Abstract
The concentrations of potential toxic elements (PTEs) such as cadmium (Cd), copper (Cu), lead (Pb) and zinc (Zn) were measured in five different stations of Rameswaram (RM) coast, Gulf of Mannar (GoM), India, in coastal waters in sediments and tissues of Adocia pigmentifera. Concentration of PTEs (Cd, Cu, Pb and Zn) was found to be highest in sediments followed by A. pigmentifera and seawater samples collected from the different stations of RM coast. The pattern of accumulation of PTEs in the three sample types is of the following order Zn < Pb < Cu < Cd. The seasonal variation of these PTEs also followed the same pattern. One-way analysis of variance (ANOVA) with Tukey’s HSD post hoc test has revealed significant differences among stations S1–S4 when compared with the station S5 which is considered as the reference site. Correlation coefficient study showed no significant correlation in the concentration of PTEs in A. pigmentifera, seawater and sediment samples. The concentration of all the PTEs in different sample types has exceeded the FAO/WHO/sediment background values, except for the concentration of Zn in A. pigmentifera and sediment sample, which did not exceed the limits. The contamination factor (CF) and geoaccumulation index (I geo) values indicated significant contamination of PTEs in the sediments from different stations of RM coast, India. Variations found between the sample types during the studies could be due to changes in levels of pollution discharge over time, availability of PTEs for adsorption as well as variations in the sampling season. Increasing urban sprawl and release of effluents both from domestic and industrial sources are the main sources of pollution at RM coast and are the prime reasons for the loss of existing diverse ecosystem.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Every year a new chemical is seen entering the market and most of them are seen finding their way into the marine environment (Rao et al. 2006). The ever increasing list of chemical pollutants released into the marine ecosystem on the whole includes potential toxic elements (PTEs), hydrocarbons both aliphatic, aromatic, and radio nucleotides. Among all these contaminants, the pollution by PTEs such as cadmium (Cd), copper (Cu), lead (Pb) and zinc (Zn) is considered potentially hazardous to human health (Cachada et al. 2012). The contamination of the marine environment by PTEs has become a global phenomenon because of their persistence and accumulation in the tissues of the inhabiting organisms (Gochfeld 2003). These PTEs belong to the priority of contaminants according to the European Water Framework Directive 2000/60/EC (Mylopoulos et al. 2008; Arsene et al. 2009). The concentration of the PTEs is usually low in the marine environment, but due to the anthropogenic activity their concentrations are increasing significantly and causing a threat to the aquatic environments. Our previous studies have clearly revealed that marine sponges serve as ideal biomonitors for the evaluation of PTEs in the coastal ecosystems (Rao et al. 2006, 2007, 2009) and studies by other researchers have also proved that sponges are the best model organisms for evaluating the health status of the marine environment (Pan et al. 2011; de Mestre et al. 2012; Genta-Jouve et al. 2012). Adocia pigmentifera is a violet marine repent sponge that has large oscula and that often grows as an encrusting form on other organisms. A. pigmentifera is also known for its diuretic and insecticidal properties (Rao et al. 2008). It is found in the subtidal zones of southeast coast of India. Individual sponges have a length ranging from 2.5 to 20 cm and are suitable for collection. Most of the demosponges serve as ideal biomonitors for monitoring contaminants in the marine environment. Until now no reports on the behavior and monitoring of PTEs have been reported on this sponge, which is the prime reason of choosing this species in the current research. The widespread distribution of A. pigmentifera in the coastal regions of GoM also makes it one of the most feasible sponges for biomonitoring. Gulf of Mannar is at the tip of the southeast coast of India and consists of 21 islands starting from Rameswaram (RM) to Tuticorin. The gulf is declared as the Marine Biosphere Reserves (GoMBR) by the Ministry of Environment and Forest, Government of India (Rao et al. 2009). It is one of the natural harbours for various marine flora and fauna such as oysters, coral reefs, various fishes and different types of sea grasses, seaweeds which are seen inhabiting the coast. During recent past there has been vast increase in the tourism facilities around RM and existence of a number of small and large scale industires which have contributed to significant increase in the level of contaminants around the coast. The sediments of RM are dominated by carbonates and poor in silicates which consists of dune sands flaky, rock-forming minerals like chlorite, biotite and muscovite. The sands are underlain by calcareous sandstones. The entire coastal tract consists of gypsum, limestone and magnesium compounds. The RM island consists of freshwater aquifers which dry up quickly in summer. The ground water quality usually varies from alkaline to highly saline. The saline aquifers in coastal tract occur to a depth of up to 80 m below ground level. The great challenges which are experienced by the existing diverse ecosystem at RM coast are due to overexploitation, mismanagement and the entry of untreated sewage both from domestic and industries surrounding the coast (Sulochanan et al. 2007; Rao et al. 2009). The concentrations of PTEs in seawater are Cd (0.07), Cu (0.82), Pb (0.13) and Zn (0.24) μg/l, and in tissues of Sigmadocia fibulata are Cd (0.4), Cu (5.53), Pb (0.45) and Zn (0.62) μg/g, which have been reported from our earlier studies (Rao et al. 2007). Similar studies on marine sediments conducted by Sulochanan et al. (2007) from the same coast have indicated an increase in the concentrations of PTEs such as Cd (1.66), Cu (3.61), Pb (15.56) and Zn (2.82) μg/g dry weight of sediment. Previous studies conducted by us involved only the measurement of PTEs in seawater and sponge tissues only (Rao et al. 2007, 2009) from a different coast of GoM, India. The current study highlights the monitoring of seasonal variation of Cd, Cu, Pb and Zn concentrations in the three sample types collected from different stations of RM coast and also to evaluate the degree of contamination in the chosen area in comparison to others. Interestingly, there is lack of information on the seasonal distribution of PTEs in the coastal region of RM, India. There exists paucity on the distribution of PTEs in different seasons among the three sample types (seawater, A. pigmentifera and sediment) from the coastal regions of RM.
Materials and methods
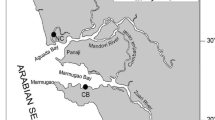
Marine sponge A. pigmentifera (Family: Chalinidae, Order: Haplosclerida, Class: Demospongia) were collected along the coastal regions of Rameswaram coast, India. A. pigmentifera was collected by snorkeling and skin diving during the months of March to December (premonsoon, monsoon and post monsoon). The collected sponges are from five different stations which are as follows: station S1 is bath ghat (9.17°N 79.24°E); S2 shankumaal (9.20°N 79.22°E); S3 sewage outlet (9.13°N 79.23°E); S4 is boat house (9.13°N 79.25°E) and S5 station is opposite to television tower (9.20°N 79.17°E) (Fig. 1). A. pigmentifera samples after collection were washed thoroughly with natural seawater to remove all the intact mud and other particles; later the samples were frozen by placing them in liquid nitrogen container until the analytical work was initiated. The voucher specimens were submitted to National Institute of Oceanography (NIO), Goa for depository purpose and were identified by Dr. P. A. Thomas as A. pigmentifera at Vizhinjam Research Centre of Central Marine Fisheries Research Institute (ICAR), Vizhinjam, Thiruvananthapuram, India. The collected sponges before analysis were removed from the liquid nitrogen cans and dried in oven before further processing was carried out.
Water and sediment sampling
Seawater samples from chosen stations were collected in fresh autoclaved and acid-rinsed bottles labelled appropriately and immediately preserved at 4 °C to prevent further changes in the PTEs concentration. Freshly deposited marine surface sediments from the upper 5 cm layer were collected. The sediment samples were collected using a stainless steel scooper, with an internal diameter of 9 cm. The collected sediments were then placed in polyethylene bags, labelled appropriately and refrigerated at 4 °C immediately to avoid changes in PTEs distribution. These samples were then carried to the work station until further analysis. These samples were then wet fractionated using <63 μm sieve (USA Standard Testing Sieve, A.S.T.M.E-11 specification, OH, USA); before this process the large calcareous debris and rock fragments were carefully removed using plastic tweezers.
Treatment of tissue samples for PTEs analysis
The oven dried and weighed samples of A. pigmentifera each were used for the analysis of PTEs by the method of Mc Carthy and Ellis (1991). The samples were placed in Teflon® beakers and digested with 10 ml of 70 % nitric acid (HNO3) in microwave vessel in an IFB (Indian Fine Blanks Ltd.) microwave oven, India. The programme was repeated once again to ensure the total digestion of all samples. After completing the heating programme, the vessel was cooled to room temperature and carefully vented in fume hood. The contents of each vessel were quantitatively transferred to another Teflon® beaker and evaporated to incipient dryness. The residues were re-dissolved in 5 ml of 1:1 HNO3: milli-Q water and brought to a final volume of 50 ml. Clear solutions were obtained in all cases, simultaneously blanks and standards were prepared.
Open acid digestion of marine sediments for PTEs analysis
The sediment samples of <63 μm were analyzed for PTEs by the method of Begum et al. (2007). Briefly, 0.5 g of the sample was added to Teflon® beaker. Each sample was moistened with a few drops of milli-Q water, and then 10 ml of acid mixture containing hydrofluoric acid, nitric acid and perchloric acid (HF: HNO3: HClO4) in the following ratio (7:3:1) was added to each sample. All the chemicals used were of analytical grade. Samples were swirled until completely moist. The beakers were covered with lids and kept overnight for digestion after adding 5 ml of 1 μg/ml 103Rh (rhodium) solution (as an internal standard). The following day the beakers were heated on a hot plate at 220 °C for about 1 h, the lids were removed and the contents were evaporated to incipient dryness until a crystalline paste was obtained. The evaporation process was repeated after adding 5 ml of the above acid mixture in each case. The remaining residues were then dissolved using 20 ml of 1:1 HNO3: milli-Q water. Clear solutions were obtained for all samples. After cooling to room temperature, the volume was made up to 250 ml with milli-Q water and stored in polyethylene bottles until analysis was performed. A procedural blank solution was also prepared.
Seawater analysis
The PTEs in seawater is determined by a simple and rapid method by complexation with ammonium pyrrolidine dithiocarbamate (APDC) and extraction into methyl iso-butylketone (MIBK), following the method of Brooks et al. (1967). In this study, after the APDC/MIBK extraction of PTEs from seawater, samples were analyzed using inductively coupled plasma mass spectroscopy (ICP-MS). The collected seawater samples were passed through 0.4 μm filter paper prior to analysis to eliminate the additional contribution of metal concentration in seawater. The pH of filtered seawater was adjusted to 2 with dilute HNO3 followed by the addition of 1 ml of 1 % APDC and 5 ml of MIBK. The samples are shaken vigorously for 2 min and allowed to stand for 10 min for phase separation. The aqueous phase was drained and the MIBK was transferred in a separating funnel. Five milliliters of 25 % HNO3 was added and shaken for back extraction. After phase separation, the aqueous phase was transferred in a test tube for further analysis by ICP-MS (Model: Perkin-Elmer SCIEX, Model ELAN® DRC II ICP-MS (Toronto, ON, Canada). The inter-elemental effects during analysis were taken care of using built in Elan 2.1 version software. The ICP-MS used in the present study is the most precise and highly sensitive instrument for the detection of PTEs even at nanogram concentration. The concentration of Cd, Cu, Pb, and Zn was highest, and the other PTEs were below the detection limit, hence only these PTEs are considered in the present study. A total of 19 numbers of samples were seen collected during sampling and are analyzed for the existing concentration of PTEs. The salinity of seawater samples collected at different stations were measured using a hand held salinity refractometer (Atago, Japan, S/Mill-E 2442-W06), temperature in the seawater samples was recorded using thermal sensitive probe attached to multimeter (Consort, Belgium, Model C932) and conductivity was measured using calibrated electrode (Consort, Belgium, SK10T JJ7) attached to multimeter.
The accuracy and precision of all the analytical methods was assessed by multi-replicate analysis using NBS marine sediment standard reference materials (MESS-3, PACS-4 and DORM-2 obtained from Canada) and blanks. Standards and blanks were digested and analyzed using the same procedure as the experimental samples were performed; measured values were of 99 % within the standards range values.
Statistical analysis
All values are presented as mean ± SE. The statistical difference between PTEs concentration in the tissues of A. pigmentifera, seawater and sediment samples collected from different stations of Rameswaram coasts was determined using one-way analysis of variance (ANOVA) with Tukey’s HSD post hoc test using Origin 8.5 version software. Results were considered with significance levels of 5 % on each PTEs to test the significant differences between the stations among the PTEs.
Results
Physiochemical properties of seawater
The pH, salinity, temperature and electrical conductivity of the seawater samples collected at different stations of Rameswaram are presented in the Table 1. The pH of seawater samples in stations S1 and S5 are seen ranging from 7.4 to 8.2, moreover the pH at stations S2 and S4 are varying from 7.0 to 8.0. At station S3 the pH is in the range of 7.4–8.0. The salinity among the different stations of RM is ranging from 26 to 34 practical salinity units (psu) at the same time temperature recorded in the different station is 16–26 °C. The electrical conductivity was varying from 48 to 55 mS/cm.
Concentration of PTEs in seawater
Cadmium concentration in seawater samples is varying from 3.13 to 9.02 μg/l. The lowest concentration of Cd 3.13 μg/l was recorded at station S1 in October month, whereas the highest concentration 9.02 μg Cd/l was noticed in station S4 during the month of March with a mean value of 6.43 μg/l. The concentration of Cu in the seawater collected from the different stations of RM coast ranged between 4.23 and 6.21 μg/l, with the highest concentration recorded at station S3, moreover the lowest value recorded at station S5 during the month of March. The concentration of Pb 3.23 μg/l which is highest at station S3 during the month of March and the least value being 1.01 μg Pb/l recorded at station S2 in the month of November (Fig. 2). Concentration of Zn in seawater samples is varying from 0.28 to 0.62 μg/l, with a mean value of 0.346 μg/l, with highest being recorded at station S1 and the least at station S3 both the highest and least values are recorded in the same month (October). The concentration of PTEs in the seawater decreased in the following pattern Zn < Pb < Cu < Cd (Table 2).
The concentration of potential toxic elements in the tissues of Adocia pigmentifera (μg/g), seawater (μg/l) and sediment (μg/g) samples in the five different stations of Rameswaram coast, Gulf of Mannar, India. Asterisk in the graph represents significant difference among stations when compared with S5, ns represents not significant among the stations when compared with S5
Concentration of PTEs in A. pigmentifera
The accumulation pattern of PTEs in the tissue of A. pigmentifera is as follows Zn < Pb < Cu < Cd (Table 2). The Cd concentration is ranging from 30.21 to 55.21 μg/g dry tissue weight. Higher concentration of Cd 55.21 μg/g was recorded at station S2 in the month of October and the least value of 30.21 μg/g recorded at Station S5 during the month of March. Concentration of Cu varied between 15.48 and 33.85 μg/g dry tissue weights. Station S2 recorded the highest concentration in the month of March, moreover the lowest is recorded at station S2 during the month of October (Fig. 2), with a mean value of 23.47 μg/g. Lead concentration in the tissues of A. pigmentifera is seen ranging from 8.09 to 16.31 μg/g, the highest concentration was noticed during the month of October at station S1 and minimum was observed during the month of March at station S2. Of all the studied PTEs the concentration of Zn is seen recorded least in the tissues of A. pigmentifera, which is ranging from 3.07 to 5.01 μg/g. The least concentration was recorded in the station S2, whereas the highest concentration was recorded in station S3 which is the sewage disposal site (Figs. 2, 3).
Concentration of PTEs in sediment
The order of accumulation of all the four PTEs analyzed in all the three sample types (A. pigmentifera, seawater and sediment) followed the same pattern without any deviation Zn < Pb < Cu < Cd (Table 2). The concentration of PTEs was highest in the sediment samples when compared to seawater and tissue of A. pigmentifera and the pattern among the sample types is as follows (seawater < A. pigmentifera < sediment). The highest concentration of Cd 100.45 μg/g dry weight of sediment was observed at station S5 during October month, whereas the lowest (59.27 μg Cd/g) is recorded at station S4 in the month of April. Concentration of Cu varied between 21.81 and 45.24 μg/g with the highest concentration being noticed at station S2, whereas the lowest being noticed at station S1, with a mean value of 31.51 μg Cu/g dry weight of sediment. The concentration of Pb was ranging from 9.05 to 22.09 μg/g dry weight of sediment. The highest concentration of Pb 22.09 μg/g was recorded in the month of October at station S5, whereas the lowest value was recorded at station S4 with a mean value of 9.05 μg Pb/g during March. The concentration of Zn noticed at station S1 is 12.01 μg/g and the least value 6.04 μg/g is seen recorded at station S3 with a mean value of 9.21 μg/g Table 2.
Correlation coefficient of metals in seawater, sediment and A. pigmentifera
Correlation coefficient for seawater samples among the stations showed no significant difference among the locations (p < 0.05), the two PTEs Pb and Zn have shown positive correlation (p < 0.05) with A. pigmentifera and also with seawater and sediment samples collected along the coast of RM, similar results were observed with Cu and Pb in relation to A. pigmentifera and sediment samples collected from the different sites of RM coast (Fig. 4a–c). There was no significant correlation among the PTEs in A. pigmentifera, sediment and seawater samples (Table 2).
Discussion
The current study is an attempt to present the seasonal distribution of PTEs (Cd, Cu, Pb and Zn) and the degree of contamination in seawater, A. pigmentifera and sediment samples collected from different stations (S1–S5) of RM coast, India. The concentration of Cd, Cu, Pb and Zn followed the same trend in the three sample types analyzed, but their distribution varied among different seasons (months). Seasonal changes were observed in all the stations with respect to PTEs. These changes are the result of fluctuation in environmental parameters of the A. pigmentifera habitat (salinity, temperature, pH, electrical conductivity, dissolved oxygen etc.). Similar observation was noticed in the Ulva rigida species by Ustunada et al. (2011). Significant difference in the concentration of Cu, Cd, Pb and Zn was noticed among different stations of RM coast (Fig. 2). The difference in distribution of PTEs in five stations is due changes in levels of pollution discharge over time, availability of PTEs for adsorption as well as variations in the sampling season. Concentration of PTEs in seawater is usually high during the months of March/May and our results are in agreement to the results of Sulochanan et al. (2007) and Rao et al. (2007, 2009). Similar findings were noticed in the coastal waters of Kalpakkam which indicated high concentration of other PTEs during the month of March (Satpathy et al. 2008). But our results were contradictory to that of Chakraborty et al. (2009) who noticed high concentration of PTEs during the moths of September/October and low concentration during the period of march/may. There exists several reasons which attribute to the high concentration of PTEs which include huge run off from the surrounding locations and also several other properties of seawater which are seen influencing the distribution of PTEs in different seasons (Chakraborty et al. 2009; Barua et al. 2011). The seasonal variation of the PTEs in seawater samples collected at different stations are significantly influenced by the physiochemical properties of seawater such as pH, temperature, salinity, electrical conductivity and dissolved oxygen (Wong et al. 2000). At the same time the solubility of PTEs is mainly regulated by the seawater pH, concentration and nature of the organic ligands, metal oxidation state and redox conditions existing in the environment (Lalah et al. 2008). In the current study the concentration of PTEs in seawater are significantly higher in the month of March this high concentrations could be due to the increase in the rate of PTEs accumulation due to the increase in temperature and decrease in salinity at the same time lower concentration in the month of October might be due to the lower temperature and sedimentation which results in decrease in the concentration of PTEs (Coulibaly et al. 2012). The concentration of PTEs in seawater exceeded the environmental impact assessment reports, FAO/WHO limits (Yigit et al. 2002; Bashir et al. 2012) indicating significant contamination of PTEs in the seawater samples during all the studied seasons.
Sediment samples revealed high concentration of PTEs at different stations in the month of October in the current study which is entirely contradictory when compared among other coastal regions (Gaspic et al. 2009; Wang et al. 2010; Fang and Yang 2011). Monsoon season in the coastal regions of RM begins during the month of October and ends in December (Krishna Kumar et al. 2010); during this period there is heavy rainfall in the coast. Turbulent conditions appear in the monsoon season during which the fresh water stream from different sources dilutes the sea. Due to the dilution of the seawater, variation in salinity is seen occurring, which is complicated by mixing of waters brought into the region by currents causing turbulent exchange. The sea becomes turbid due to the resuspension of sediment which results in the enhancement of PTEs concentration in the environment (Jonathan et al. 2004; Barua et al. 2011; Rajan et al. 2012; Jagadeesan et al. 2013). Further, increase in the concentration of nutrients, phosphates, organic compounds, organic matter and changes in the salinity during monsoon period causes increase in the concentration of PTEs in the ambient medium and leads to the deposition in the sediments (Martin et al. 2012). Earlier reports have indicated high concentration of PTEs in the core sediments of GoM, India, during the month of December (Sundararajan and Srinivasalu 2010). High concentrations of Cu, Pb and Zn are reported from other coastal regions (Ravera et al. 2003; Franca et al. 2005; Papastergios et al. 2010). Contrary to the present results, the concentrations of the following PTEs were relatively low in the month of October in the west coast regions of India (Chakraborty et al. 2009). Natural background levels of PTEs exist in the majority of sediments due to mineral weathering and natural soil erosion. It is due to the anthropogenic activities which accelerate or antagonize this process that the background levels are increased resulting in pollution of the environment (Koigoora et al. 2013). When comparing among different stations one should be aware of many factors (grain size) influencing their distribution and mineralogical composition of the sediment (Koigoora et al. 2013). The contamination of sediment with Cd, Cu, Pb and Zn is reported from the coastal regions of Palk Bay, GoM and RM, India, by Sulochanan et al. (2007), but the reported concentrations were relatively low for Cd and Cu, but at the same time the concentrations of Pb and Zn were higher in the same station irrespective of the season. The concentration of Cd and Cu presented in the current study is several times higher when compared to the previous studies from the southeast coast of India (Jonathan and Ram Mohan 2003; Jonathan et al. 2004; Stephen-Pichaimani et al. 2008; Sankar et al. 2010). Except for the concentration of Zn, rest of all the PTEs concentrations exceeded the sediment background values (Bervoets and Blust 2003) in all the seasons indicating significant contamination of these PTEs in the marine sediments of RM coast of India. The contamination factor (CF) as reported by Turekian and Wedepohl (1961), is an effective tool in the evaluation of significant contamination over certain period of time. High CF value for Cd revealed significant enrichment of these PTEs in the sediments of RM coast. The contamination levels of Cu and Pb are moderate and moreover the contamination of Zn was significantly low in the coast of RM as presented in the Table 3. The current CF values measured for the PTEs are in agreement with the sediment background values which indicated the same pattern of contamination. Geoaccumulation index (I geo) originally defined by Muller (1969), is a quantitative measure of the PTEs pollution in the marine sediments (Koigoora et al. 2013). It is used to determine the contamination levels in the study area. The measured I geo values for the different PTEs in the sediments of RM coast indicate high contamination of Cd followed by unpolluted levels of rest of the PTEs (Table 3).
The uptake of PTEs in A. pigmentifera was higher when compared to Sigmadocia fibulata and Haliclona tenuiramosa collected from GoM, India (Rao et al. 2007, 2009). The concentrations of Cd, Cu and Pb are higher in the present study when compared to those reported in Suberites cf. diversicolor reported from Australia (de Mestre et al. 2012) and in Spongia officinalis collected from the coast of Antarctica (Negri et al. 2006). Similar kind of studies performed in France reported higher concentrations of Cu, Pb and Zn in S. officinalis (Perez et al. 2005). The relative abundance of PTEs in the tissues of A. pigmentifera reflects uptake in proportion to that of the PTEs in sediments and seawater. High concentration of PTEs in the tissues of A. pigmentifera reflects the capacity of the sponge to accumulate these PTEs. Similar kind of observation in other sponge species was noticed from our earlier studies conducted at GoM, India (Rao et al. 2006, 2007, 2009). Coral skeletal tissues displayed high concentration of Cd and Pb collected from coastal regions of GoM, India (Krishna Kumar et al. 2010). Urban sprawl, storm water runoff and sewage inputs both from domestic and industrial sources have resulted in the high concentrations of Cd and Pb in the coral tissues (Krishna Kumar et al. 2010). Our results are in agreement with the results of Krishna Kumar et al. (2010) who observed high concentrations of Cu and Zn from the coastal regions of GoM, India. Seasonal variation of PTEs (Cd, Cu, Pb and Zn) in A. pigmentifera is also associated with growth dynamics. The concentrations of PTEs were low during the months March–April and higher concentrations have been reported during the months October–December; this is due to the presence of young individuals of A. pigmentifera which are seen growing very fast during their young stage and similar increase in growth rate was observed in several species of sea grass (Ward 1987; Nobi et al. 2010; Manikandan et al. 2010). Seasonal variation of PTEs in A. pigmentifera might be due to the fact that these are loosely bound to the suspended particulate fraction and at the same time existing low pH has also caused the increased solubility and mobility of PTEs which increase their availability (Violante et al. 2010). The variation of PTEs is also affected by the total concentration in seawater and sediments; it seems that some of the PTEs are found to be cleaned from the surface of the sediments by sponge tissues and some from the suspended seawater particles. A comparative study of PTEs concentration in different species of sponges that are in other geographical areas has been presented in Table 4. The concentration of Zn in the present study is lower when compared to all the other studied areas, whereas the concentration of Cu are reported to be higher in the tissue of sponges from Australia, France and Saudi Arabia, whereas the Pb concentration was higher only at France (Table 4).
Conclusion
Rameswaram is one of the marine biosphere reserve hotspots of the southeast coast of India with a diverse ecosystem. With the obtained current results, it is apt that RM is contaminated with PTEs in sediment, A. pigmentifera and seawater samples. This initial finding supports the notion that A. pigmentifera can differently accumulate PTEs from seawater and sediment of the RM coast during different seasons, even though the concentration of PTEs in A. pigmentifera at different stations was changing widely, reflecting contamination in both sediment and seawater column. The difference in the concentration of PTEs in the different sample types is due to changes in the level of pollution discharge over time, availability of PTEs for adsorption as well as variation in the sampling seasons will contribute to the variation in their concentration. Except the concentration of Zn in A. pigmentifera and sediment rest of all the PTEs have exceeded the FAO/WHO/sediment background values representing a significant contamination of these PTEs to the inhibiting biota and could be at potential risk. Proper measures need to be taken in order to protect the fragile ecosystem of RM from contaminants and also reduce environmental risk. The current study provides a base line data on RM coast for future research.
References
Abdullah MH, Sidi J, Aris AZ (2007) Heavy metals (Cd, Cu, Cr, Pb and Zn) in Meretrix meretrix roding, water and sediments from estuaries in Sabah, North Borneo. Int J Environ Sci Edu 2(3):69–74
Alyahya H, EI-Gendy AH, Farraj SA, EI-Hedeny M (2011) Evaluation of heavy metal pollution in the Arabian Gulf using the clam Meretrix meretrix Linnaeus, 1758. Water Air Soil Pollut 214(1–4):499–507
Arsene C, Bougatioti A, Mihalopoulos N (2009) Sources and variability of non-methane hydrocarbons in the Eastern Mediterranean. Global NEST J 11:333–340
Barua P, Mitra A, Banerjee K, Chowdhury MSN (2011) Seasonal variation of heavy metals accumulation in water and oyster (Saccostrea cucullata) inhabiting central and western sector of Indian Sundarbans. Environ Res J 5(3):121–130
Bashir FA, Othman MS, Mazlan AG (2012) Evaluation of trace metal levels in tissues of two commercial fish species in Kapar and Mersing coastal waters, peninsular Malaysia. J Environ Public Health 2012:1–9
Begum Z, Balaram V, Ahmad SM, Satyanarayanan M, Gnaneshwar Rao T (2007) Determination of trace and rare earth elements in marine sediment reference materials by ICP-MS: comparison of open and closed acid digestion methods. At Spectrosc 28(2):41–50
Bervoets L, Blust R (2003) Metal concentrations in water, sediment and gudgeon (Gobio gobio) from a pollution gradient: relationship with fish condition factor. Environ Pollut 126(1):9–19
Brooks R, Presley BJ, Kaplan IR (1967) APDC-MIBK extraction system for the determination of trace elements in saline waters by atomic-absorption spectrophotometry. Talanta 14(7):809–816
Cachada A, Dias A, Pato P, Mieiro C, Rocha-Santos T, Pereira M, Ferreira da Silva E, Duarte A (2012) Major inputs and mobility of potentially toxic elements contamination in urban areas. Environ Monit Assess 185(1):279–294
Chakraborty R, Zaman S, Mukhopadhyay N, Banerjee K, Mitra A (2009) Seasonal variation of Zn, Cu and Pb in the estuarine stretch of West Bengal. Indian J Mar Sci 28:104–109
Choi SC, Wai OWH, Choi TWH, Li XD, Tsang CW (2006) Distribution of cadmium, chromium, copper, lead and zinc in marine sediments in Hong Kong waters. Environ Geol 51:455–461
Coulibaly S, Atse BC, Koffi KM, Sylla S, Konan KJ, Kouassi NJ (2012) Seasonal accumulations of some heavy metal in water, sediment and tissues of black-chinned tilapia Sarotherodon melanotheron from Biétri Bay in Ebrié Lagoon, Ivory Coast. Bull Environ Contam Toxicol 88(4):571–576
de Mestre C, Maher W, Roberts D, Broad A, Krikowa F, Davis AR (2012) Sponges as sentinels: patterns of spatial and intra-individual variation in trace metal concentration. Mar Pollut Bull 64(1):80–89
Demirak A, Yilmaz F, Tuna AL, Ozdemir N (2006) Heavy metals in water, sediment and tissues of Leuciscus cephalus from a stream in southwestern Turkey. Chemosphere 63(9):1451–1458
Fang GC, Yang HC (2011) Comparison of heavy metals in marine sediments from coast areas in East and Southeast Asian countries during the years 2000–2010. Toxicol Ind Health 27(8):754–759
FAO/WHO (1989) Evaluation of certain food additives and the contaminants mercury, lead and cadmium, WHO Technical Report Series No. 505
Franca S, Vinagre C, Caçador I, Cabral HN (2005) Heavy metal concentrations in sediment, benthic invertebrates and fish in three salt marsh areas subjected to different pollution loads in the Tagus Estuary (Portugal). Mar Pollut Bull 50(9):998–1003
Gaspic ZK, Bogner D, Ujević I (2009) Trace metals (Cd, Pb, Cu, Zn and Ni) in sediment of the submarine pit Dragon ear (Soline Bay, Rogoznica, Croatia). Environ Geol 58(4):751–760
Genta-Jouve G, Cachet N, Oberhänsli F, Noyer C, Teyssié JL, Thomas OP, Lacoue-Labarthe T (2012) Comparative bioaccumulation kinetics of trace elements in Mediterranean marine sponges. Chemosphere 89(3):340–349
Gochfeld M (2003) Cases of mercury exposure, bioavailability, and adsorption. Ecotoxicol Environ Safety 56(1):174–179
ITS (2000) The Ministry of the Agriculture of Turkey, Rep. 5
Jagadeesan L, Jyothibabu R, Anjusha A, Mohan AP (2013) Ocean currents structuring the mesozooplankton in the Gulf of Mannar and the Palk Bay, southeast coast of India. Prog Oceanograp 110:27–48
Janaki-Raman D, Jonathan MP, Srinivasalu S, Armstrong-Altrin JS, Mohan SP, Ram-Mohan V (2007) Trace metal enrichments in core sediments in Muthupet mangroves, SE coast of India: application of acid leachable technique. Environ Pollut 145(1):245–257
Jonathan MP, Ram Mohan V (2003) Heavy metals in sediments of the inner shelf off the Gulf of Mannar, South East Coast of India. Mar Pollut Bull 46(2):263–268
Jonathan MP, Ram-Mohan V, Srinivasalu S (2004) Geochemical variations of major and trace elements in recent sediments, off the Gulf of Mannar, the southeast coast of India. Environ Geol 45(4):466–480
Khoshnoud MJ, Mobini K, Javidnia K, Hosseinkhezri P, Jamshid KA (2011) Heavy metals (Zn, Cu, Pb, Cd and Hg) contents and fatty acids ratios in two fish species (Scomberomorus commerson and Otolithes ruber) of the Persian Gulf. Iran J Pharm Res 7(3):191–196
Koigoora S, Ahmad I, Pallela R, Janapala VR (2013) Spatial variation of potentially toxic elements in different grain size fractions of marine sediments from Gulf of Mannar, India. Environ Monit Assess 185(9):7581–7589
Krishna Kumar S, Chandrasekar N, Seralathan P (2010) Trace elements contamination in coral reef skeleton, Gulf of Mannar, India. Bull Environ Contam Toxicol 84(1):141–146
Lalah JO, Ochieng EZ, Wandiga SO (2008) Sources of heavy metal input into Winam Gulf, Kenya. Bull Environ Contam Toxicol 81(3):277–284
Manikandan S, Ganesapandian S, Singh M, Kumaraguru A (2010) Seagrass diversity and associated flora and fauna in the coral reef ecosystem of the Gulf of Mannar, Southeast Coast of India. Res J Environ Earth Sci 3(4):321–326
Martin GD, George R, Shaiju P, Muraleedharan KR, Nair SM, Chandramohana Kumar N (2012) Toxic metals enrichment in the surficial sediments of a eutrophic tropical estuary (Cochin Backwaters, Southwest Coast of India). Sci World J 2012:17
Mc Carthy HT, Ellis PC (1991) Comparison of microwave digestion with conventional wet ashing and dry ashing digestion for analysis of lead, cadmium, chromium, copper, and zinc in shellfish by flame atomic absorption spectroscopy. J Assoc Off Anal Chem 74(3):566–569
Muller G (1969) Index of geoaccumulation in the sediments of the Rhine River. Geol J 2:108–118
Mylopoulos Y, Kolokythas E, Vagiona D, Kampragou E, Eleftheriadou E (2008) Hydrodiplomacy in practice: transboundary water management in northern Greece. Global NEST J 10:287–294
Negri A, Burns K, Boyle S, Brinkman D, Webster N (2006) Contamination in sediments, bivalves and sponges of McMurdo Sound, Antarctica. Environ Pollut 143(3):456–467
Nobi EP, Dilipan E, Thangaradjou T, Sivakumar K, Kannan L (2010) Geochemical and geo-statistical assessment of heavy metal concentration in the sediments of different coastal ecosystems of Andaman Islands, India. Est Coastal Shelf Sci 87(2):253–264
Pan K, Lee OO, Qian PY, Wang WX (2011) Sponges and sediments as monitoring tools of metal contamination in the eastern coast of the Red Sea, Saudi Arabia. Mar Pollut Bull 62(5):1140–1146
Papastergios G, Filippidis A, Fernandez Turiel JL, Gimeno D, Sikalidis C (2010) Distribution of potentially toxic elements in sediments of an industrialized coastal zone of the Northern Aegean Sea. Environ Forensics 11(3):282–292
Perez T, Longet D, Schembri T, Rebouillon P, Vacelet J (2005) Effects of 12 years’ operation of a sewage treatment plant on trace metal occurrence within a Mediterranean commercial sponge (Spongia officinalis, Demospongiae). Mar Pollut Bull 50(3):301–309
Raj SM, Jayaprakash M (2008) Distribution and enrichment of trace metals in marine sediments of Bay of Bengal, off Ennore, south-east coast of India. Environ Geol 56:207–217
Rajan S, Firdaus N, Appukutty M, Ramasamy K (2012) Effects of climate changes on dissolved heavy metal concentrations among recreational park tributaries in Pahang, Malaysia. Biomed Res 23(1):23–30
Rao JV, Kavitha P, Reddy NC, Rao TG (2006) Petrosia testudinaria as a biomarker for metal contamination at Gulf of Mannar, southeast coast of India. Chemosphere 65(4):634–638
Rao JV, Kavitha P, Srikanth K, Usman P, Rao T (2007) Environmental contamination using accumulation of metals in marine sponge, Sigmadocia fibulata inhabiting the coastal waters of Gulf of Mannar, India. Toxicol Environ Chem 89(3):487–498
Rao JV, Usman PK, Kumar JB (2008) Larvicidal and insecticidal properties of some marine sponges collected in Palk Bay and Gulf of Mannar waters. Afr J Biotechnol 7(2):109–113
Rao JV, Srikanth K, Pallela R, Rao T (2009) The use of marine sponge Haliclona tenuiramosa as bioindicator to monitor heavy metal pollution in the coasts of Gulf of Mannar, India. Environ Monit Assess 6(1–4):451–459
Ravera O, Cenci R, Beone G, Dantas M, Lodigiani P (2003) Trace element concentrations in freshwater mussels and macrophytes as related to those in their environment. J Limnol 62(1):61–70
Sankar R, Ramkumar L, Rajkumar M, Sun J, Ananthan G (2010) Seasonal variations in physico-chemical parameters and heavy metals in water and sediments of Uppanar estuary, Nagapattinam, India. J Environ Biol 31(5):681–686
Satpathy K, Natesan U, Sarguru A, Mohanty A, Prasad M, Sarkar S (2008) Seasonal variations in mercury concentrations in the coastal waters of Kalpakkam, southeast coast of India. Curr Sci 95(3):374–381
Stephen-Pichaimani V, Jonathan M, Srinivasalu S, Rajeshwara Rao N, Mohan S (2008) Enrichment of trace metals in surface sediments from the northern part of Point Calimere, SE coast of India. Environ Geol 55(8):1811–1819
Sulochanan B, Krishnakumar P, Prema D, Kaladharan P, Valsala K, Bhat G, Muniyandi K (2007) Trace metal contamination of the marine environment in Palk Bay and Gulf of Mannar. J Mar Biol Assoc India 49(1):12–18
Sundararajan M, Srinivasalu S (2010) Geochemistry of Core Sediments from Gulf of Mannar, India. Int J Environ Res 4(3):861–876
Turekian KK, Wedepohl KH (1961) Distribution of the elements in some major units of the earth’s crust. Geol Soc Am 72:175–192
Ustunada M, Erduğan H, Yılmaz S, Akgul R, Aysel V (2011) Seasonal concentrations of some heavy metals (Cd, Pb, Zn, and Cu) in Ulva rigida J. Agardh (Chlorophyta) from Dardanelles (Canakkale, Turkey). Environ Monit Assess 177(1–4):337–342
Violante A, Cozzolino V, Perelomov L, Caporale A, Pigna M (2010) Mobility and bioavailability of heavy metals and metalloids in soil environments. J Soil Sci Plant Nutr 10(3):268–292
Wang S, Jia Y, Wang S, Wang X, Wang H, Zhao Z, Liu B (2010) Fractionation of heavy metals in shallow marine sediments from Jinzhou Bay, China. J Environ Sci (China) 22(1):23–31
Ward TJ (1987) Temporal variation of metals in the seagrass Posidonia australis and its potential as a sentinel accumulator near a lead smelter. Mar Biol 95(2):315–321
Wong CKC, Cheung RYH, Wong MH (2000) Heavy metal concentration in green lipped mussels collected from Tolo Habour and markets in Hong Kong and Shenzhen. Environ Pollut 109(1):165–171
Yigit N, Colak E, Ketenoglu O, Kurt L, Sozen M, Hamzaoglu E, Karatas AS, Ozkurt S (2002) Environment effect valuation (CED). Ankara, pp 354–381
Acknowledgments
The authors are thankful to Department of Biotechnology (DBT), Government of India for financial assistance, and also thankful to the Director, IICT for providing the facilities and his constant encouragement. The author K. Srikanth is thankful to CSIR, for providing the Senior Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Srikanth, K., Rao, J.V. Spatial and seasonal variation of potential toxic elements in Adocia pigmentifera, seawater and sediment from Rameswaram, southeast coast of India. Environ Earth Sci 72, 2905–2916 (2014). https://doi.org/10.1007/s12665-014-3194-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-014-3194-9