-
1.
The slope of the LMWL in Shahrood, northeastern Iran, is 5.47 which is the typical of arid and semi-arid regions.
-
2.
The local air temperature and the amount of precipitation collectively control the isotopic content of the precipitation in Shahrood.
-
3.
Precipitation in Shahrood is highly enriched; its salinity is controlled by precipitation amount and precipitation interval.
-
4.
Long-term draughts are capable of salinizing the precipitation because they reduce the amount while increasing the interval of precipitation.
-
5.
Calcium and sulfate are the major ions in Shahrood’s precipitation.
-
6.
Enrichment factor values show that most ions have major non-sea sources.
-
7.
Shahrood precipitation is generally alkaline, reflecting the neutralizing role of aerosol particles emanated from the local extensive carbonate outcrops.
-
8.
Acid precipitation is not expected in Shahrood in the near future although sulfur producing sources are appreciable.
-
9.
Due to an explosion in the number of cars moving in the streets, the salinity of atmospheric precipitation in Shahrood is on the rise, rising by 13 % in 12 years. Draughts are responsible for additional 38 % increase in the salinity of precipitation during this period, toping up the rise to 51 %.
References
Achotegui-Castells A, Sardans J, Ribas A, Peñuelas J (2013) Identifying the origin of atmospheric inputs of trace elements in the Prades Mountains (Catalonia) with bryophytes, lichens, and soil monitoring. Environ Monit Assess 185:615–629
Aqayan H (2011) Statistics of petroleum products consumption during 1927–2010. National Iranian Oil Products Distribution Company, Public Relation Office, Tehran [In Persian]
Blackburn G, McLeod S (1983) Salinity of atmospheric precipitation in the Murray-Darling Drainage Division, Australia. Aust J Soil Res 21:411–434
Cao YZ, Wang S, Zhang G, Luo J, Lu S (2009) Chemical characteristics of wet precipitation at an urban site of Guangzhou, South China. Atmos Res 94:462–469
Clark I, Fritz P (1997) Environmental isotopes in hydrogeology. CRC Press/Lewis Publishers, Boca Raton
Das R, Das SN, Misra VN (2005) Chemical composition of rainwater and dustfall at Bhubaneswar in the east coast of India. Atmos Environ 39(32):5908–5916
Farahmandkia Z, Mehrasbi MR, Sekhavatjou MS (2011) Relationship between concentrations of heavy metals in wet precipitation and atmospheric PM10 particles in Zanjan, Iran. Iran J Environ Heal Sci Eng 8(1):49–56
Galloway JN, Aber JD, Erisman JW, Seitzinger SP, Howarth RH, Cowling EB, Cosby BJ (2003) The nitrogen cascade. Bioscience 53:341–356
Huang Y, Lu X, Chen K (2013) Wet atmospheric deposition of nitrogen: 20 years measurement in Shenzhen City, China. Environ Monit Assess 85:113–122
Kamani H, Mahvi A, Hosseini M, Ansari H, Nazmara Sh, Safari Gh (2013) Evaluation of heavy metals concentration in the atmospheric precipitation in Tehran and finding their origin by factor analysis. In: Abstracts of the 16th national symposium of environmental health, Iran. Tabriz University of Medical Sciences, Abstract No. 13223
Kazemi GA (2004) Temporal changes in the physical properties and chemical composition of the municipal water supply of Shahrood, northeastern Iran. Hydrogeol J 12:723–734
Kazemi GA (2011) Impacts of urbanization on groundwater resources in Shahrood, Northeastern Iran: comparison with other Iranian and Asian cities. Phys Chem Earth 36:150–159
Kazemi GA (2013) Isotopic composition (deuterium and oxygen-18) of atmospheric precipitation in Shahrood. In: Abstracts of the first national conference on application of stable isotopes, Ferdowsi University, Mashad, Iran, p 17
Kazemi GA, Mehdizadeh H (2001) Solute content and chemical composition of atmospheric precipitation of Shahrood Region. In: Proceed. of 5th symposium of the Geological Society of Iran, Tehran, September 2001, pp 401–405
Khosropour E, Attarod P, Shirvani A, Matinzadeh M, Bayramzadeh V (2011) Rainfall interception loss and chemical composition of throughfall in Cupressus Arizonica planatation in Chitgar Forest Park. Res J Forest Sci Eng 1(2):31–39
Madhavi Latha K, Badarinath VS (2004) Characterization of aerosols and its radiative impacts over urban and rural environments: a case study from Hyderabad and Srisailam. Environ Pollut 132:463–468
Mazor E (2004) Applied chemical and isotopic groundwater hydrology, 3rd edn. Marcel Dekker, New York
Moller D (1990) The Na/Cl ration in rainwater and the seasalt chloride cycle. Tellus B 423:254–262
Panettiere P, Cortecci G, Dinelli E, Bencini A, Guidi M (2000) Chemistry and sulfur isotopic composition of precipitation at Bologna, Italy. Appl Geochem 15(10):1455–1467
Road Police chief report (2011) http://www.asriran.com/fa/news/183344. Accessed 25 Oct 2012
Roberts JM, Osthoff HD, Brown SS, Ravishankara AR (2008) N2O5 oxidizes chloride to Cl2 in acidic atmospheric aerosol. Science 321(5892):1059
Saeedi M, Pajooheshfar SP (2012) Acid rain examination and chemical composition of atmospheric precipitation in Tehran, Iran. Environ Asia 5(1):39–47
Seinfeld JH, Pandis SN (2006) Atmospheric chemistry and physics, from air pollution to climate change, 2nd edn. John Wiley & Sons, New York
Skjelkvåle BL, de Wit HA (2011) Trends in precipitation chemistry, surface water chemistry and aquatic biota in acidified areas in Europe and North America from 1990 to 2008. In: ICP Waters report, OR-6218, NIVA, Oslo, Norway
Teixeira EC, Migliavacca D, Filho SP, Machado ACM, Dallarosa JB (2008) Study of wet precipitation and its chemical composition in South of Brazil. Anais da Academia Brasileira de Ciências 80(2):381–395
Thimonier A, Schmitt M, Waldner P, Schleppi P (2008) Seasonality of the Na/Cl ratio in precipitation and implications of canopy leaching in validating chemical analyses of throughfall samples. Atmos Environ 42:9106–9117
World Bank (2008) Website of the world bank. http://data.worldbank.org/indicator/IS.VEH.NVEH.P3/countries?page=1. Accessed 10 Feb 2012
Yang F et al (2011) Five year record of precipitation chemistry in Beijing. Atmos Chem Phys Discuss 11:28097–28124
Acknowledgments
Mr Tanoue and Mr Mototaka, MSc students of Isotope Hydrology Laboratory of Kumamoto University have helped with isotope and hydrochemical analysis of the samples. They are therefore gratefully thanked.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kazemi, G.A., Ichiyanagi, K. & Shimada, J. Isotopic characteristics, chemical composition and salinization of atmospheric precipitation in Shahrood, northeastern Iran. Environ Earth Sci 73, 361–374 (2015). https://doi.org/10.1007/s12665-014-3430-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-014-3430-3