Abstract
The Wujiang River is an important tributary to the Changjiang River that has been intensively impounded for hydropower exploitation. To understand the potential impact of reservoir construction on the riverine inorganic carbon transport, seasonal longitudinal sampling was conducted in four reservoirs Hongjiadu (HJD), Dongfeng (DF), Suofengying (SFY) and Wujiangdu (WJD) along the Wujiang River from April 2006 to January 2007. Results indicated that damming the river induced an obvious discontinuity of water chemistry in the warmer seasons. δ 13C of dissolved inorganic carbon (DIC) ranged from −3 to −11.4 ‰, likely as the results of photosynthesis, respiration and carbonate weathering. During periods of thermal stratification, the addition of CO2 from respiration to hypolimnion and the deep water release for hydropower generation led to higher pCO2 downstream, as well as 13C depletion in DIC and undersaturated to calcite. An estimate of DIC budget indicated that only DF reservoir was the sink for DIC while reservoirs HJD, SFY and WJD were the sources for DIC. However, when the retained water was taken into account, for the reason of water storage occurring mainly in HJD and DF, all reservoirs became the sources for DIC with exporting rates of 26.68, 7.97, 6.22 and 11.80 % for HJD, DF, SFY and WJD, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rivers are usually a chief medium between the terrestrial and oceanic ecosystems and play an important role in the transport of carbon from land to ocean. Despite the fact that fluvial carbon export is only a small portion (~1015 g C year−1) of the global carbon cycle (Meybeck 1982; Aucour et al. 1999), it contributes an important percentage to the regional carbon budget. Fluvial carbon also has a close relationship with chemical weathering in drainage basin (Gaillardet et al. 1999; Raymond and Cole 2003), which has been known as a control of the concentration of atmospheric CO2 at the geological timescale (>106 y). Thus, research on the river carbon cycling is crucial to understand the geochemical processes in drainage basins, specifically in estimating the weathering rate of drainage basin and the atmospheric CO2 consumption as well as in identifying different sources of carbon input. This study focuses on the impact of river impoundment on the transport and transformation of riverine inorganic carbon.
However, the geochemical information on river chemistry is not only an integrated response to the geochemical processes in drainage basin, but can also be altered by physical, chemical and biological processes in the aquatic system. The increasing anthropogenic activities further complicate carbon cycling in rivers, for instance, river damming and eutrophication are widely regarded as the most remarkable and extensive events imposed on river systems, but how these factors alter river carbon speciation and fluxes have not been well-documented. Consequently, chemical data must be approached with caution, particularly when evaluating the carbon cycling at the catchment scale.
Impoundment converts a river into an “artificial lake”, and generally leads to a decrease in suspended solids and turbidity, an increase in water residence times, and the development of thermal stratification in the water column. As a result, the river’s pristine status in hydrological condition and the nutrient concentrations would gradually shift to “lacustrine/reservoir” type (Saito et al. 2001). The major biological processes (e.g. photosynthesis and respiration) in reservoirs have significant impact on the transformation among carbon species, as well as on the thermodynamic equilibrium of carbonate in the water column. Due to the commonly adopted deep water discharge and the counter-season impounding (i.e. hydropower reservoirs generally maintain a low water level at flood season, and a high water level during the period when a river is at its normal level) hydropower reservoirs potentially bring a discontinuity in the water chemistry along the river channel. Consequently, the nature and quantity of fluvial nutrient fluxes to oceans have experienced enormous changes by hydrological alterations in the last few decades (Ittekkot et al. 2000).
Currently, the “damming effect” on dissolved silicon retention, greenhouse gas emissions, downstream fishery resources, fluvial flux of nutrients as well as on ecological risks has been well-documented (such as, Li and Zhang 1997; Campo and Sancholuz 1998; Conley et al. 2000; Abril et al. 2005; Avilés and Niell 2007; Devi et al. 2008). In comparison, the impact of river impoundment on the carbon transformation and the changes in fluvial carbon flux are not fully understood. To take full advantage of waterpower, the river was continuously dammed creating a reservoir chain. The “impounded river” has become an important hydrological landscape imposed on rivers; for example, over 48,000 reservoirs are presently in operation in Changjiang River drainage basin, China. Undoubtedly, this phenomenon can profoundly disturb the natural cycling of river carbon. This study investigated a reservoir chain constructed on the Wujiang River system in karstic area. The main objective was to investigate (1) main processes control the longitudinal discontinuity of riverine inorganic carbon cycle in reservoir and river system; (2) the carbonate equilibrium system in water varies in this area, and (3) the budget of DIC in these reservoirs.
Methods
Study area
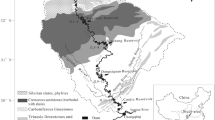
The Wujiang River is the largest tributary to the upper reaches of the Changjiang River and ends at Fuling City, where the waterway continues to join the Changjiang River (Fig. 1). With a total length of 1,037 km, a drainage basin of 88,267 km2 and a mean water discharge of 1,690 m3/s, the Wujiang is the largest river in the Guizhou Province (Han and Liu 2004; Yu et al. 2008).
The Wujiang basin is located in karstic areas with an average annual temperature of 12.3 °C. The coldest month is January with an average temperature of 3.5 °C, and the hottest month is July with an average temperature of 26 °C. The annual precipitation in this area ranges from 1,100 to 1,300 mm with roughly 75 % of that falling between May and October. The fall is almost 1,000 m between its upper reach (with an altitude of 1,500 m) and lower reach. Along the upper reaches of the Wujiang River, the catchment is developed Permian and Triassic carbonate rocks and coal-bearing formations. The middle reaches of the Wujiang River watershed contain Permian and Triassic limestone, dolomitic limestone and dolomites, while the lower reaches of the river contain mainly carbonate rocks, shale, sands shale and siltstones (Han and Liu 2004).
The Wujiangdu (WJD) Reservoir, Dongfeng (DF) Reservoir, Hongjiadu (HJD) Reservoir and Suofengying (SFY) Reservoir are all situated along the middle and upper reaches of the Wujiang River. They were impounded in 1979, 1994, 2004 and 2006, respectively. The main features of these reservoirs are described in Table 1.
Sampling and analysis
Water samples were collected in April, July and October 2006, as well in January 2007, which represent spring, summer, autumn and winter. The sampling stations are indicated in Fig. 1. In each reservoir, samples of the inflow and downstream waters were collected 0.5 m under the water surface, while water samples along the water column were carried out in the central part of the reservoir using a Niskin bottle. For the tributaries, water was taken 0.5 m under the water surface. During sampling seasons, the subsidiary facilities of SFY Reservoir were still under construction. Therefore, this reservoir’s water profile was not sampled.
The pH and temperature (T) were measured in situ with a portable YSI 6600V2 (YSI Inc., USA). Alkalinity was titrated with HCl in the field. For δ 13CDIC determination, 100 mL of water was filtered through a 0.45 μm polycarbonate membrane poisoned with a few drops of saturated HgCl2 solution immediately after sampling, and stored in 100 mL plastic bottles in a cool box. δ 13CDIC determination was performed following the method described by Atekwana and Krishnamurthy (1998). 15 mL of water sample is injected into the closed evacuated glass vessels already containing approximately 2 mL of concentrated phosphoric acid and was then heated at 50 °C for CO2 extraction. Extracted CO2 was quantitatively frozen into an evacuated vial cooled with liquid nitrogen, and the resulting gas was analyzed on an MAT252 mass spectrometer with the analytical precision at ±0.1 ‰.
Major cations (Mg2+, Ca2+, K+ and Na+) were analyzed by inductively coupled plasma-optical emission spectrometry (ICP-OES; Vista MPX, Varian Inc.) and the anions (SO4 2−, Cl− and NO3 −) by high performance liquid chromatography. The typical precisions of major ions are about ±5 %.
pCO2 was calculated from HCO3 − and pH. Henry’s constant (K H) and first dissociation constants for CO2 gas in water (K 1) were corrected by temperature and ionic strength (Stumm and Morgan 1981; Raymond et al. 1997; Barth and Veizer 1999; Telmer and Veizer 1999). Calcite saturation index (SIc) was calculated from Eq. 1:
K c is the temperature-dependent equilibrium constant for calcite dissociation.
The diffusion flux of CO2 can be calculated with the Eq. 2:
where, F is the diffusion flux of CO2 between water and the atmosphere, pCO2w is the partial pressure of CO2 in the surface water and gassat is the equilibrium concentration of atmospheric CO2 (390 μatm, http://www.esrl.noaa.gov/gmd/ccgg/globalview/co2/co2_whatsnew.html) in water. K H is Henry’s constant corrected via temperature. The piston velocity, k (cm/h), of CO2 at the water–air interface can be affected by different factors such as river runoff, turbidity, velocity of flow, wind speed and water depth (Abril et al. 2006; Wanninkhof 1992). Considering the similar hydrographic features between studied reservoirs and other reservoirs on the Maotiao River (which belong to the Wujiang River, see Fig. 1), in this study, the k value (k = 3 cm/h for the reservoir surface and k = 10 cm/h for the downstream the dam) as reported by Wang et al. (2011) was used and is referenced therein.
Hydrological data (water discharges) was provided by the Guizhou Bureau of hydrology, as well as by the administrative offices of local reservoirs. DIC mass load for this data was estimated using the following procedure: DIC concentrations of the sampling month were multiplied by the total discharge of the 3 months of the respective season. Precipitation contribution was not included in the estimate because direct precipitation on the water area of HJD reservoirs was <3 % of its total incoming water, and just 0.3, 0.1 and 0.7 % in DF, SFY and WJD reservoirs, respectively. Furthermore, no flood discharge occurred in these reservoirs during the sampling year. As a result, the calculation of DIC load is based on the inflow and outflow. For the reservoirs in series, the head water reservoir (HJD in this study) generally has more than 1-year storage capacity, while the downstream reservoirs have smaller storage capacities (i.e. both DF and WJD are seasonal storage reservoirs, and SFY is a daily storage reservoir). In our sampling year, the ratio of output to input of water in the HJD was 79.3 %. The ratios were 91.4, 100 and 95.6 % for DF, SFY and WJD reservoirs, respectively. So, both the actual DIC retention/release rates, as well as the assumed rate were calculated.
To qualify the contribution of organic matter decomposition to downstream, the following 13C mass balance Eq. (3) was used:
δ 13CDIC: δ 13C of DIC in the discharge water; δ 13Ccc: δ 13C of DIC from chemical weathering (−8.5 ‰); δ 13Corg: δ 13C of organic matter which was decomposed in reservoirs. f is the fraction. This study used −23 ‰ as the isotopic composition of the organic matter here (Li et al. 2008), and assumed that all CO2 decomposed from organic matter dissolves into DIC.
Results
Major water chemistry
Due to the widespread carbonate rocks in the studied area, HCO3 − was the dominant anion in the Wujiang River with a range from 1.1 to 2.87 mmol L−1 and an average value of 2.22 mmol L−1. The second major anion was SO4 2− with an average concentration of 0.81 mmol L−1. Cl− and NO3 − concentrations ranged from 0.05 to 0.26 mmol L−1 and from 0.05 to 0.34 mmol L−1, respectively. In most samples, HCO3 − and SO4 2− accounted for more than 90 % of total anions.
Ca2+ and Mg2+ were the most prevalent cations accounting for more than 80 % of the total cations. The prevalence of Ca2+, Mg2+ and HCO3 − revealed extensive dissolution of carbonate rocks. However, in the Wujiang River, Ca2+ and Mg2+ were largely in excess with respect to HCO3 −, and excess Ca2+ and Mg2+ could be balanced by SO4 2−. Dissolution of sulfate evaporite and oxidation of sulfide minerals were considered to be the possible sources of SO4 2− in this area (Han and Liu 2004; Li et al. 2008).
Water temperature and pH
Water temperature ranged from 11.1 to 30.5 °C with a mean of 19.2 °C, and pH ranged from 7.1 to 9.0 with a mean value of 8.2. Generally, temperature and pH in the surface water showed a similar trend along the main channel of the Wujiang River (Fig. 2). With the exception of winter when the water mixed through the water columns in these reservoirs, water with lower pH and temperature was released from the dams for the rest of the seasons. For instance, the maximum temperature difference between epilimnion and downstream the dam was up to 8 °C with a pH of 1.2 in the Wujiangdu Reservoir in July. Both pH and temperature in the water gradually increased during its journey to the downstream reservoir.
Inorganic carbon system
The concentrations of different species and the isotopic compositions of DIC are listed in Table 2. Seasonally, DIC had the highest concentration in spring with an average of 2.61 mmol L−1. In summer, autumn and winter, the mean concentrations of DIC were 2.04, 2.19 and 2.35 mmol L−1, respectively. DIC concentrations increased downriver in July and October, but showed insignificant changes in April and January (Table 2). In addition, there was a significant increase in DIC concentration in downstream dams, particularly in July and October. The maximum difference of DIC concentrations between the epilimnion of reservoir and its downstream was observed in HJD reservoir in October with a value of 0.36 mmol L−1.
The partial pressure of CO2 (pCO2) in the surface water ranged from 71 to 8,480 μatm, and demonstrated a significant seasonal and longitudinal discontinuity (Fig. 3). In most samples, pCO2 was above the atmospheric level (i.e. around 390 ppm). However, samples taken in April had a mean pCO2 of 375 μatm indicating a status of undersaturation. In July and October, pCO2 had a significant increase in water downstream the dams (Fig. 3). In January, only DF and WJD reservoirs had high pCO2 in their discharge, and in April, the differences in pCO2 decreased in each reservoir. Consequently, the CO2 flux was significantly different between reservoir surface and downstream the dam (Table 2).
Seasonal variations of pCO2 in the surface water along the mainstream of Wujiang. The horizontal dash line is the level of atmospheric CO2. Legends of 1, 2, 3 and 4 are same as in Fig. 2
Controlled by the pCO2, the saturation index of calcite (SIc) also demonstrated an obvious seasonal discontinuity along the river channel. The SIc results of most of the samples were above zero indicating that the water was oversaturated with CaCO3. As a result, precipitation of calcite was thermodynamically favored along the river. However, SIc in the downstream the dams showed an abrupt decrease down to below zero in July and October (Table 2). During its transport downstream, SIc increased again to above zero. In January, only the DF reservoir released water with SIc below zero.
In summer and autumn, δ 13CDIC also showed a discontinuity between epilimnion in reservoir and downstream waters. δ 13CDIC ranged from −3 to −11.4 ‰ with a mean value of −7.8 ‰ (Table 2; Fig. 4), indicating that the major source of DIC is from carbonate weathering. While δ 13CDIC decreased downriver in all seasons, this trend was more notable in July and October. A “sudden fall” of δ 13CDIC was observed in the downstream of each reservoir, especially in the upstream reservoir (HJD).
Seasonal variations of δ 13CDIC in surface water along the mainstream of Wujiang. Legends of 1, 2, 3 and 4 are same as in Fig. 2
As shown in Fig. 5, seasonal thermal stratification of the water columns developed in the studied reservoirs. Generally, the temperature gradient began to form from April in these reservoirs, and thermal stratification could persist from July to October. In January, the water mixed well vertically. Contrary to water temperature, DIC concentrations along the water column tended to increase from the epilimnion to hypolimnion, particularly in warmer seasons. In January and April, DIC concentrations were significantly higher than those in July and October (Fig. 5). However, during all seasons in the WJD reservoir, DIC concentration was relatively stable along the water column.
Figure 6 shows the seasonal distribution of pCO2 and δ 13CDIC. There were significant variations of pCO2 in these reservoirs. Hypolimnion had high pCO2 in the warmer seasons, especially in the summer. Due to the elimination of thermal stratification during the cold seasons there was little variation in pCO2 in the water column. Comparatively, the headwater reservoirs (HJD) had the highest mean pCO2, while the WJD reservoir had the lowest average among the reservoirs. δ 13CDIC showed a similar distribution to that of pCO2 in the studied reservoirs (Fig. 6). Epilimnion had more positive δ 13CDIC than hypolimnion. Among these reservoirs, δ 13CDIC in WJD reservoir was the lowest, indicating that the largest contribution of DIC comes from respired carbon. In cold seasons, the difference of δ 13CDIC between top and bottom water lessened due to the mixing of the water column.
DIC budget
Based on the result of DIC fluvial flux and CO2 emission flux, only DF Reservoir retained 6.16 % of DIC. On an annual basis, HJD, SFY and WJD reservoirs all exported an excess of 0.7, 11.8 and 0.4 kton of DIC, respectively (Table 3). These estimates from the sampling year showed different source/sink effect on DIC in these reservoirs partly due to the water imbalance in HJD and DF reservoirs.
In fact, river water will finally reach a balance on the over-year scale. Thus, the assumed DIC budget was also estimated by taking into account the imbalance of water volume between inflow and outflow, given that the retained water in a reservoir had been released and the balance of water volume had been reached. Under this assumption, all reservoirs become sources to the DIC. For example, about 26.6 % more of the DIC was discharged downstream than that annually entering the HJD reservoir. The assumed exporting rates for DF, SFY and WJD reservoirs were 7.9, 6.2 and 11.8 %, respectively (Table 3). The main possible source for these excess DIC is from the microbial transformation of organic carbon to inorganic carbon in these reservoirs (Table 4). Furthermore, calcite precipitation possibly impacts on the DIC fluxes, but it was not estimated here.
Discussion
Factors controlling δ 13CDIC in the impounded river
In general, DIC in the river water are mainly derived from: (1) the dissolution of carbonates, (2) soil CO2, (3) respiration process in the river and (4) direct input from precipitation. Source (4) generally has a minor contribution to the river DIC pool (Telmer and Veizer 1999). The atmospheric CO2 invasion may also contribute to the river DIC pool, when pCO2 in river water is lower than that in atmosphere. In this study, most samples had pCO2 considerably higher than atmospheric level with the exception of a few samples in April (Table 2). Thus, sources (1), (2) and (3) should be the major DIC sources to the studied reservoirs. In these sources, marine limestone carbonate typically has an average δ 13C of 0 ‰ (Andrews et al. 2001); δ 13C of soil CO2 in this area was reported to be about −23 ‰ with a range of −28.7 to −17.9 ‰, likely originated from the decomposition of organic matter produced by C-3 plants (Li et al. 2008). The dissolution of soil CO2 in soil water will lead to an enrichment of 13C giving soil water an average δ 13C of −17 ‰ (Telmer and Veizer 1999). Dissolution of marine carbonates (δ 13C ≈ 0 ‰) in soil water generally produces DIC with δ 13C around −8.5 ‰. As a result, due to the karstic lithological background in this study, chemical weathering should be the main contribution to river DIC, with δ 13CDIC around −8.5 ‰. Consequently, δ 13CDIC deviating from this value will reflect other biogeochemical processes or the input of respired CO2.
Rivers do not passively transport carbon to the oceans. The presence of lakes and reservoirs has significant impacts on the fluxes and compositions of riverine DIC (Brunet et al. 2005; Wachniew 2006). First, after a river is impounded, algal photosynthesis will be enhanced due to both the improvement of transparency in the reservoir and the decrease in flow speed. Photosynthetic assimilation of CO2 leads to the enrichment of 13C in the remaining DIC pool toward ~1 ‰ of δ 13CDIC (Fig. 7), which is only the result of the equilibrium fractionation of CO2 exchange between atmosphere and the river (Yang et al. 1996; Aucour et al. 1999). CO2 outgassing may also lead to 13C enrichment in DIC (Doctor et al. 2008). Figure 7 showed that the most samples in July and October were above the dissolution line of carbonate indicating the influence of photosynthesis and CO2 outgassing. This is because during these two seasons, pCO2 in the river was significantly higher than the atmospheric level. The decomposition of autochthonous and allochthonous organic matter in these reservoirs releases DIC (δ 13C < −25 ‰) into the DIC pool. As a result, δ 13CDIC values will fall below the dissolution line of carbonate, and higher pCO2 should be observed in the water column (Fig. 7; Table 2). For instance, water downstream the dams during summer and autumn had δ 13CDIC below the carbonate dissolution line, and also showed higher DIC concentrations (Fig. 7; Table 2). The high pCO2 in the discharged water was possibly a result of increasing contribution from microbial respiration. From winter to spring, samples of mainstream and tributaries showed less variation in DIC concentration and δ 13CDIC. This can be explained by the water column mixing during this period. Figure 7 plots DIC concentration versus δ 13CDIC, from which main processes and DIC sources that influenced river δ 13CDIC could be explained.
Mechanisms for the seasonal discontinuity of inorganic carbon system
Due to the deep incision by the river on the karstic background, reservoirs on the Wujiang are quite deep, usually with a maximal depth of more than 100 m. As a result, the stratification of both temperature and water chemistry is easy to develop, as shown in Figs. 5 and 6. Due to the hypolimnetic discharge for hydropower in these reservoirs, released water from the dams had similar physical and chemical characteristics of deep water of the same reservoirs; the discharged water contained the characteristics of water found below 20 m in reservoirs (Fig. 8) with higher pCO2 and more negative δ 13CDIC as the result of the addition of respired carbon, which fall into the lower right of Fig. 8.
However, photosynthesis and the CO2 degassing in the surface water of the river and the reservoirs can both lower pCO2. Both processes also cause the increase in δ 13CDIC values, and these waters fall into the upper-left of Fig. 8. Doctor et al. (2008) also reported that isotopic fractionation of DIC due to CO2 outgassing may be the primary cause of increased δ 13CDIC values downstream when pCO2 of surface water exceeds twice the atmospheric CO2 concentration. In colder seasons (winter to spring), most water samples are located near the line of carbonate dissolution (Fig. 7) indicating the control of carbonate weathering.
The physical obstruction of the dam has significant impacts on the inorganic carbonate equilibrium system particularly in the warmer seasons. The apparent discontinuity of inorganic carbon species could be observed when water was released from the dam. The transformation among carbon species in the reservoirs should be the main effect on the evolution of inorganic carbon along these reservoirs. On the whole, reservoirs tend to increase the export of DIC downstream due to the decomposition of organic matter in the deep water. Our results showed that reservoirs with longer hydrological retention time have a higher discharge rate of DIC (Table 3).
Conclusions
Dams have a major impact on the flux and isotopic compositions of riverine DIC, as well as the major physical and chemical parameters. The carbon chemistry in the river water showed seasonal and longitudinal variations along the river course. In warm seasons, photosynthesis and CO2 evasion into the atmosphere were the major pathways for altering the inorganic carbon equilibrium in surface water. These processes also led to the surface water over saturating to calcite. In the hypolimnion, respiration became more important which led to higher pCO2 and lower δ 13CDIC. Consequently, water released from the dams contained significant differences in chemical composition to that of the upstream waters. In colder seasons, water was well mixed and dam effect on downstream water chemistry weakens. These reservoirs were the source for DIC, if the imbalance of water is taken into account. The headwater reservoir (HJD) is the largest source for DIC with an exporting rate of 26.7 % followed by WJD, DF and SFY reservoirs.
References
Abril G, Guérin F, Richard S, Delmas R, Galy-Lacaux C, Gosse P, Tremblay A, Varfalvy L, Santos MAD, Matvienko B (2005) Carbon dioxide and methane emissions and the carbon budget of a 10-year old tropical reservoir (Petit Saut, French Guiana). Glob Biogeochem Cycles 19:GB4007
Abril G, Richard S, Guérin F (2006) In situ measurements of dissolved gases (CO2 and CH4) in a wide range of concentrations in a tropical reservoir using an equilibrator. Sci Total Environ 354:246–251
Andrews JE, Greenaway AM, Dennis PF, Barnes-Leslie DA (2001) Isotopic effects on inorganic carbon in a tropical river caused by caustic discharges from bauxite processing. Appl Geochem 16:197–206
Atekwana EA, Krishnamurthy RV (1998) Seasonal variations of dissolved inorganic carbon and δ 13C of surface waters: application of a modified gas evolution technique. J Hydrol 205:265–278
Aucour AM, Sheppard SMF, Guyomar O, Wattelet J (1999) Use of δ 13C to trace origin and cycling of inorganic carbon in the Rhône river system. Chem Geol 159:87–105
Avilés A, Niell FX (2007) The control of a small dam in nutrient inputs to a hypertrophic estuary in a Mediterranean climate. Water Air Soil Pollut 180:97–108
Barth JAC, Veizer J (1999) Carbon cycle in St. Lawrence aquatic ecosystems at Cornwall (Ontario), Canada: seasonal and spatial variations. Chem Geol 159:107–128
Brunet F, Gaiero D, Probst JL, Depetris PJ, Lafaye FG, Stille P (2005) δ 13C tracing of dissolved inorganic carbon sources in Patagonian rivers (Argentina). Hydrol Process 19:3321–3344
Campo J, Sancholuz L (1998) Biogeochemical impacts of submerging forests through large dams in the Rĭo Negro, Uruguay. J Environ Manage 54:59–66
Conley DJ, Stålnacke P, Pitkänen H, Wilander A (2000) The transport and retention of dissolved silicate by rivers in Sweden and Finland. Limnol Oceanogr 45:1850–1853
Devi R, Tesfahune E, Legesse W, Deboch B, Beyene A (2008) Assessment of siltation and nutrient enrichment of Gilgel Gibe dam, Southwest Ethiopia. Bioresour Technol 99:975–979
Doctor DH, Kendall C, Sebestyen SD, Shanley JB, Ohte N, Boyer EW (2008) Carbon isotope fractionation of dissolved inorganic carbon (DIC) due to outgassing of carbon dioxide from a headwater stream. Hydrol Process 22:2410–2423
Gaillardet J, Dupré B, Louvat P, Allégre CJ (1999) Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem Geol 159:3–30
Han G, Liu CQ (2004) Water geochemistry controlled by carbonate dissolution: a study of the river waters draining karst-dominated terrain, Guizhou Province, China. Chem Geol 204:1–21
Ittekkot V, Humborg C, Scháfer P (2000) Hydrological alterations and marine biogeochemistry: a silicate issue? Bioscience 50:776–782
Li F, Zhang B (1997) Possibility of reservoir induced seismicity round Three Gorges Dam on Yangtze River. Int J Rock Mech Min Sci 34:76
Li SL, Liu CQ, Lang YC, Tao F, Zhao Z, Zhou Z (2008) Stable carbon isotope biogeochemistry and anthropogenic impacts on karst ground water, Zunyi, Southwest China. Aquat Geochem 14:211–221
Meybeck M (1982) Carbon, nitrogen, and phosphorus transport by World Rivers. Am J Sci 282:401–405
Raymond PA, Cole JJ (2003) Increase in the export of alkalinity from North America’s largest river. Nature 301:88–91
Raymond PA, Caraco NF, Cole JJ (1997) Carbon dioxide concentration and atmospheric flux in the Hudson River. Estuaries 20:381–390
Saito L, Johnson BM, Bartholow J, Hanna RB (2001) Assessing ecosystem effects of reservoir operations using food web-energy transfer and water quality models. Ecosystems 4:105–125
Stumm W, Morgan JJ (1981) Aquatic chemistry. Wiley, New York
Telmer K, Veizer J (1999) Carbon fluxes, pCO2 and substrate weathering in a large northern river basin, Canada: carbon isotope perspectives. Chem Geol 159:61–86
Wachniew P (2006) Isotopic composition of dissolved inorganic carbon in a large polluted river: The Vistula, Poland. Chem Geol 233:293–308
Wang F, Wang B, Liu CQ, Wang Y, Guan J, Liu X, Yu Y (2011) Carbon dioxide emission from surface water in cascade reservoirs system on the Maotiao River, southwest of China. Atmos Environ 45:3827–3834
Wanninkhof R (1992) Relationship between wind speed and gas exchange over the ocean. J Geophys Res 97(C5):7373–7382
Yang C, Telmer K, Veizer J (1996) Chemical dynamics of the “St. Lawrence” riverine system: δDH2O, δ 18OH2O, δ 13CDIC, δ 34Ssulfate, and dissolved 87Sr/86Sr. Geochim Cosmochim Acta 60:851–866
Yu Y, Liu C, Wang F, Wang B, Li J, Li S (2008) Dissolved inorganic carbon and its isotopic differentiation in cascade reservoirs in the Wujiang drainage basin. Chin Sci Bull 53:3371–3378
Acknowledgments
We are grateful to Binhe Gu and two reviewers for their valuable comments and suggestions on this manuscript. This research was funded by the National Natural Science Foundation of China (Nos. 41273128 and 41021062) and the Shanghai Municipal Education Commission (12YZ017).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, F., Liu, CQ., Wang, B. et al. Influence of a reservoir chain on the transport of riverine inorganic carbon in the karst area. Environ Earth Sci 72, 1465–1477 (2014). https://doi.org/10.1007/s12665-014-3051-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-014-3051-x