Abstract
The δ13C values of 23 unevenly spaced guano samples from a 17-cm long clay sediment profile in Gaura cu Muscă Cave (GM), in SW Romania, made it possible to preliminarily characterize the Medieval Warm Period summer hydroclimate regime. The beginning of the sequence (AD 990) was rather wet for more than a century, before becoming progressively drier. After a brief, yet distinct wet period around AD 1170, drier conditions, with a possible shift from C3 to a mixed C3-dominated/C4 type vegetation (2 ‰ lower δ13C values), prevailed for almost half a century before the climate became colder and wetter at the onset of the Little Ice Age, when bats left the cave. The guano-inferred wet and dry intervals from the GM Cave are mirrored by changes in the color and amount of clay accumulated in the cave. They also agree well with reconstructions based on pollen and charcoal from peat bogs and δ13C and δ18O on speleothems from other Romanian sites. Overall, these results indicate that the δ13C of bat guano can provide a sensitive record of the short-term coupling between local/regional climate and the plant–insect–bat–guano system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Caves host a variety of clastic, chemical, and organic deposits from which past environmental and climatic information can be recovered (Sasowsky and Mylroie 2007; White 2007). A relatively new, yet untapped, but important repository for paleoclimate information in caves is represented by the accumulation of bat guano (Mizutani et al. 1992a; Bird et al. 2007; Wurster et al. 2008). Such deposits are especially valuable in those karst areas where other environmental archives are either poor, of low quality, or completely missing.
Worldwide, the most significant bat guano deposits are concentrated in caves at low- and mid-latitude (Kunz et al. 2012). When building up large heaps, guano is regarded as a cave sediment that is composed of organic (mainly insect remains) or/and inorganic matter (various phosphate and carbonate minerals, silt, clay, etc.) accumulated in a loose form, similarly to any other unconsolidated sediment. Weathering processes typical of surface environments are minimized in caves, making ideal circumstances for preservation of these materials. In addition, further from the entrance, the cave environment is characterized by constant topoclimatic conditions (temperature, relative humidity, air currents); and therefore, large guano deposits remain undisturbed for long periods of time, preserving their original stratigraphy. The inner structure of guano heaps can potentially be altered by bioturbation, post-deposition diagenetic processes, or if underground streams flow/flood the subterranean passages in which guano accumulated over time.
As early as 1980, Des Marais et al. recognized the paleoecological significance of bat guano, suggesting that there is a connection between its carbon isotopic signature and local climatic conditions. This is because bats are feeding on insects whose dietary preferences reflect the local vegetation of which distribution is controlled by the local/regional climate. Plants use one of three different photosynthetic pathways (C3, C4, or the Crassulacean acid metabolism, CAM; Smith and Epstein 1971; Osmond et al. 1973; Kennedy and Laetsch 1974; O’Leary 1981), each type producing a characteristic range of δ13C values. Typically, C4 type plants have a mean of 12.5 ± 1.1 ‰ whereas the δ13C values for C3 vegetation averages 26.7 ± 2.3 ‰ (O’Leary 1981; Cerling et al. 1997; error represents 1σ standard deviation); CAM plants typically show δ13C values between these two endmembers. As the carbon isotopic composition of insect tissue such as chitin reflects the isotopic composition of their diet, and as the δ13C of plants is highly specific to the photosynthetic pathway, changes in the isotopic composition of guano should also reflect changes in the δ13C values of vegetation consumed by the insects. Thus, the δ13C values of bulk guano or chitin recovered from guano deposits represents a weighted average of insect diet before being consumed by bats, and can therefore be considered an implicit proxy for paleovegetation in the investigated region (Des Marais et al. 1980; Wurster et al. 2007).
The first report on the isotopic composition, fractionation, and mixing processes in the plant–insect–bat–guano system was published by Des Marais et al. (1980). The authors investigated the carbon isotopes of individual hydrocarbons in bat guano and estimated the abundance of C3, C4, and CAM plants in the Carlsbad region (New Mexico, USA). Mizutani et al. (1992a, b) extended the studies based on carbon isotopes, adding δ15N to reconstruct the paleo-food web and better constrain the paleoenvironmental information. Over the last decade, similar studies have incorporated measurements of C:N ratio, δ13C, δ2H, and δ15N in bulk guano or beetles chitin recovered from bat guano deposits to reconstruct paleoclimate and paleoenvironmental change through the Late Pleistocene and Holocene of USA, southeast Asia, Australia, and Jamaica (McFarlane et al. 2002; Forbes and Bestland 2006; Bird et al. 2007; Wurster et al. 2008, 2010).
Climate-driven changes in the ecosystem impact insect population, although, there is a flexible adaptation of them to different diets (Waldbauer and Friedman 1991). Bats consume different insects in the foraging range, which is commonly up to almost 9 km (Zahn et al. 2005). One of the main difficulties in interpreting the δ13C signal recovered from guano deposits derives from the fact that there is no detailed information about the isotope fractionation along the plant–insect–bat–guano system (Des Marais et al. 1980). Depending on the diet, the δ13C values of insect bodies is assumed to be enriched by about 1 ‰ relative to the plants they consumed (DeNiro and Epstein 1978). However, little detailed study of individual and species-specific variation in the trophic fractionation involved in the plant–insect–bat–guano continuum has been completed (however, see Boecklen et al. 2011 for a review of trophic fractionation). Other paleoenvironmental proxies derived from bat guano include pollen, charcoal, faunal remains, sediments, and atmospheric 36Cl (Pons and Quézel 1958; Harris 1979; Coles et al. 1989; Bui-Thi and Girard 2000; McFarlane et al. 2002; Gilbertson et al. 2005; Carrión et al. 2006; Leroy and Simms 2006; Maher 2006; Dykes 2007; Johnston et al. 2010).
Although mineralogical investigations on guano-derived cave phosphates have been abundant from Romanian cave deposits (Onac and Bengeanu 1992; Diaconu and Dumitraș 2000; Onac and Vereș 2003; Marincea et al. 2004; Onac et al. 2006), no isotopic studies have been published. Three other studies of guano deposits from Romania have been published including work on the chronology of the guano accumulation in the Adam Cave (Carbonnel et al. 1999) as well as the 36Cl/Cl content and pollen stratigraphy of the 800-year-old guano heap in Măgurici Cave (Johnston et al. 2010; Geantă et al. 2012).
With this paper, the authors attempt to use, for the first time in Romania, and to our knowledge elsewhere in Europe, the δ13C of guano from a cave in south-western Romania to reconstruct shifts in the local to regional environment and climate. Only limited information is currently available on paleoclimate and paleoenvironmental change from terrestrial sites on this part of Romania. Most of the present data derives from pollen studies from sediments in the Danube Gorge caves. However, their utility is significantly reduced due to lack of chronology (Boşcaiu and Lupşa 1967a, b; Pop et al. 1970; Boşcaiu et al. 1971). One study does present a speleothem isotope-based paleoclimate reconstruction; however, the temporal resolution of its Late Holocene part is relatively poor (Constantin et al. 2001).
Study site, bats, and sample description
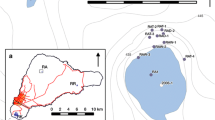
The Gaura cu Muscă Cave (hereafter GM; meaning the Cave with Flies) is located at the southeastern end of the Locvei Mountains (Southern Carpathians), part of the Reşiţa-Moldova Nouă karst plateau (Fig. 1a). This geomorphic unit is characterized by low elevations near GM cave, ending with some spectacular limestone cliffs facing the Danube River. Given its proximity to the Danube and especially because of the large entrance and the presence of a natural “window” (suspended entrance), the cave was known and inhabited at least since the time of the Hallstatt Culture. The cave was fortified, several times since the fourteenth century, with stone walls that are still visible (Boroneanţ 2000).
Map of the Gaura cu Muscă Cave (modified after Botoșăneanu et al. 1976) showing the sampling site (black star). a Location of the Gaura cu Muscă Cave on the karst regions map of Romania; 1 Poleva Cave, 2 Urşilor Cave, 3 Grota Haiducilor Cave, 4 Bihor Mountains peat bogs. b Evolutionary stages of the Bats Gallery on a N–S transect
The climate in the cave region is temperate continental with significant sub-mediterranean influences. The mean annual temperature is between 10 and 11 °C and the amount of annual precipitation ranges between 700 and 800 mm (mean 750 mm), with most rainfall occurring during summer (Munteanu and Bălănescu 1999; Sandu et al. 2008). The vegetation around the cave consists of thermophylous low shrubs, pastures, and deciduous C3-type mixed forests of Fagus sylvatica with Quercus ceris and Q. petrea, or Fagus with Carpinus betulus (Matacă 2003). The plant community in this region also includes C4-type xerophytic shrubs (Boşcaiu and Resmeriţă 1969); however, the exact ratio between C3 and C4 plants is not assessed. Summer precipitations are important for forbs, grasses, and shrubs that emerge and flower from mid-April to late July (growing season). This interval overlaps a significant part of the foraging period when bats are most active.
The cave opens on the left bank of the Danube Gorge, 3 km downstream the village of Coronini (Caraş-Severin County, SW Romania) at 92 m above sea level (Botoșăneanu et al. 1967). It is a short cave (254 m in length) consisting of a main Water Gallery, two short side passages, one near the entrance and the other one at the far end of the cave, respectively (Dry and Clay galleries). At 55 m from the entrance, opens the largest room in the cave, Bats Chamber (6 × 4 m) that continues with the Bats Gallery, a short and rather low height (<1.5 m) corridor that bypasses a lake along the stream passage (Fig. 1).
The cave air temperature (yearly average) measured during several visits between 1962 and 2013 (covering all seasons) is fairly constant (~13.7 °C), whereas the relative humidity is 93–96 %, except for the Dry Gallery where it always stays below 90 % (Negrea and Negrea 1979).
The most commonly reported bat species in GM Cave are Myotis myotis, M. capaccinii, M. oxygnathus, M. emarginatus, Miniopterus schreibersii, Rhinolophus ferrum-equinum, R. euryale, and R. blasii (Dumitrescu et al. 1962–1963; Decu et al. 2003; Coroiu pers. comm.). The diet of these species consists of small to medium size insects (beetles, spiders, flies, crickets, cranefly, etc.). They forage between April and October in a wide variety of different habitats, including waterways, open woodlands, pastures, and shrubs all available in the cave vicinity.
All faunal observations available from this cave mention the presence of either maternity or hibernation roosts of tens to several hundreds bats (Méhely 1900; Dumitrescu et al. 1962–1963; Negrea and Negrea 1979; Coroiu pers. comm.). Apart from these assemblies, small colonies and isolated individuals were also noticed along the Water Passageway, Bats Gallery, and Clay Gallery. The largest nursery roost for Myotis capacinii (and consequently guano deposit underneath) is in the ceiling of the Bats Chamber. Unfortunately, the underground stream is constantly flooding and removing part of the guano from this location, hence not a proper site for sampling.
The seasonal activity of bats, i.e., active during summer and entering a torpid state (hibernation) in winter is well reflected by the guano heap underneath colonies. Fresh guano continuously accumulates during late-spring and summer; however, when bats enter the hibernation period or move away from a particular cave site, it begins to desiccate (if cave microclimate allows). A new layer of guano will be laid down when bats resume their activities. Therefore, the presence of massive guano deposits or thinner layers interbedded within other cave sediments is always related to the period between April and early October when bats are most active. The volume/thickness of guano in a given cave location largely depends on whether that is a permanent maternity roost and on its size. However, the amount of guano accumulated does not always reflect the size and/or permanency of the bat colony; natural (water, gravitation) or anthropic (small to large scale guano mining) factors are responsible for this situation.
A 25-cm deep excavation was dug in the clayey floor of the Bats Gallery below a section of the ceiling easily distinguishable as bat roost by dark ceiling stains (hydroxylapatite coatings) and some guano accumulation on the floor (Fig. 1, black star). The opened profile was carefully cleaned and a 20 × 7 × 25 cm (width × depth × height) rectangular slice was extracted, photographed, documented, and described (stratigraphy, color, sedimentology) in the cave. The recovered sediment sequence consists of two distinct types of clays [yellow in the upper 7.6 cm (Munsell color system: 10YR 7/6) and brownish yellow (10YR 6/6) between 7.6 and the bottom of the profile] in which very dark brown (7.5YR 2.5/2) guano layers or lenses are interbedded (Fig. 2a). The thickest of these guano horizons is between 14 and 15.6 cm below the surface. One other thick horizon appears between (6.5 and 7.6 cm), followed upward by three more, each under 1 cm in thickness. Millimeter-size black shiny fragments of charcoals are scattered throughout the entire profile. As the lower 8 cm of the profile contains no guano, the present study focuses on its upper 17 cm.
a Synthetic profile of the sediment (yellow and brownish yellow)/guano (black layers) deposit within the Bats Gallery. The location of the radiocarbon (empty starts), guano (white rectangles), and charcoal (black rectangles) samples is also shown. b Carbon-isotope composition of the guano samples and the MAP reconstruction (upper X-axis) based on Kohn’s (2010) equation (see text for explanations) c The Medieval Warm Period δ13C profile for stalagmites PU-2 from Urşilor Cave (Onac unpubl. data)
The particular stratigraphy of this profile (alternations of guano and clay, with or without charcoal fragments) suggests that over the accumulation period, the southern end of the Bats Gallery repeatedly acted as a dam behind which clay sediments settled on top of guano deposits (Fig. 1b, stages 1 and 4). The charcoal fragments are very light; thus, they floated at the surface of the ponding water until the dam completely dried up. At this point, the charcoal was laid down on top of the freshly accumulated clay sediments (Fig. 1b, stages 2 and 3). The reason for the modest amount (thickness) of organic matter in the sequence is twofold: (i) part of the guano might have been removed when the gallery flooded; or (ii) the size of the nursery roost was small (plausible hypothesis considering the overall space available).
Materials and methods
Carbon isotopic composition
For the analysis of carbon isotopic composition, each guano horizon or lens at 4 mm intervals were sampled, except for the thin layers from which only one sample was recovered. In addition to the 23 guano samples, nine charcoal fragments were extracted from clay and guano layers. The guano samples were prepared for isotopic analysis using a modified method of Wurster et al. (2007). To avoid contaminations from organic materials (e.g., plastics) glass vials and watch glasses were used throughout the entire procedure. The organic material was dried for 3 h at 40 °C, a temperature that is lower than the bats maximum body temperature (Hock 1951), thus preventing any loss of organic material. The dried samples were ground in an agate mortar, homogenized and weighed. Half the quantity of each sample was mixed with 300 μl distilled water and 1 ml HCl (10 %) and left for 3 h at 25 °C to remove any carbonate minerals. No visible bubbles resulted from these reactions. After drying at 40 °C overnight, the organic material was ground and homogenized again. Both the acid treated and non-treated samples were analyzed. The Student’s t test indicated that at the significance level of 0.01, there is no major difference between the carbon isotopic compositions of the two sample populations, thus confirming that the samples were free of carbonates.
The carbon isotopic composition was measured in the Stable Isotope Laboratory of the Babeş-Bolyai University (Cluj, Romania), using the Combustion Module-Cavity Ring Down Spectroscopy (CM-CRDS) technique (Busch and Busch 1999; Brand et al. 2009). The combustion module designed by Costech Analytical Technologies Inc. delivers the CO2 to the CRDS analyzer (Picarro G2101-i type), which was used with the default factory calibration. Corrections were carried out on each sample measurement batch, using two-point calibration with internal standards (atropine and acetanilide) and a control standard (B2151) for consistency verification. The internal standard was calibrated against ANU Sucrose and two organic analytical standard reference materials, B2155 Protein (Casein) and B2151 high organic sediment (Elemental Microanalysis, Ltd), respectively. All δ13C values are expressed relative to the VPDB standard, where:
For each sample, three (in case of charcoal) or four (in case of guano) measurements were carried out and the average reported. Precision is estimated to be better than ± 0.06 ‰ (1σ) based on replicate internal standards in each run. The reproducibility between replicate standards in each run was better than ± 0.1 ‰ (1σ).
Sequence chronology
The dating control of the upper 17 cm sediment sequence in GM Cave is based on three accelerator mass spectrometry radiocarbon measurements performed on a twig charcoal fragment (GM3 A) and two pre-treated carbonate-free guano samples (GM3 A–B and GM3 B) at the Poznan Radiocarbon Laboratory and 14Chrono Center, Queens University Belfast, respectively. Radiocarbon dates were calibrated using OxCal 4.1 and the INTCAL09 dataset of Reimer et al. (2009) and are reported as calibrated years AD. Because the dated sequence is highly peculiar (i.e., unevenly spaced layers of guano or charcoal pieces within clays), constructing an age-depth model is not suitable for this site.
Results and discussion
14C dates
Results from the radiocarbon measurements reveal that all three ages (AD 1280, AD 1170, and AD 990) are in stratigraphic order (Table 1). The two calibrated radiocarbon ages at the top and bottom of the profile are likely to be AD 1280 and AD 990, respectively, thus constraining the Medieval Warm Period.
One could argue that this youngest age obtained on charcoal may carry an “inbuilt” error, defined as the amount of time elapsed from the death of the tree to its burn (McFadgen 1982). It has been abundantly documented that if this is the case the inbuilt age always biases 14C ages to be older (up to hundreds of years) than the time of the fire (Waterbolk 1983; Gavin 2001). Therefore, the inbuilt-age error impacts the authors’ ability to safely distinguish, using 14C dating of charcoal, the minimum interval between two fires. In this case, dating any of the charcoal fragments in the upper 7 cm of the profile, spanning ~106 years, would have produced similar 14C ages, but definitely older than the other two available. In addition, however, the authors’ upper two ages were obtained on different materials, a piece of burned twig (known to be significantly less affected by inbuilt error; McFadgen 1982) and bulk guano, respectively. Considering this and the age-stratigraphy of the sediment sequence, an inbuilt error was excluded for the top age of the profile.
Mean annual precipitation reconstruction from carbon isotopic composition
In a recent paper, Kohn (2010) presented the following equation to estimate the mean δ13C value of C3 plants (™δ13CC3) using the altitude, absolute latitude, and the mean annual precipitation of a given region:
where MAP stands for the mean annual precipitation. Substituting altitude, MAP and latitude terms pertaining to the GM Cave (102 m; 800 mm/year; 44.66472º) the authors estimate that the mean δ13CC3 in the region should be −27.89 ‰. This is within the analytical error of the mean value of −27.85 ‰ obtained on terrestrial grasses and leaves from trees and bushes collected nearby the cave (Onac et al. unpubl. data). The difference between the calculated (and present-day) δ13CC3 and the δ13C value of fresh guano in GM Cave (−26.63 ‰) is 1.26 ‰. This value may be used as an estimation of the fractionation along the plant–insect–guano path, comprising a difference in δ13C values for two trophic levels (plant–insect and insect–bat, DeNiro and Epstein 1978). Having tested the Kohn (2010) equation that relates δ13CC3 to environment, the authors rearranged it and reconstructed paleoprecipitation (MAP) based on the measured δ13C of guano through the Medieval Warm Period (MWP) (Fig. 2b).
Isotope data interpretation
The δ13C values of guano from GM cave range from 27.1 to 25.2 ‰ for bulk guano, and from 27.4 to 24.5 ‰ in charcoal, and is 26.63 ‰ in modern guano collected at the surface of the site (Fig. 2b). Beneath 0.4 cm, the δ13C value of the guano increases rapidly to 25.2 ‰ at 3 cm before decreasing to almost 27 ‰ at 6.7 cm. Within the next 0.7 cm, the δ13C values are once again increasing to 25.8 ‰, then drop to 27.1 ‰. Between 11 cm and the base of the profile, the δ13C values only change about 0.15 ‰ on both sides of 27 ‰. In order to understand the paleoclimatic signal encoded in the δ13C values of the GM Cave guano, the authors attempted to compare this time series with those available in SW Romania for this period.
Overall, the trend of guano and charcoal δ13C values parallel each other, bearing similarity to the δ18O trend in the PP9 stalagmite from Poleva Cave situated within a few kilometers from the GM Cave (Fig. 1a, site 1). Constantin et al. (2007) interpreted this record to reflect swings from cold to warm climates. In particular, for the time frame investigated in this study, the variation in δ18O values from Poleva Cave suggest a rapid warming at the transition from the Dark Ages to the MWP. The climate remained warm until the cooling associated with the LIA begun. On the other hand, the δ13C isotope record from PP-9 (despite the low resolution data set) is thought to document shifts from wetter to drier conditions (Constantin, unpubl. data). Furthermore, comparing the δ13C records from GM Cave and Urşilor Cave PU-2 stalagmite from NW Romanian (Fig. 1a, site 2; Onac et al. 2002; Onac et al. in prep.), the authors noticed the two profiles overlap each other (within data uncertainty) very well. Individual spikes of 13C-depleted δ13C values were interpreted as indicative of periods of heavy rainfall, based on the speleothem calcite fabric changes (Onac et al. 2002). Corroborating the above observations, the authors are tempted to conclude that the more positive/negative δ13C values in both bulk guano and charcoal from the GM Cave, document dry/wet periods. Thus, the 17-cm isotopic profile reveals a shift from wetter summer conditions in the first half of the MWP towards more drier over the last century of it. These major hydroclimatically different intervals are also mirrored by changes in the color (from yellowish during the dry period to dark brown when wet conditions prevailed) and amount of clay accumulated in the Bats Passage.
Conclusions
The time interval identified in this sequence is short (<300 years) and bracketed by two cold periods know as the Dark Ages and LIA, respectively. Paleorecords of this period from SW Romania are not only scant but either completely lacking chronology or originate from high altitude locations (e.g., Boşcaiu and Lupşa 1967a, b; Pop et al. 1970; Rösch and Fischer 2000). Under these circumstances, little information is available for evaluation and comparison purposes. Although accumulated at uneven sedimentation rates, the organic layers/lenses or fragments preserved within the clay sequence at GM Cave allowed to tentatively unravel the summer hydroclimatic changes using the δ13C signal in bulk guano, in addition to being used as a proxy for paleovegetation in the investigated region.
Though 14C ages can be useful in establishing the chronology of guano deposits, given the distinctiveness of this site, the temporal precision is rather coarse. Therefore, the radiocarbon dating of GM Cave guano and charcoal primarily constrains the cave occupancy by bats and document flood events, respectively. The ages suggest bats dwelt in the cave during both wet and arid periods; however, apparently, the production of guano was higher at the beginning of the MWP. Around AD 1170, the δ13C values in the GM Cave guano record a short-lived (few years) wet period also seen in the PU-2 stalagmite and known from many other locations throughout Europe (Marusek 2010). Towards the upper part of the profile, corresponding to the demise of MWP, the guano layers are thinner and completely vanish at the onset of the LIA.
The alternating dry/wet periods is further supported by the pollen assemblage (older-middle to younger Subatlantic Period) described from nearby Grota Haiducilor Cave sediments (Fig. 1a, site 3) by Boşcaiu and Lupşa (1967b). A regional charcoal study (concentration and rate of accumulation) conducted by Feurdean et al. (2012), shows a general rise over the MWP followed by a drop in the LIA, suggesting biomass burning was modulated by climate. The wet/dry spells inferred from the δ13C record in GM Cave agrees well with the charcoal concentration in two of the investigated sites (Molhaşul Mare and Călineasa, Bihor Mountains; Fig. 1a, site 4). The MWP summer hydroclimatic variations in SW Romania inferred from the δ13C of bat guano, also correlate well with the tree ring-based reconstructed summer precipitations totals of Central Europe (Büntgen et al. 2011).
References
Bird MI, Boobyer EM, Bryant C, Lewis HA, Paz V, Stephens WE (2007) A long record of environmental change from bat guano deposits in Makangit Cave, Palawan, Philippines: Earth and Environmental Science Transactions of the Royal Society of Edinburgh 98:59–69
Boecklen WJ, Yarnes CT, Cook BA, James AC (2011) On the use of stable isotopes in trophic ecology. Annu Rev Ecol Evol Syst 42:411–440
Boroneanţ V (2000) The archeology of the Romanian’s caves and mines. cIMeC, Bucharest, p 327 (in Romanian)
Boşcaiu N, Lupşa V (1967a) Palynological studies in Veterani Cave, Danube Gorge. Contribuţii Botanice: 39–46 (in Romanian)
Boşcaiu N, Lupşa V (1967b) Palynological research in the “Grota Haiducilor” Cave near the Herculaneum Spa (Romania). Rev Roum Biol Botanique 12(2–3):137–140
Boşcaiu N, Resmeriţă I (1969) Xerophylous herbaceous vegetation of the Eşelniţa-Mraconiei valleys alluvia in the Danube Gorge. Stud Cerc Biol Seria Bot 21(3):209–216 (in Romanian)
Boşcaiu N, Lupşa V, Boroneanţ V (1971) Sporo-pollen analysis of sediment in Climente Cave (Danube Gorge). St Cerc Biol Ser Bot 23(5):401–403 (in Romanian)
Botoșăneanu L, Negrea A, Negrea Ș (eds) (1967) Recherches sur les grottes du Banat et l’Oltenie, Roumanie (1959-1962). Centre National de la Recherche Scientifique, Paris, p 392
Brand WA, Geilmann H, Crosson ER, Rella CW (2009) Cavity ring-down spectroscopy versus high-temperature conversion isotope ratio mass spectrometry; a case study on δ2H and δ18O of pure water samples and alcohol/water mixtures. Rapid Commun Mass Spectrom 23:1879–1884
Bui-Thi M, Girard M (2000) Les guanos de chauve-souris d’âge médiéval de la Grande Grotte d’Arcy-sur-Cure (Yonne). Analyse pollinique et implications paléobotaniques. Géologie Alpine Mémoire HS 31:83–95
Büntgen U, Tegel W, Nicolussi K, McCormick M, Frank D, Trouet V, Kaplan JO, Herzig F, Heussner K-U, Wanner H, Luterbacher J, Esper J (2011) 2500 Years of European climate variability and human susceptibility. Science 331:578–582
Busch KW, Busch MA (1999) Cavity-ringdown spectroscopy: an ultratrace-absorption measurement technique. American Chemical Society, Washington, p 269
Carbonnel JR, Olive P, Decu VG, Klein D (1999) Datation d’un dépôt de guano holocène dans la Carpates méridionales (Roumanie), Implications tectoniques. CR Acad Sci Paris 328:367–370
Carrión JS, Scott L, Marais E (2006) Environmental implications of pollen spectra in bat droppings from southeastern Spain and potential for palaeoenvironmental reconstructions. Rev Palaeobot Palynol 140:175–186
Cerling TE, Harris JM, MacFadden BJ, Leakey MG, Quade J, Eisenmann V, Ehleringer JR (1997) Global vegetation change through the Miocene-Pliocene boundary. Nature 389:153–158
Coles GM, Gilbertson DD, Hunt CO, Jenkinson RDS (1989) Taphonomy and the palynology of cave deposits. Cave Sci 16:83–89
Constantin S, Onac BP, Fleitmann D, Tamas T (2001) Past vegetation changes in karst areas as revealed by a comparative isotopic study of two Holocene speleothems from Romania. In: Cheng Z, Changhong Y, Fong G (eds) World Correlation of Karst Ecosystems Newsletter. IGCP Project 448, Karst Dynamics Laboratory, Guilin, pp 67–73
Constantin S, Bojar A-V, Lauritzen S-E, Lundberg J (2007) Holocene and Late Pleistocene climate in the sub-Mediterranean continental environment: a speleothem record from Poleva Cave Southern Carpathians, Romania. Palaeogeogr Palaeoclim Palaeoecol 243:322–338
Decu V, Murariu D, Gheorghiu V (2003) The bats of Romania. Institutul de Speologie “Emil Racoviţă” şi Muzeul de Istorie Naturală “Grigore Antipa”, Bucureşti, p 521 (in Romanian)
DeNiro MJ, Epstein S (1978) Influence of diet on the distribution of carbon isotops in animals. Geochim Cosmochim Acta 42:495–506
Des Marais DJ, Mitchell JM, Meinschein WG, Hayes JM (1980) The carbon isotope biogeochemistry of the individual hydrocarbons in bat guano and the ecology of the insectivorous bats in the region of Carlsbad, New Mexico. Geochim Cosmochim Acta 44:2075–2086
Diaconu G, Dumitraş D (2000) Sur la présence de l’ardéalite dans la Grotte de Topolnita (Plateau de Mehedinti, Roumanie). Theor Appl Karstol 13–14:67–73
Dumitrescu M, Tanasacche J, Orgidan T (1962–1963) The distribution of bats in R.P.Română, Lucr Inst Speo “Emil Racoviţă” 1–2:509–576 (in Romanian)
Dykes AP (2007) Mass movements in cave sediments: geotechnical investigation of a 42,000-year old guano mudflow inside Niah Great Cave, Sarawak, Borneo. Landslides 4:279–290
Feurdean A, Spessa A, Magyari EK, Willis KJ, Veres D, Hickler T (2012) Trends in biomass burning in the Carpathian region over the last 15,000 years. Quat Sci Rev 45:111–125
Forbes MS, Bestland EA (2006) Guano-derived deposits within the sandy cave fills of Naracoorte, South Australia. Altering. Australas J Palaeontol 30:129–146 (special issue 1)
Gavin DG (2001) Estimation of inbuilt age in radiocarbon ages of soil charcoal for fire history studies. Radiocarbon 43(1):27–44
Geantă A, Tanţău I, Tămaș T, Johnston V (2012) Palaeoenvironmental information from the palynology of an 800 year old bat guano deposit from Măgurici Cave, NW Transylvania (Romania). Rev Palaeobot Palynol 174:57–66
Gilbertson DD, Bird M, Hunt C, McLaren S, Mani Banda R, Pyatt B, Rose J, Stephens M (2005) Past human activity and geomorphological change in a guano-rich tropical cave mouth: initial interpretations of the Late Quaternary succession in the Great Cave of Niah, Sarawak. Asian Perspect 44(1):16–41
Harris AH (1979) Reconstruction of mid-Wisconsin environments in southern New Mexico. Natl Geogr Res 3:142–151
Hock RJ (1951) The metabolic rates and body temperatures of bats. Biol Bulletin 101:289–299
Johnston VE, McDermott F, Tămaș T (2010) A radiocarbon dated bat guano deposit from N.W. Romania: implications for the timing of the Little Ice Age and Medieval Climate Anomaly. Palaeogeogr Palaeoclimatol Palaeoecol 291:217–227
Kennedy RA, Laetsch WM (1974) Plant Species Intermediate for C3, C4 Photosynthesis. Science 184:1087–1089
Kohn MJ (2010) Carbon isotope compositions of terrestrial C3 plants as indicators of (paleo)ecology and (paleo)climate. PNAS 107:19691–19695
Kunz TH, Murray SW, Fuller NW (2012) Bats. In: White WB, Culver DC (eds) Encyclopedia of Caves. Academic Press, Oxford, pp 40–54
Leroy SAG, Simms MJ (2006) Iron age to medieval entomogamous vegetation and Rhinolophus hipposideros roost in South-Eastern Wales (UK). Palaeogeogr Palaeoclimatol Palaeoecol 237:4–18
Maher LJJ (2006) Environmental information from guano palynology of insectivorous bats of the central part of the United States of America. Palaeogeogr Palaeoclimatol Palaeoecol 237:19–31
Marincea Ș, Dumitraș DG, Diaconu G, Bilal E (2004) Hydroxylapatite, brushite and ardealite in the bat guano deposit from Pestera Mare de la Meresti, Persani Mountains, Romania. N Jb Miner Mh 10:464–488
Marusek JA (2010) A chronological listing of early weather events. Science & Public Policy Institute, Reprint Series, p 580
Matacă SȘ (2003) Porțile de Fier Natural Park. Flora, vegetation, and nature protection. Unpubl. PhD Thesis, Romanian Academy, Institute of Biology, Bucharest
McFadgen BG (1982) Dating New Zealand archaeology by radiocarbon. NZ J Sci 25:379–392
McFarlane DA, Lundberg J, Fincham AGA (2002) Late Quaternary paleoecological record from caves of southern Jamaica: West Indies. J Cave Karst Stud 64:117–125
Méhely L (1900) Monographia Chiopterorum Hungariae. Budapest, p 372
Mizutani H, McFarlane DA, Kabaya Y (1992a) Nitrogen and carbon isotope study of a bat guano core from Eagle Creek Cave, Arizona, USA. Mass Spectrosc 40:57–65
Mizutani H, McFarlane DA, Kabaya Y (1992b) Carbon and nitrogen isotopic signatures of bat guanos as a record of past environments. Mass Spectrosc 40:67–82
Munteanu R, Bălănescu D (1999) Air and rainfall temperature regime in the space of Banat in the year 1997. Proc Regional Conf of Geography “Danube-Criș-Mureș-Tisa Euroregion-Geoeconomical space of sustainable development”. West University, Timișoara, pp 141–155
Negrea A, Negrea Ș (1979) The caves of the Danube Gorge and their terrestrial fauna. In: Orghidan T, Negrea Ș (eds) Speologia. Academiei RSR, București, pp 30–75 (in Romanian)
O’Leary MH (1981) Carbon isotope fractionation in plants. Phytochemistry 20:553–567
Onac BP, Bengeanu M (1992) Phosphate minerals in few caves from Padurea Craiului Mountains (Romania)—Preliminary note. Nymphaea XXII:23–28
Onac BP, Vereș DȘ (2003) Sequence of secondary phosphate deposition in a karst environment: evidence from Magurici Cave (Romania). Eur J Mineral 15:741–745
Onac BP, Constantin S, Lundberg J, Lauritzen SE (2002) Isotopic climate record in a Holocene stalagmite from Urșilor Cave (Romania). J Quat Sci 17:319–327
Onac BP, Zaharia L, Kearns J, Vereș DȘ (2006) Vashegyite from Gaura cu Muscă Cave. Int J Speleol 35(2):67–73
Osmond CB, Allaway WG, Sutton BG, Troughton JH, Queiroz O, Luttge U, Winter K (1973) Carbon isotope discrimination in photosynthesis of CAM plants. Nature 246:41–42
Pons A, Quézel P (1958) Premières remarques sur l’étude palynologique d’un guano fossile du Hoggar, Compte Rendus Hebdomadaires des Séances de l’Académie des Sciences 246: 2290–2293
Pop E, Boşcaiu N, Lupşa V (1970) Spoor-pollen analysis of sediments from Cuina Turcului—Dubova. St cerc ist veche 21(1):31–34 (in Romanian)
Reimer PJ, Baillie MGL, Bard E, Bayliss A, Beck JW, Blackwell PG, Bronk Ramsey C, Buck CE, Burr GS, Edwards RL, Friedrich M, Grootes PM, Guilderson TP, Hajdas I, Heaton TJ, Hogg AG, Hughen KA, Kaiser KF, Kromer B, McCormac FG, Manning SW, Reimer RW, Richards DA, Southon JR, Talamo S, Turney CSM, van der Plicht J, Weyhenmeyer CE (2009) IntCal09 and Marine09 radiocarbon age calibration curves, 0–50, 000 years cal BP. Radiocarbon 51:1111–1150
Rösch M, Fischer E (2000) A radiocarbon dated Holocene profile from the Banat mountains (Southwestern Carpathians, Romania). Flora 195:277–286
Sandu I, Pescaru VI, Poiană I (eds) (2008) The climate of Romania. Editura Academiei Române, București, p 365 (in Romanian)
Sasowsky ID, Mylroie J (2007) Studies of cave sediments. Physical and chemical records of paleoclimate. Springer, Berlin, p 340
Smith BN, Epstein S (1971) Two categories of 13C/12C ratios for higher plants. Plant Physiol 47:380–384
Waldbauer GP, Friedman S (1991) Self-selection of optimal diets by insects. Ann Rev Entomol 36:43–63
Waterbolk HT (1983) Ten guidelines for the archaeological interpretation of radiocarbon dates. In: Mook WG, Waterbolk HT (eds) Proc 1st Int Symp 14C Archaeology, Groningen 1981, PACT8:57–70
White WB (2007) Cave sediments and paleoclimate. J Cave Karst Stud 69:76–93
Wurster CM, McFarlane DA, Bird MI (2007) Spatial and temporal expression of vegetation and atmospheric variability from stable carbon and nitrogen isotope analysis of bat guano in the southern United States. Geochim Cosmochim Acta 71:3302–3310
Wurster CM, Patterson WP, McFarlane DA, Wassenaar LI, Hobson KA, Athfield NB, Bird MI (2008) Stable carbon and hydrogen isotopes from bat guano in the Grand Canyon, USA, reveal Younger Dryas and 8.2 ka events. Geology 36:683–686
Wurster CM, McFarlane DA, Bird MI, Ascough P, Ashfield NB (2010) Stable isotopes of subfossil bat guano as a long-term environmental archive: insights from a Grand Canyon cave deposit. J Cave Karst Stud 72(2):111–121
Zahn A, Haselbach H, Güttinger R (2005) Foraging activity of central European Myotis myotis in a landscape dominated by spruce monocultures. Mammalian Biol 70:265–270
Acknowledgments
The authors thank the administration of the Porţile de Fier Natural Park for granting permission to recover the guano/clay profile from Gaura cu Muscă Cave. Special thanks to Dr. I. Coroiu for discussing the aspects of bat ecology with the authors. This research was funded by CNCS grant PN-II-ID-PCE 2011-0-0588 to BPO.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Onac, B.P., Forray, F.L., Wynn, J.G. et al. Guano-derived δ13C-based paleo-hydroclimate record from Gaura cu Musca Cave, SW Romania. Environ Earth Sci 71, 4061–4069 (2014). https://doi.org/10.1007/s12665-013-2789-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-013-2789-x