Abstract
This paper deals with the possible impact of hydraulic fracturing (fracking), employed in the exploitation of unconventional shale gas and tight gas reservoirs, on groundwater, which is the most important source of drinking-water in Germany and many other European countries. This assessment, which is part of an interdisciplinary study by a panel of neutral experts on the risks and environmental impact of hydraulic fracturing, is based mainly on data obtained from three ExxonMobil drilling sites in northern Germany. First, the basic technical aspects of fracking and its relevant water fluxes are explained. The type, purpose and fate of the constituents of the fracking fluids are discussed. The chemicals used in the fracking fluids are assessed with regard to their hazardous properties according to the Regulation (EC) No. 1272/2008 of the European Parliament and of the Council on the classification, labelling and packaging of substances and mixtures (CLP regulation) and the German “Water Hazard Classes”. Contamination of groundwater by ingredients of fracking fluids may occur from under ground or may result from above-ground accidents associated with the transport, storage and handling of hazardous substances used as additives in fracking fluids. The degree of groundwater contamination cannot be predicted in a general way. Therefore, different dilutions of the fracking fluid in groundwater are considered. It is shown that the concentrations of most ingredients resulting from a 1:10,000 up to 1:100,000 dilution of the fracking fluid in groundwater are below health-based reference values such as the limit values of the European Drinking Water Directive, the WHO Guideline Values for Drinking-water Quality, and other health-based guide values for drinking-water. Regarding the salinity of fracking fluids, a dilution of 1:1,000 is sufficient to reach concentrations which are acceptable for drinking-water. From the human-toxicological point of view, the constituents of flowback water are more problematic with respect to drinking-water produced from groundwater than those of the fracking fluids. The few reliable data which have become available, as well as hydrogeological considerations, point in the direction of considerable salt concentrations and toxic constituents, e.g., Hg, As, Pb, Zn, Cd, BTX, PAHs, or even radioactive elements. The identification and assessment of reaction products and metabolites, which are produced as a result of the fracking operation and the metabolic activity of microorganisms, are important topics for further research. The recommendations include the need for a better understanding of the environmental impact of fracking operations, especially with regard to the development of sustainable rules for planning, permission, performance and management of fracking, and for the monitoring of groundwater quality around fracked drilling sites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Growing world population and industrialisation are the main drivers for an increase of energy demand. However, other needs such as environmental quality, climate protection, and the protection of drinking-water resources are essential issues for the quality of human life. In many cases, severe conflicts of interests may arise in modern societies. Therefore, the needs of environmental protection must be taken into account from the very beginning of the exploitation of new energy resources. In recent years, the unconventional methane production by hydraulic fracturing (commonly known as “fracking”) has been added to the manifold options for the exploitation of the earth’s energy resources and became a topic of discussion because of its possible environmental impacts (e.g., Lechtenböhmer et al. 2011), especially with regard to water management (Gregory et al. 2011) and water quality (Osborn et al. 2011).
In Germany, hydraulic fracturing operations have been applied for the exploitation of tight gas reservoirs in the deep sandstone of Lower Saxony since the 1980s. However, due to recent plans by ExxonMobil to explore shale gas reservoirs and coalbed methane with substantially shallower target horizons of about 1,000 m, a public debate on the risks and safety of hydraulic fracturing arose. Thus, following ExxonMobil’s initiative, an interdisciplinary panel of neutral experts was assembled to elaborate a risk assessment of fracking, focussing on various aspects and drawing conclusions from the facts known so far (Ewen et al. 2012). The aspects that were considered include the drilling and technical operation conditions, the respective geological and hydrogeological formations, the interactions and reactions under ground (Kissinger et al. 2013; Lange et al. 2013), the aqueous fluids’ qualities and their management (Olsson et al. 2013; Rosenwinkel et al. 2012), the technical risk aspects under ground and above-ground (Uth 2012), the possibility of groundwater contamination, infrastructure and landscape planning (Schneble et al. 2012), and finally the legal situation (Rossnagel et al. 2012, 2013).
The specific topic of this paper is the human-toxicological assessment of the ingredients of fracking fluids and flowback fluids, which in case of an accident may penetrate into groundwater bodies which are used as drinking-water resources. The objectives are in detail (1) to assess the constituents of the fracking fluids from a human-toxicological point of view, (2) to consider the influence of flowback, (3) to consider the possible hazards for groundwater as the most important resource for drinking-water in Germany, and (4) to advise on future procedures to minimize environmental hazards.
Hydraulic fracturing operation and its water relevant aspects
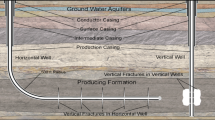
In connection with the technique of deep horizontal drilling, hydraulic fracturing permits natural gas to be extracted from so-called unconventional reservoirs, where the gas does not flow spontaneously, but is trapped in minute pores in the rock (EPA 2011a; Wood et al. 2011). This procedure consists of a fluid being injected into the borehole at high pressure. This fluid then penetrates the perforations in the pipe in the horizontal borehole and induces cracks in the formation rock. The fracking fluid contains proppants, i.e. sand or ceramic beads about 1 mm in diameter. Their function is to “prop” the pores open after pressure release, when the liquid medium is withdrawn. Very often, water is employed as the liquid medium. In order to keep the proppants suspended in the liquid phase on their way down to the horizontal pipe and until they are deposited in the cracks, fracking fluids contain chemical additives, usually added to the water on-site at the drilling location. The water fluxes involved in a hydrofracking operation are given in Fig. 1.
The liquid withdrawn after pressure release and during the extraction phase following the fracking event, called produced water or flowback water, differs in its composition from the originally injected fracking fluid. Most of the sand and proppants stay in the deep geological formation; some of the chemical additives react under the conditions of the process, e.g., high temperature, and/or due to microbial activity to form degradation and reaction products. Additionally, the geochemical nature of the formation and the fossil deposit is reflected in the produced water to some degree (Orem et al. 2007; Pees Coleman 2011). Salts and other geogenic element species can become mobilised in the dry or water-saturated fractures and reach the earth’s surface in dissolved or gaseous form, either due to extraction of formation water, or as a result of the fracking fluid dissolving components of the geological formation. Handling and disposal of produced water is also an issue for conventional natural gas exploitation. In the framework of the aforementioned panel of experts, aspects of produced-water management and treatment are discussed in detail by Olsson et al. (2013) and Rosenwinkel et al. (2012).
A major concern with respect to fracking activities is whether the employed chemicals might be hazardous to drinking-water resources, especially to groundwater. Despite the fact that fracking operations are performed in depths of 1,000 m or more, it has to be taken into account that the shallow, usable aquifer containing freshwater must be penetrated for this purpose.
Normally, safety measures, e.g., the asphalt coating of drilling sites, additional spill containments for tanks containing the bulk chemicals, or several casings of the borehole, especially near the upper usable aquifer, prevent fracking chemicals or components of flowback water from entering the soil and groundwater (see also Uth 2012). Nevertheless, accidents, e.g., spilling or leakages, can occur. Therefore, in this paper, the compounds involved are assessed for their hazard potential from a human-toxicological viewpoint. Ecotoxicological aspects are dealt with in the paper by Riedl et al. (2013) and Schmitt-Jansen et al. (2012).
Database
The formulas of fracking fluids (ingredients and employed amounts) used by ExxonMobil in fracking operations in Germany in the past 30 years are published on the internet (http://www.erdgassuche-in-deutschland.de/technik/hydraulic_fracturing/frac_fluessigkeiten.html). Meanwhile, chemicals used in the USA are also disclosed on the web (http://fracfocus.org/chemical-use/what-chemicals-are-used) and can be followed for each well. In Canada, the public can access fracfocus.ca for industry disclosures. Not all fracking chemicals listed on the internet have been used for the Exxon fracks in Germany, e.g., naphthalene has not been employed, nor have the biocidal agents glutaraldehyde and tetrakis hydroxymethylphosphonium sulphate, which have been used in the USA. About 150 substances with different chemical identities could be identified in the fracking formulas published by ExxonMobil for Germany, 119 of them specified by their CAS number (Riedl et al. 2013; Schmitt-Jansen et al. 2012). Additionally to these fracking formulas which have already been applied, ExxonMobil has disclosed an example for a formula of a fracking fluid intended for future shale gas fracking operations. As a further chemical database, analytical results for groundwater analyses in the fracking area and analyses of flowback and formation waters were made available to the panel of experts.
The presented assessment is based on the fracking fluids as they were used in recent, representative hydrofracking operations at three drilling sites. The drilling sites and the conditions of the operations are given in Table 1. Further information is also available in Olsson et al. (2013) or Rosenwinkel et al. (2012).
The composition of the fracking fluids employed and the function of their constituents are given in Table 2.
The function of the fracking fluid is to transport and deposit the proppants down to the target horizon and to transfer the pressure of the frack. Its design has to suit the properties of the formation, e.g., permeability, fissuring, clay content, water saturation, capillary effects, and the conditions of the hydrofracking operation (e.g., pressure and temperature in the underground) and is thus adapted individually for each drilling site and fracking operation. Various approaches to fracking fluid design, the chemicals applied and their function are treated in EPA (2011b) by Degner (2011), McCurdy (2011) and Satya Gupta (2011).
The main components of the three fracking fluids are water and proppants. The water was taken from the public water supply or a nearby well and thus was of drinking-water quality. In the frack C Z3a, liquid CO2 was additionally applied as an energizing component of the fluid phase. Both fracks B T12 and C Z3a are gel-based with the proppant making up 28 or 12.5 % of the total applied mass, respectively. The frack D 3 is a so-called slickwater frack. This kind of operation is possible in relatively shallow horizons. The proppant is kept suspended in the water phase by application of a high pumping rate. To facilitate this, a friction reducer is added to the water. Notable is the high water demand of about 4,000 m3 per single frack, which is due to the fissured nature of the shale formation, and the relatively low concentration of proppants in the fluid.
Chemicals account for about 3, 1.1 or 0.16 % of the fracking fluids (calculated without proppants) for the fracking operations B T12, C Z3a or D 3, respectively. They are listed in Table 2. Whereas 18 or 22 different frack additives were employed in the gel-based deep sandstone fracks B T12 and C 3a, respectively, only nine different chemicals were added for the shale-gas frack D 3. For selected chemicals in Table 2, some comments will be given in the following sections.
The biocidal agents 5-chloro-2-methyl-2H-isothiazol-3-one (CIT) and 2-methyl-2H-isothiazol-3-one (MIT) are common constituents of the three fracking fluids. The addition of magnesium chloride and—nitrate by the manufacturer stabilises the preparation. Biocides are regular ingredients of fracking fluids (Brandon et al. 1995; Rimassa et al. 2011). They are applied in order to prevent (1) microbial degradation of the gel and a subsequent drop of viscosity; (2) biofilm formation, and (3) microbiologically induced corrosion. Biofilms in the pores of the formation and on the proppants hinder dewatering and thus extraction of the gas (Bottero et al. 2010). Microbiologically induced corrosion of drilling facilities by sulphate-reducing bacteria producing H2S or other acid-producing bacteria under ground is a common problem in natural gas and oil production (Nemati and Voordouw 2000). The mixture of CIT and MIT in a ratio of 3:1 is sold under the trade name Kathon® and is used as a bactericide, fungicide and algicide in many commercially available products, such as water-based dispersion paints for indoor and outdoor use, various glues as well as hygiene products, for example liquid soaps and ointments. A German ordinance allows cosmetic products to contain up to 0.0015 % (equivalent to 15 mg/kg) of Kathon® as a preservative. Further applications are the production of paper and the conservation of technical liquids. Kathon®, like 2-bromo-2-nitropropane-1,3-diol (Bronopol), a biocide formerly employed by ExxonMobil and frequently used in cooling systems, belongs to the group of chlorine and bromine releasing, non-oxidising biocides. Data on its environmental behaviour were published by Jacobson and Williams (2000). In the IUCLID data sheet, it is assessed as readily biodegradable based on a modified Zahn-Wellens test (OECD 302 B). Furthermore, DT50 values are given, amounting 4 days for CIT and <2 days for MIT, respectively (ESIS 2000a). In a recent survey on the occurence of isothiazolines in the aquatic environment, MIT and CIT could not be detected in the effluent of a wastewater treatment plant, although concentrations of 0.5 μg/l were determined in the influent. In water samples from the rivers Rhine, Neckar and Danube, as well as from some smaller rivers highly influenced by wastewater, MIT or CIT could not be detected (Rafoth et al. 2007). In a study on the relevance of biocides for the drinking-water supply initiated by the German Association on Gas and Water from 2012, Kathon®—despite being used in many consumer products—was assessed as of minor concern for drinking-water supply because of its low DT50 values (Thoma and Sacher 2012). This assessment, however, implies an above-ground pathway of immission. Because of its high polarity, especially MIT is assessed a micropollutant not being easily removable by drinking water treatment, as it is not likely to adsorb on activated carbon (Thoma and Sacher 2012). In the European Union, the limit for biocidal agents in drinking-water is 0.1 μg/l per single compound and 0.5 μg/l for the total of biocides.
Common to all three fracking operations is the use of a clay stabiliser. Clay stabilisers prevent water-sensitive clay particles present in the formation from swelling when in contact with the water-based fracking fluid and thus clogging flow paths. In B T12 this is achieved by salting the fracking fluid by means of potassium chloride. In the fracks C 3a and D 3, tetramethylammonium chloride was employed, which prevents adsorption of water by coating the clay particles.
An essential functional component of the slickwater frack D 3 is the friction reducer, ethoxylated octylphenol, which is added to the fracking water in a hydrocarbon-based preparation.
A main functional component of the gel-based fracks B T12 and C Z3a is the carbohydrate-based thickener. In both fracks, carboxymethyl hydroxypropyl guar (CMHPG) polymer was employed. CMHPG is produced by derivatisation of guar flour. Guar flour consists mainly of the polysaccharide guar which is composed of mannose and galactose. Guar flour is modified by etherification and esterification of the hydroxy groups to give CMHPG which has a better solubility, better stability and higher salt tolerance.
Gel formation is supported by inorganic salts being able to form three-dimensional networks. In both gel-based fracks B T12 and C Z3a, inorganic borate is used as crosslinking agent. Inorganic borate often is employed in the form of borax, sodium tetraborate (Na2B4O7·10 H2O), a naturally occurring mineral that is produced in large quantities and is used in the production of glass and the glazing of porcelain and earthenware, as well as a component of detergents. Furthermore, borax is used for fire-proofing wood and textiles, to stiffen fabrics and as an additive in bleaching creams, skin-care products and other cosmetics. Recent animal experiments have shown that boron compounds are harmful to reproduction and to foetuses. Since 2010, inorganic borates and boric acid therefore are included in the REACH candidate list of substances of very high concern (SVHC) (ECHA 2010). The highest dose without effect (no-observed effect level, NOEL) for rats is given as 9.6 mg boron-equivalents per kg of body weight per day (Fail et al. 1998). This corresponds to a dose of 85 mg of borax per kg of body weight per day. Application of a safety factor of 100 delivers a tolerable daily intake of 0.85 mg per kg of body weight per day. Humans are unlikely to consume such a large quantity. In the European Union, the limit for drinking-water is 1 mg/l, expressed as boron.
For the frack B T12, the gelling agent also contains citrus terpenes. This product is extracted from the peel of lemons and other citrus fruits. The main component is limonene, which belongs to the group of terpenes, and other hydrocarbons—some of which are derivatives of terpene. Citrus terpenes, such as limonene, are used in many cosmetic and cleaning products as fragrances. As a bulk substance, limonene is toxic to aquatic life.
In order to maintain suitable conditions for longer phases of the fracking operations, stabilisers are added. One of the agents employed for this purpose in both gel fracks B T12 and C Z3a is tetraethylenepentamine. This substance is a volatile, oily liquid which is easily water soluble. The aqueous solution is very alkaline. It is mainly used as a solvent for sulphur, acidic gases, resins and paints, as a dispersing agent in motor oil, as an additive for ceramic, cement and concrete as well as a stabiliser for polymers. As a bulk substance, it is toxic to aquatic life.
After pressure release, the viscosity of the gel hinders the withdrawal of the fracking fluid. To reduce the viscosity, breakers, often inorganic oxidants, are used in gel-based fracking fluids. Sodium bromate is employed in both B T12 and C Z3a. According to EC regulation No 1272/2008, this substance is not classified as carcinogenic. However, potassium bromate is listed as a group 2B carcinogen (possibly carcinogenic to humans) by the International Agency of Research on Cancer (IARC 1999). The carcinogenic agent in this case is the bromate ion, which is known to be a strong oxidising agent. This classification is based on experiments performed with rats that were exposed to bromate in drinking-water throughout their lifespan, and developed tumours at different locations. Similar experiments on mice revealed no elevated tumour frequencies. It is still unclear whether the carcinogenicity of bromate is specific to rats. No epidemiological data are available on the carcinogenicity of bromate in humans. The US Environmental Protection Agency is cautious about whether long-term consumption of bromate poses a cancer risk to humans (US-EPA 2001). In the European Union, the limit for drinking-water is 10 μg/l.
A further large-scale component of the fracking fluids B T12 and C Z3a is the solvent 2-butoxyethanol. Colourless 2-butoxyethanol is miscible with water and most organic liquids. It has a slight ether-like smell and a boiling point of 171 °C. This substance is used in industrial and household water-based paints, detergents, polishes, inks and cosmetic products. The production capacity of EU member states is estimated to be 70,000–90,000 tonnes per year (in 1998). Due to the many areas of domestic use of this chemical, widespread dermal and inhalatory exposure is to be expected.
Five chemicals in the three formulas were only specified as “not classified as hazardous according to the directive 1999/45/EC” (EC 2008a) and no further information on their chemical identity was available.
Assessment of fracking fluids and their constituents
Assessment according to hazardous substances legislation
The assessment of the chemicals applied in the fracking fluid was performed according to the European Union’s Regulation No. 1272/2008 on classification, labelling and packaging of substances and mixtures (CLP) (EC 2008a). Hazardous, as defined by the regulation, are substances and/or mixtures which show at least one of the following properties: explosive; oxidising; extremely flammable, highly flammable or flammable; very toxic, toxic or harmful; corrosive; irritant; sensitising; carcinogenic; mutagenic; toxic for reproduction; dangerous for the environment. The regulation distinguishes between physical, health and environmental hazards. Hazard statements for the bulk fracking chemicals, which assign hazard class and hazard category, are given in Table 2. Classified as toxic to humans are methanol, tetramethylammonium chloride and the active ingredients of the biocide Kathon®. This biocide is also classified as toxic to aquatic life. Further constituents classified as toxic to aquatic organisms are citrus terpenes and tetraethylenepentamine. Twelve substances out of the 30 listed fracking additives in Table 2 are not classified as hazardous as bulk chemicals.
For hazardous substances as ingredients of mixtures, the CLP regulation specifies concentration limits for classification. Below these limits, the ingredients are classified as not relevant. With respect to acute toxicity, ingredients with a mass fraction <1 % are specified as not relevant. This roughly corresponds to a mass concentration of 10,000 mg/l. For some other hazard classes, the concentration limits for ingredients of mixtures are 5 % for skin irritation; 3 % for irreversible eye effects; 1–3 % for reversible eye effects; 0.1 % for skin sensitisation; 0.1 % for mutagenicity; 0.1 % for carcinogenicity; and 0.3 % for toxicity with regard to reproduction. From Table 3, giving the approximated mass concentrations of the additives classified as hazardous in the fracking fluid, it can be deduced that 2-butoxyethanol is the only constituent with a mass fraction exceeding 0.1 %. Since 2-butoxyethanol is neither skin-sensitising, nor mutagenic, nor carcinogenic, nor toxic for reproduction and its content is below 1 %, this ingredient is also not relevant according to the European Union’s Regulation No. 1272/2008. Thus, the fracking fluids as mixtures are not to be classified as hazardous according to the EU-CLP regulation.
Aside from the classification according to the CLP regulation, water protection aspects are, in Germany, additionally considered by classification of hazardous substances on the basis of the “Administrative Regulation on the Classification of Substances Hazardous to Waters into Water Hazard Classes (Verwaltungsvorschrift wassergefährdende Stoffe)” (UBA 2005). There are three water hazard classes (WGK): WGK 1, low hazard to waters; WGK 2, hazard to waters; WGK 3, severe hazard to waters. The water hazard class of a mixture of substances can be either determined by using a calculating rule and the WGK of each compound of the mixture, or on basis of ecotoxicological test data determined on the mixture. Table 2 shows that most of the chemicals used in the fracking fluids are classified as WGK 1. Ethoxylated alcohols and tetraethylenepentamine are assigned as WGK 2 and the biozide Kathon® with its two active components CIT and MIT is a representative of WGK 3. Thus, special care must be taken when handling these substances. The fracking fluids as mixtures are classified as WGK 1.
Since they are not soluble in water, the proppants are not classified as hazardous to the aquatic environment.
Assessment with respect to drinking-water
In case of an accident, the concentration levels in which ingredients of fracking fluids may appear in groundwater which is used as a drinking-water resource depend on the actual circumstances and cannot be predicted in a general way. Under ground, the concentrations in the fracking fluids represent the upper concentration limit. Thus, concentrations in the fracking fluids are assessed by regarding the dilutions necessary to meet limit values for drinking-water.
In Table 3, the mass concentrations of hazardous chemicals in the respective fracking fluids, approximated by dividing the mass of ingredient by the total mass of fracking fluid without proppants, are given and compared to health-related reference values from different assessment approaches for drinking-water. First, the European Drinking Water Directive (EC 1998) was consulted, which in Germany has been transferred to national law by the drinking-water ordinance (TrinkwV 2001). The parametric values of its Annex I are established in order to ensure that human health is protected from adverse effects caused by drinking-water contaminants. These parametric values are mostly based on the guideline values for drinking-water established by the World Health Organisation WHO (WHO 2011). For anthropogenic contaminants that have no function in drinking-water treatment or stabilisation they are sometimes even more restrictive than required from a human-toxicological point of view, following the so-called ALARA-principle, i.e., as low as reasonably (mostly technically) achievable. An example is the parametric value of 0.1 μg/l set for single biocidal compounds (pesticides) that are not included in the list of disinfectants permitted for drinking-water treatment, which has been oriented at the analytical detection limit (technical zero) at the time of its implementation.
The WHO guide values (GV) are health-based guide values to protect human beings from adverse effects by drinking-water constituents based on a lifelong water consumption of 2 l/day. For substances with an effect threshold, they are derived in a way that ingestion by drinking-water may not exceed 10–20 % of the tolerable daily intake (TDI). For substances for which a TDI cannot be given, e.g., carcinogens, levels that are associated with an additional cancer risk of 10−5–10−6 for a lifelong (70-year) exposure are regarded as acceptable.
For the majority of the fracking additives, neither a parametric value of the Drinking Water Directive nor a WHO guide value for drinking-water was available, because these substances are not expected to occur in raw waters. For some compounds, having been classified as hazardous, but not as carcinogenic, mutagenic or toxic to reproduction (cmr), health-related guide values (GV) were calculated from TDI values taken from the literature, assuming a drinking-water consumption of 2 l a day, a body weight of 70 kg and a 10 % fraction of TDI allocated to water consumption (see Table 4). In the table, the uncertainty factor (UF) that was used to calculate the TDI from no-observed adverse effect level (NOAEL), no-observed effect level (NOEL) or lower confidence limit of benchmark dose (BMDL) is also given.
For several fracking additives, TDI values are not available. These compounds were assessed according to the HRIV approach of the German Federal Environment Agency (Dieter 2011), a pragmatic default approach for new analytes in drinking-water with an incomplete human-toxicological data base assigning health-related indication values (HRIV) for lifelong exposure at four concentration steps between 0.1 and 3 μg/l. For the fracking additives with incomplete human-toxicological data base, a HRIV of 0.3 μg/l was set. This is the value for contaminants known to be devoid of a genotoxic potential in the absence of any other information.
Table 3 shows that after a 1:10,000 and up to 1:100,000 dilution of the fracking fluid the concentrations of fracking additives classified as hazardous would be below legal norms or health-based guide values for drinking-water. For some constituents, a higher dilution would be necessary to meet the low precautionary health-related indication values used for assessment because of incomplete human-toxicological database. HRIVs, however, are chosen in a manner that future scientifically health-based guide values will most likely be higher than the HRIV, but definitely not stricter.
Further constituents of the fracking fluids, mainly inorganic compounds, are not classified as hazardous substances, but are nevertheless regulated as constituents of drinking-water. As the salt content of the fracking fluids also has been subject of public concern, the concentrations of inorganic ions in the fracking fluids, calculated from the respective inorganic constituents, are listed in Table 5 and compared to reference values for drinking-water. The parametric value for nitrate is based on a health-based WHO guideline value. Sodium, potassium, magnesium and chloride are natural constituents of raw water and normally occur in concentrations below those of health concern. They are regulated for reasons of taste or corrosiveness. As for potassium and magnesium, no parametric values are available in the current European Drinking Water Directive, the former version of the directive (EC 1980) was consulted to roughly estimate the order of magnitude of a reference value. Thiosulphate and peroxodisulphate are not constituents of natural waters but are applied for drinking-water treatment and are included in the German “white list” of permitted additives for treatment (UBA 2012). The maximum permitted residual levels after treatment specified there are taken as a basis for assessment.
Table 5 shows that in many cases the concentrations of ions are already below the reference values for drinking-water in the undiluted fracking fluids. In every case, the requirements are met after a dilution of 1:1,000. The salt content is highest in the fracking fluid of B T12, as potassium chloride was used for clay stabilisation there.
A further indicative parameter for the assessment of drinking-water is the total organic carbon (TOC). As organic substances can support the growth of microorganisms in water and be precursors for disinfection by-products, low organic carbon content is desirable in drinking-water, as it is the case, e.g., in well-protected groundwaters. The European Drinking Water Directive specifies no limit but stipulates that this parameter should not exhibit abnormal changes. The TOC concentration of most German drinking-waters ranges from 0.5 to 2 mg/l. As the majority of fracking additives used in the considered recent fracks are organic compounds, the TOC concentration, calculated from the composition of the fracking fluids, is given in the table for comparison. The TOC concentrations in the fracking fluids are higher than the typical TOC values of highly polluted domestic wastewaters (about 300 mg/l). TOC concentrations in a range typical for drinking-waters would result after a 1:1,000 dilution for the slickwater frack D 3 or a 1:10,000 dilution for the gel-based fracks B T12 and C Z3a. These comparisons are made only to estimate the order of magnitude of organic load. It should be kept in mind that the drinking-water parameter TOC refers mostly to natural organic matter in water bodies and is not intended to be regarded as an upper limit for hazardous or undesired ingredients.
Assessment with respect to groundwater
Since groundwater is a major resource for drinking-water, a thorough assessment of the possible influences of fracking fluids on groundwater and drinking-water quality is one of the key issues. Beyond this aspect, groundwater needs protection as a natural water body. In Germany, local and limited groundwater contaminations are evaluated on the basis of so-called “thresholds of low concern”, which correspond (a) to the limit values for drinking-water or (b) to the predicted no effect concentrations for aquatic organisms (Länderarbeitsgemeinschaft Wasser—LAWA 2004). The assessment of the groundwater contaminant in question usually is performed with respect to the stricter of both values. This paper focuses mainly on groundwater as a drinking-water resource. The complementary ecotoxicological aspects are covered by Riedl et al. (2013).
Boron is the only constituent of the three fracking fluids of this study for which a “threshold of low concern” for groundwater has already been specified: 750 μg/l (Länderarbeitsgemeinschaft Wasser—LAWA 2004). After a dilution of 1:10, the boron concentrations of the boron-containing fracking fluids of this study (B T12 and C Z3a) would be below this level.
In case of a groundwater contamination, the groundwater will not be used as raw water for drinking-water production, but will be restored by suitable remediation methods on-site or off-site.
In order to assess the severeness of a groundwater contamination, not only is concentration relevant, but also the total amounts of substances that penetrate into the aquifer following a possible leakage. These loads must be removed by groundwater remediation methods. For contaminations from under ground, the maximum loads are not given by the total masses of the fracking chemicals applied, but by the volume of fracking fluid likely to seep through a leak during the time of the fracking operation and/or until the leakage is noticed and remediation starts. Uth (2012) distinguishes two scenarios of leakage during the hydrofracking operation. A major leakage in the borehole, due to pipe rupture, is detected within 5 min because of the resulting drop in pressure. The fracking operation is subsequently stopped. Until then, about 35 m3 fluid will have been released. A minor seepage leakage remains undetected during the fracking operation (e. g., up to 8 h). In this time, up to 76.6 t are likely to seep into the surrounding aquifer. The contamination can only be detected afterwards after a migration period of about 1 week by a groundwater observation well—assumed to be located 20 m away from the borehole. The released amounts correspond to about 16–35 (B T12), 0.9–2 (C Z3a), or 0.3–0.6 % (D 3) of the total fracking fluid. From this, the maximum amount of chemical load to be removed by groundwater remediation measures can be roughly estimated. Given as organic carbon (OC), this would be about 213–524 kg OC for the gel-based fracks B T12 and C Z3a, and 23–51 kg OC for the slickwater frack D 3. One consequence of these estimates is that the location of the fracking operations should be in a safe distance from wells or boreholes which are used for drinking-water production.
In order to check whether there is an influence on usable groundwater by fracking fluids, monitoring is necessary including components of the fracking fluid or their reaction products. Data on this item are scarce. Groundwater analyses from the surroundings of a fracking site in the area of this study were available for four observation wells near the site Goldenstedt Z23 for a sampling depth of about 50 m (ExxonMobil 2011b). The samples were taken several months after the fracking operation and were analysed by a certified analytical laboratory for nearly 100 single parameters, mostly oriented at the requirements for drinking-water. The analytical results did not indicate an influence of the fracking operation on groundwater quality. Additionally, the samples from six wells were analysed for the following ingredients of the fracking fluid applied: 2-butoxyethanol (ρ FF = 6,793 mg/l; LOQ = 1 mg/l), propan-2-ol (ρ FF = 307 mg/l; LOQ = 1 mg/l), methanol (ρ FF = 238 mg/l; LOQ = 10 mg/l), CIT (ρ FF = 5.26 mg/l; LOQ = 0.05 mg/l), and MIT (ρ FF = 1.75 mg/l; LOQ = 0.05 mg/l). The concentrations of these ingredients in the groundwater were below the limit of quantification (LOQ) for all wells. However, the LOQs were relatively high. This matches findings in the literature, where the unsatisfactory sensitivity of available analytical methods substantially limited the evaluation of glycols like ethylene glycol and 1,2-propylene glycol as sentinel compounds for fracking fluid influence even in flowback waters (Pees Coleman 2011).
Development of fracking fluids design: aspects and outlook
Analysis of the fracking formulas available (ExxonMobil 2011a) back to the 1980s shows that the amount and concentration of chemicals in the fracking fluids applied for comparable drilling sites have been be reduced from about 5–10 % in the beginning to about 1–3 % for the recent tight gas fracks B T12 and C Z3a dealt with in this paper. For shale gas fracks, lower concentrations and a lower number of additives are generally necessary for technical reasons.
Perceived to be of major concern with respect to safety of drinking-water and groundwater are chemicals classified as toxic to humans, toxic to aquatic life or as a severe hazard to water. There have already been efforts to replace those substances (see also Ewers et al. 2012). For instance, diethanolamine (CAS No. 111-42-2) has been in discussion to replace the more toxic methanol, still employed in the frack C Z3a as the solvent for the surfactant. A technically challenging but urgent task will be the substitution of inorganic borate in gel-based tight-gas fracking fluids. This additive is not required for the shallow horizons of shale-gas fracks.
To illustrate in which direction the development could go, a draft formula for future hydrofracking operations in shale gas reservoirs in Germany is given in Table 6. ExxonMobil made this draft available to the panel of experts towards the end of the dialogue process. In the table, two options of design are considered: slickwater frack and linear-gel frack. The table gives the maximum amounts considered to be employed.
For clay stabilisation, neither the neutral salt potassium chloride is intended, which is of ecotoxicological concern in the concentrations usual for this purpose (Riedl et al. 2013; Schmitt-Jansen et al. 2012), nor tetramethylammonium chloride, which is toxic. Instead, choline chloride will be employed. The acute toxicity of choline chloride (Oral LD50 for rats: 3,400 mg/kg) (ESIS 2000b) is significantly lower than that of tetramethylammonium chloride (Oral LD50 for rats: 50 mg/kg) (GESTIS 2012). Choline occurs naturally in animals and in the human body.
As an additive in both slickwater and linear-gel frack, polyethylene glycol monohexyl ether (ethoxylated hexan-1-ol) is intended. Polyethylene glycol-octylphenyl ether will no longer be employed. Concern was voiced during the public discussion that octylphenol, which is a priority pollutant, could be released as a degradation product, in case the biocide fails to suppress microbial activity.
As the thickener for the option linear-gel frack, a carbohydrate derivative, classified as non-hazardous, will be employed like in the other gel fracks already discussed in this paper.
As the friction reducer, almost non-toxic 2-(2-butoxy-ethoxy)-ethanol (“butyl diglycol”) shall be employed instead of the hydrotreated light petroleum distillates used for the slickwater frack D 3.
The highly effective biocide Kathon®shall be substituted by the biocide (ethylenedioxy)dimethanol (other names: [1,2-ethylene-diylbis-(oxy)]-bis-methanol; 1,6-dihydroxy-2,5-dioxy-hexane) which is not classified as toxic, but as harmful. By the German WGK classification, this biocide is classified as WGK 1 (low hazard), whereas Kathon® is classified as WGK 3 (severe hazard). The biocidal action of this agent is based on the release of formaldehyde. It is used, for example, in fluids for metalworking (De Groot et al. 2010). The substitution may be beneficial from the viewpoint of occupational safety. However, (ethylenedioxy)dimethanol must be employed at about one thousand times higher concentrations than Kathon® to achieve the same effect. With respect to the low limit for biocides for drinking-water, this is disadvantageous.
The employment of biocides in frackings fluid is a major item of criticism, especially due to the low limit values for drinking-water. Meanwhile, studies are being performed on the tailor-made dosing of biocides suitable to maintain the biocidal action for the duration of the fracking operation but the biocide not persisting in flowback water beyond a few days, as biocidal components in the flowback may be adverse for some options for disposal (Rimassa et al. 2011).
As an alternative option to the addition of biocides, disinfection by UV radiation is in discussion. This option, however, is only efficient for transparent media. A technical rule for UV disinfection of drinking-water specifies the following requirements to the water: spectral absorption coefficient ≤10/m (λ = 254 nm), spectral extinction coefficient ≤15/m (λ = 254 nm), turbidity ≤0.3 FNU (DVGW 2006). Thus, only the plain fracking water can be disinfected in this way, but not the final fracking fluid. Furthermore, no long-term disinfection capacity is provided by this method.
As an alternative to water-based fracking fluids, non-aqueous fracking technologies for shale gas production are in discussion, especially the use of liquefied propane (LPG) as fracking fluid is considered as an environmentally friendly alternative (Rogala et al. 2013). As the composition of each fracking fluid has to be adapted to the specific conditions of the actual formation and drilling site and technical progress will provide new options, the development of fracking formulas which take human-toxicological and environmental aspects into account is a continuous process.
Consequences and further research topics
As a precautionary measure for groundwater and drinking-water protection, fracking activities should be monitored by groundwater observation wells in the fracking area. A baseline for groundwater quality should be documented before fracking activities start. Further work has to be done to specify suitable target analytes which may serve as sentinel compounds for fracking fluid influence. For this, sufficiently sensitive analytical methods should be applied with detection limits in the range of 0.0001–0.01 mg/l. Another analytical option to be considered is non-target screening, which permits comparison of patterns and fingerprints of organic ingredients by capturing a large spectrum of organic micropollutants in a single analytical run (Müller et al. 2011). In a recent publication, 3D fluorescence spectroscopy (excitation emission mapping) was proposed as a method to identify groundwater contamination from coalbed methane production (Dahm et al. 2013).
The assessment in this paper is limited to the fracking chemicals as they were employed. It is likely that many of the chemicals undergo transformations during the fracking operation and under ambient conditions under ground. For instance, bromate, which is applied as an oxidant, is likely to react to give bromide with all the options of further reactions. Furthermore, microbial transformations might occur, when the efficiency of the biocide has decayed. A detailed analysis of flowback composition for possible degradation or transformation products would be necessary. Formate and acetate were found in some flowback waters (Rosenwinkel et al. 2012; Olsson et al. 2013), but much work still has to be done on the material balances of fracking operations.
The employment of biocides evidences that microorganisms and microbial processes are relevant in hydrofracking. A considerable amount of the injected fracking fluid (Olsson et al. 2013; Rosenwinkel et al. 2012) will remain under ground. Little is known about the influence of these materials on the autochthonous microbial population in the deep surface (Krumholz 2000).
Constituents of flowback water and their assessment
In the framework of the panel of experts, the issue of flowback and formation waters was covered in detail by Olsson et al. (2013) and Rosenwinkel et al. (2012), especially with regard to the neutral salt content of these waters and options for their disposal or treatment. In this paper, only the aspect of micropollutants is addressed with respect to the requirements for groundwater and drinking-water. To do so, the results of 13 flowback water analyses from different tight gas drilling sites were evaluated with respect to heavy metals and different hydrocarbons (see Tables 7, 8) and compared with legal norms for drinking-water and groundwater. Additionally, environmental quality standards for surface waters are given where available. To consider the aspects of surface water is also sensible, since parts of the flowback water management, e.g., transport, is performed above ground, until the produced water is disposed by deep well injection.
The concentrations of toxic heavy metals in the formation water samples are mainly in the range below 0.1 mg/l. In most cases, a dilution of 1:100 is sufficient to meet the requirements for drinking-water or the threshold values of low concern for local and limited groundwater contaminations. For antimony, mercury and zinc, a dilution of 1:1,000 would be necessary. The mercury and cadmium levels are relevant with respect to environmental quality standards for surface waters.
As with coalbed formation waters (Orem et al. 2007), the results of the analyses vary considerably over time. This especially holds for the results for organic micropollutants given in Table 8.
The total sum of BTEX hydrocarbons covers a wide range (0.07–19.4 mg/l). The main component is benzene, which was measured in quantities of up to 13 mg/l.
For the total concentration of PAHs, values of up to 10 mg/l were determined in the produced waters. The relatively highly water soluble PAHs, such as naphthalene, fluorene and phenanthrene dominate. Several micropollutants listed in Table 8 would meet the threshold values for groundwater only after a dilution of 1:1,000 or more. Of special concern are naphthalene, benzene and the total concentration of the PAHs, where a dilution of 1:100,000, in extreme cases of 1:1,000,000 would be necessary. The latter especially holds for the assessment of PAH with respect to the requirements for drinking-water. For several organic micropollutants of Table 8 environmental quality standards for surface waters are also set, since these substances are priority pollutants in the field of water policy.
According to the CLP regulation, Annex 1, No. 4.1.3.3, the produced waters for which analytical data were available to the panel of experts are neither to be classified as hazardous mixtures, nor as mixtures which are acutely or chronically hazardous for aquatic systems. Nevertheless, release of produced waters into the ground and groundwater, as well as pollution of surface water bodies by these waters must be avoided by suitable safety measures. As formation water pipelines and disposal boreholes also might be subject to leakages (Uth 2012), the management of flowback water requires high safety standards and thorough monitoring.
Conclusions and recommendations
This paper deals with the human-toxicological assessment of substances used and mobilised in the hydraulic fracturing process, especially with regard to a possible influence on groundwater quality. The preservation of groundwater quality is of essential importance, since groundwater is the most important resource of drinking-water in Germany and many other European countries. Fracking fluids consist mainly of water and proppants. Usually, they contain about 1–3 % (w/w) of chemical additives. A number of these additives fulfil the criteria relating to physical hazards, health hazards or environmental hazards laid down in Parts 2–5 of Annex I of Regulation (EC) No. 1272/2008. As a consequence, these additives must be classified in relation to the respective hazard classes provided in the aforementioned annex. The concentrations of the additives employed in frack fluids are usually below 0.1 % and thus below the threshold limits at or above which the presence of that substance in a mixture leads to the classification as hazardous mixture.
Contamination of groundwater by ingredients of fracking fluids may occur from under ground or may result from above-ground accidents associated with the transport, storage and handling of hazardous substances used as additives for fracking fluids. The degree of groundwater contamination cannot be predicted in a general sense. Therefore, different dilutions of the fracking fluid in groundwater were considered. Three selected fracking fluids were evaluated. It was calculated that the concentrations of various ingredients in fracking fluids are 1:10,000 up to 1:100,000 times above the limit values of the European Drinking Water Directive, the WHO Guideline Values for Drinking-water Quality, and other health-based guide values. Regarding the salinity of fracking fluids, a dilution of 1:1,000 is sufficient to reach concentrations acceptable for drinking-water.
From a human-toxicological point of view, the ingredients of flowback water are more problematic with respect to drinking-water aspects than those of fracking fluids. Flowback water is influenced by the organic constituents of the fossil deposit and it contains heavy metals. Some of the substances in flowback water are priority pollutants in the field of water policy.
The conclusions from the results are:
-
The synthetic fracturing fluids should contain only environmentally friendly constituents with low toxicity
-
Geogenic salts, toxic and radioactive species which are expected to be mobilised by the fracking process and other reaction products which reach the surface as flowback should be kept in closed systems to prevent pollution of soil, groundwater and surface water. The management and disposal of flowback and formation waters is not specific for hydraulic fracturing, but a general problem in natural gas exploitation.
-
Safety measures may fail. Therefore, a sufficient distance of drilling operations and above-ground handling of hydrofracking chemicals to wells used for drinking-water production should be assured. Fracking activities should not interfere with vital water protection measures, especially with drinking-water protection areas and should be excluded in geological regions with disturbance zones that possibly allow fracking fluids to ascend from the deep underground into the shallow usable aquifer.
There is no doubt that due to the uniqueness of the role of water for life and mankind, its protection is of highest priority. Therefore, it is advisable to:
-
Include competent water quality specialists from the very beginning of hydraulic fracturing planning.
-
Involve governmental water authorities in the process of hydraulic fracturing.
-
Improve the state of the art of environmentally friendly hydraulic fracturing and to develop standard methods for the procedure itself, for additives and material balances, for comprehensive monitoring and for the management of produced wastewater and risk factors.
-
Investigate and publish on the impact of hydraulic fracturing on nature and especially on water resources.
-
Use an open information and discussion strategy amongst all peers and the public from early stages of planning onwards.
References
Bottero S, Picioreanu C, Enzien M, van Loosdrecht MCM, Bruining H, Heimovaara T (2010) Formation damage and impact on gas flow caused by biofilms growing within proppant packing used in hydraulic fracturing. Paper SPE 128066, from SPE International Symposium on Formation Damage in Lafayette
Brandon DM, Fillo JP, Morris AE, Evans JM (1995) Biocide and corrosion inhibition use in the oil and gas industry: effectiveness and potential environmental impacts. Paper SPE29735 presented at the Houston 1995 E&P Environmental Conference
Dahm KG, Van Straaten CM, Munakata-Marr J, Drewes JE (2013) Identifying well contamination through the use of 3-D fluorescence spectroscopy to classify coalbed methane produced water. Environ Sci Technol 47:649–656. doi:10.1021/es303866k
De Groot A, Geier J, Flyvholm MA, Lensen G, Coenraads PJ (2010) Formaldehyde-releasers: relationship to formaldehyde contact allergy. Metalworking fluids and remainder. Part 1. Contact Dermat 63:117–128
Degner DL (2011) Hydraulic fracturing fluid considerations in Marcellus Shale completions. In: Proceedings of the technical workshops for the hydraulic fracturing study: chemical and analytical methods. EPA 600/R-11/066. http://www.epa.gov/hfstudy/chemworkshop.html. Accessed 31 Aug 2012, pp 15–17
Dieter HH (2011) Drinking water toxicology in its regulatory framework. In: Frimmel FH (ed) Aquatic chemistry and microbiology, treatise on water science, Vol. 3 (ed. Wilderer P). Elsevier, Amsterdam, pp 377–415
DVGW (2006) Technische Regel—Arbeitsblatt W 294-1—UV-Geräte zur Desinfektion in der Wasserversorgung; Teil 1: Anforderungen an Beschaffenheit, Funktion und Betrieb (Ultraviolet disinfection equipment for drinking-water treatment. Part 1: requirements for type, performance and operation). DVGW Deutsche Vereinigung des Gas- und Wasserfaches e. V., Bonn
EC (1980) Council Directive of 15 July 1980 relating to the quality of water intended for human consumption (80/778/EEC). Off J Eur Commun L 229:11–29
EC (1998) Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. Off J Eur Commun L 330:32–54
EC (2008a) Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006
EC (2008b) Directive 2008/105/EC of the European Parliament and of the Council on environmental quality standards in the field of water policy, amending and subsequently repealing Council Directives 82/176/EEC, 83/513/EEC, 84/156/EEC, 84/419/EEC, 86/280/EEC and amending Directive 2000/60/EC. Off J Eur Commun L 348:84–97 (24 December 2008)
ECHA (2010) http://echa.europa.eu/web/guest/candidate-list-table. Accessed 31 Aug 2012
EPA (2011a) Proceedings of the technical workshops for the hydraulic fracturing study: well construction and operations. EPA 600/R-11/046. http://www.epa.gov/hfstudy/wellconstructworkshop.html. Accessed 31 Aug 2012
EPA (2011b) Proceedings of the technical workshops for the hydraulic fracturing study: chemical and analytical methods. EPA 600/R-11/066. http://www.epa.gov/hfstudy/chemworkshop.html. Accessed 31 Aug 2012
ESIS (2000a) http://esis.jrc.ec.europa.eu/doc/IUCLID/data_sheets/26172554.pdf. Accessed 31 Aug 2012
ESIS (2000b) http://esis.jrc.ec.europa.eu/doc/IUCLID/data_sheets/67481.pdf. Accessed 31 Aug 2012
Ewen C, Borchardt D, Richter S, Hammerbacher R (2012) Hydrofracking risk assessment. Executive summary. Study concerning the safety and environmental compatibility of hydrofracking for natural gas production from unconventional reservoirs. http://dialog-erdgasundfrac.de/sites/dialog-erdgasundfrac.de/files/Ex_HydrofrackingRiskAssessment_120611.pdf. Full-length surveys in German: http://dialog-erdgasundfrac.de/gutachten. Accessed 31 Aug 2012
Ewers U, Frimmel FH, Gordalla B (2012) Humantoxikologische Bewertung der beim Fracking eingesetzten Chemikalien im Hinblick auf das Grundwasser, das für die Trinkwassergewinnung genutzt wird (Human-toxicological assessment of chemicals employed for hydrofracking operations with respect to groundwater to be used for drinking-water production). Survey within the framework of the dialogue and information dissemination process concerning the health and environmental aspects of hydrofracking. http://dialog-erdgasundfrac.de/gutachten. Accessed 31 Aug 2012
ExxonMobil (2011a) http://www.erdgassuche-in-deutschland.de/technik/hydraulic_fracturing/frac_fluessigkeiten.html. Accessed 31 Aug 2012
ExxonMobil (2011b) http://www.erdgassuche-in-deutschland.de/files/Brunnen_Vechta_SKMBT_C20311060116050.pdf. Accessed 31 Aug 2012
Fail PA, Chapin RE, Price CJ, Heindel JJ (1998) General, reproductive, developmental, and endocrine toxicity of boronated compounds. Reprod Toxicol 12:1–18
GESTIS (2012) GESTIS-Stoffdatenbank—Gefahrstoffinformationssystem der Deutschen Gesetzlichen Unfallversicherung (Data base on hazardous substances of the German statutory accidant insurance). http://www.dguv.de/ifa/de/gestis/stoffdb/index.jsp. Accessed 31 Aug 2012
Gregory KB, Vidic RD, Dzombak DA (2011) Water management challenges associated with the production of shale gas by hydraulic fracturing. Elements 7:181–186. doi:10.2113/gselements.7.3.181
Hahn S et al (2005) Health risks from biocide-containing products and articles of daily use. Action program, environment and health project funding number (UFOPLAN) 204 61 218/05. http://www.apug.de/archiv/pdf/Abschlussbericht_Kurzfassung_Biozide_english.pdf. Accessed 31 Aug 2012
IARC—International Agency for Research on Cancer (1999) IARC monographs on the evaluation of carcinogenic risks to humans 73:481. http://monographs.iarc.fr/ENG/Monographs/vol73/mono73-22.pdf
Jacobson A, Williams T (2000) The environmental fate of isothiazolone biocides. Chimica oggi/Chem Today (October 2000)
Kissinger A, Helmig R, Ebigbo A, Class H, Lange T, Sauter M, Heitfeld M, Klünker J, Jahnke W (2013) Hydraulic fracturing in unconventional reservoirs—risks in the geological system, part 2. Environ Earth Sci. doi:10.1007/s12665-013-2578-6
Krumholz LR (2000) Microbial communities in the deep subsurface. Hydrogeol J 8:4–10
Länderarbeitsgemeinschaft Wasser—LAWA (2004): Ableitung von Geringfügigkeitsschwellen für das Grundwasser (Deduction of thresholds of low concern for local and limited groundwater contaminations). http://www.lawa.de/documents/GFS-Bericht-DE_a8c.pdf. Accessed 31 Aug 2012
Lange T, Sauter M, Heitfeld M, Schetelig K, Jahnke W, Kissinger A, Helmig R, Ebigbo A, Class H (2013) Hydraulic fracturing in unconventional reservoirs—risks in the geological system, part 1. Environ Earth Sci. doi:10.1007/s12665
Lechtenböhmer S, Altmann M, Capito S, Matra Z, Weindorf W, Zittel W (2011) Impacts of shale gas and shale oil extraction on the environment and on human health. Study of the European Parliament. http://www.europarl.europa.eu/document/activities/cont/201107/20110715ATT24183/20110715ATT24183EN.pdf. Accessed 31 Aug 2012
McCurdy R (2011) High rate hydraulic fracturing additives in non-Marcellus unconventional shales. In: Proceedings of the technical workshops for the hydraulic fracturing study: chemical and analytical methods. EPA 600/R-11/066. http://www2.epa.gov/hfstudy/high-rate-hydraulic-fracturing-additives-non-marcellus-unconventional-shales. Accessed 8 July 2013
Müller A, Schulz W, Ruck WKL, Weber HW (2011) A new approach to data evaluation in the non-target screening of organic trace substances in water analysis. Chemosphere 85:1211–1219
Nemati M, Voordouw G (2000) Identification and characterization of sulfate-reducing bacteria involved in microbially influenced corrosion in oil fields. NACE Paper 00126, presented at Corrosion 2000
Olsson O, Weichgrebe D, Rosenwinkel KH (2013) Hydrofracking wastewater in Germany: composition, treatment, concerns. Environ Earth Sci. doi:10.1007/s12665-013-2535-4
Orem WH, Tatu CA, Lerch HE, Rice CA, Bartos TT, Bates AL, Tewalt S, Corum MD (2007) Organic compounds in produced waters rom coalbed natural gas wells in the Poweder River Basin, Wyoming, USA. Appl Geochem 22:2240–2256. doi:10.1016/j.apgeochem.2007.04.010
Osborn S, Vengosh A, Warner N, Jackson R (2011) Methane contamination of drinking water accompanying gas-well drilling and hydraulic fracturing. Proc Nat Acad Sci 108(20):8172–8176. doi:10.1073/pnas.1100682108
Pees Coleman N (2011) Produced formation water sample results from Shale plays. In: EPA (ed) Proceedings of Technical Workshops for the Hydraulic Fracturing Study: chemical analytical methods. US Environmental Protection Agency. http://www.epa.gov/hfstudy/producedformationwatersampleresultsfromshaleplays.pdf. Accessed 31 Aug 2012
Rafoth A, Gabriel S, Sacher F, Brauch H-J (2007) Analysis of isothiazolinones in environmental waters by gas chromatography-mass spectrometry. J Chromatogr A 1164:74–81
Riedl J, Rotter S, Faetsch S, Schmitt-Jansen M, Altenburger R (2013) Proposal for applying a component-based mixture approach for ecotoxicological assessment of fracturing fluids. Environ Earth Sci. doi:10.1007/s12665-013-2320-4
Rimassa SH, Howard P, MacKay B, Blow K, Coffman N (2011) Case study: evaluation of an oxidative biocide during and after a hydraulic fracturing job in the Marcellus Shale. SPE International Symposium on Oilfield Chemistry, Paper SPE 141211. The Woodlands, Texas, USA, 11–13 April 2011
Rogala A, Krzysiek J, Bernaciak M, Hupka J (2013) Non-aqueous fracturing technologies for shale gas recovery. Physicochem Probl Mineral Process 49(1):313–322. doi:10.5277/ppmp130128
Rosenwinkel KH, Weichgrebe D, Olsson O (2012) Stand der Technik und fortschrittliche Ansätze in der Entsorgung des Flowback (State of the art and advanced approaches for disposal of flowback). Survey within the framework of the dialogue and information dissemination process concerning the health and environmental aspects of hydrofracking. http://dialog-erdgasundfrac.de/gutachten. Accessed 31 Aug 2012
Rossnagel A, Hentschel A, Polzer A (2012) Rechtliche Rahmenbedingungen der unkonventionellen Erdgasförderung mittels Fracking (Regulatory framework for unconventional exploitation of natural gas by hydraulic fracturing). Survey within the framework of the dialogue and information dissemination process concerning the health and environmental aspects of hydrofracking. http://dialog-erdgasundfrac.de/gutachten. Accessed 31 Aug 2012
Rossnagel A, Hentschel A, Polzer A (2013) Legal contributions to conflict resolution—the legal evaluation of unconventional natural gas extraction by means of fracking in Germany. Environ Earth Sci. doi:10.1007/s12665
Satya Gupta DV (2011) Unconventional fracturing fluids. In: Proceedings of the technical workshops for the hydraulic fracturing study: chemical and analytical methods. EPA 600/R-11/066. http://www2.epa.gov/sites/production/files/documents/unconventionalfracturingfluids-what-where-why.pdf. Accessed 8 July 2013
Schmitt-Jansen M, Aulhorn S, Faetsch S, Riedl J, Rotter S, Altenburger R (2012) Ökotoxikologische Beurteilung von beim hydraulischen Fracking eingesetzten Chemikalien (Ecotoxicological assessment of chemicals employed for hydraulic fracturing). Survey within the framework of the dialogue and information dissemination process concerning the health and environmental aspects of hydrofracking. http://dialog-erdgasundfrac.de/gutachten. Accessed 31 Aug 2012
Schneble H, Weinem K, Niethammer I (2012) Flächeninanspruchnahme, (oberirdische) Infrastruktur, Betrieb (Footprint, above-ground infrastructure, operation). Survey within the framework of the dialogue and information dissemination process concerning the health and environmental aspects of hydrofracking. http://dialog-erdgasundfrac.de/gutachten. Accessed 31 Aug 2012
Thoma A, Sacher F (2012) Studie zur Bedeutung von Bioziden für die Trinkwasserversorgung (Study on the relevance of biocides for the drinking-water supply). Abschlussbericht zum Forschungsvorhaben W 3/01/09. Veröffentlichungen aus dem DVGW-Technologiezentrum Wasser Karlsruhe, Band 53
TrinkwV (2001) Trinkwasserverordnung i. d. F. der Bekanntmachung vom 28 November 2011, die zuletzt durch Artikel 1 der Verordnung vom 5. Dezember 2012 (BGBl. I S. 2562) geändert worden ist. (Drinking-water ordinance). http://www.bgbl.de/Xaver/start.xav?startbk=Bundesanzeiger_BGBl#__Bundesanzeiger_BGBl__%2F%2F*[%40attr_id%3D%27bgbl112s2562.pdf%27]__1369244730353
UBA (2005) Verwaltungsvorschrift wassergefährdende Stoffe (VwVwS) vom 17. Mai 1999, zuletzt geändert am 27. Juli 2005 (Administrative regulation on the classification of substances hazardous to water into water hazard classes and amendment of 27 July 2005)
UBA (2012) Liste der Aufbereitungsstoffe und Desinfektionsverfahren gemäß § 11 der Trinkwasserverordnung 2001 (List of permitted additives and disinfection processes for drinking-water treatment). 17. Änderung Stand November 2012. http://www.umweltbundesamt.de/wasser/themen/downloads/trinkwasser/17_aenderung_aufbereitungsstoffe_desinfektionsverfahren_11_trinkwv_11_2012.pdf. Accessed 28 March 2013
US-EPA (1993) Integrated risk information system. Methanol (CASRN 67-56-1). http://www.epa.gov/iris/subst/0305.htm. Accessed 25 May 2012
US-EPA (2001) Toxicological review of bromate. http://www.epa.gov/iris/toxreviews/1002tr.pdf. Accessed 31 Aug 2012
US-EPA (2010) Integrated risk information system. Ethylene glycol monobutyl ether (EGBE) (2-Butoxyethanol) (CASRN. 111-76-2). http://www.epa.gov/iris/subst/0500.htm. Accessed 25 May 2012
Uth HJ (2012) Technische Sicherheit von Anlagen und Verfahren zur Erkundung und Förderung von Erdgas aus nichtkonventionellen Lagerstätten (Technical safety of installations and operations for exploration and exploitation of natural gas from unconventional reservoirs). Survey within the framework of the dialogue and information dissemination process concerning the health and environmental aspects of hydrofracking. http://dialog-erdgasundfrac.de/gutachten. Accessed 31 Aug 2012
WHO—World Health Organization (2011) Guidelines for drinking-water quality. 4th edn. http://www.who.int/water_sanitation_health/publications/2011/dwq_chapters/en/index.html. Accessed 25 May 2012
Wood R, Gilbert P, Sharmina M, Anderson K, Footit A, Glynn S, Nicholls F (2011) Shale gas: a provisional assessment of climate change and environmental impacts. A research report by The Tyndall Centre University of Manchester. http://www.tyndall.ac.uk/publications/technical-report/2011/shale-gas-provisional-assessment-climate-change-and-environmental. Accessed 31 Aug 2012
Acknowledgments
The authors thank Alejandra Lenis Parra and Jörg Mießner for help with the data and the manuscript. Fiona Crowther’s help with language polishing is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gordalla, B.C., Ewers, U. & Frimmel, F.H. Hydraulic fracturing: a toxicological threat for groundwater and drinking-water?. Environ Earth Sci 70, 3875–3893 (2013). https://doi.org/10.1007/s12665-013-2672-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-013-2672-9