Abstract
Over the past decades, the Gujarat state of India experienced intensive agricultural and industrial activities, fertilizer consumption and abstraction of groundwater, which in turn has degraded the ground water quality. Protection of aquifers from nitrate pollution is a matter of prime concern for the planners and decision-makers. The present study assessed the spatial and temporal variation of groundwater nitrate levels in areas with different land use/land cover activities for both pre- and post-monsoon period. The pre-monsoon nitrate level (1.6–630.7 mg/L) in groundwater was observed to be higher as compared to the post-monsoon level (2.7–131.7 mg/L), possibly due to insufficient recharge and evaporation induced enrichment of agrichemical salts in groundwater. High HCO3 − (200–1,000 mg/L) as well as SO4 2−/Cl− (0.111–0.992) in post-monsoon period provides a favourable environment for denitrification, and lower the NO3 levels during the post-monsoon period. The K vs NO3 scatter plot suggests a common source of these ions when the concentration is <5 mg/L, the relationships between different pollutants and nitrate also suggest that fertilizers and other sources, such as, animal waste, crop residue, septic tanks and effluents from different food processing units present in the area can be attributed to higher nitrate levels in the groundwater. Appropriate agronomic practices such as application of fertilizers based on calibrated soil tests and proper irrigation with respect to crop can minimize the requirement for inorganic fertilizers, which can bring down the cost of cultivation considerably, and also protect groundwater from further degradation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The groundwater in the state of Gujarat, situated in the west coast of India, is used for drinking, industrial and agricultural purposes, and irrigating 80 % of the land area. The state has experienced intense growth of agricultural and industrial activities in past decade. Changes in cropping pattern and agriculture have led to different types of fertilizer use and water abstraction especially for cash crops. The total N, P, K fertilizer consumption increased from 11,071 tonnes in 1960–1961 to 1716,983 tonnes in 2008–2009 (Fertilizer Association of India 2009). For sustenance of agriculture, nitrogen as an essential nutrient input is commonly provided in the study area by the nitrogen fertilizers such as ammonium chloride, diammonium phosphate, ammonium sulphate, calcium ammonium nitrate and urea. In addition to fertilizers, farmyard manure is also used. Due to regular and high application of nitrogen fertilizers, a blanket (non-point) source of nitrate has been created in the irrigated cropped land (Datta et al. 1997).
It is well known that urea and ammonium fertilizers, generally used as a source of nitrogen for paddy and other crops during irrigation, is hydrolyzed by the enzyme urease, and increase the ammonium concentration by the following equation (Widory et al. 2004):
which is ultimately converted into nitrate in the unsaturated zone as follows:
From the unsaturated zone, nitrate is leached into the groundwater depending upon soil moisture status, soil texture and fertilizer management practices (N rate, source, and timing). Nitrate dynamics is closely related to water dynamics and its complexity in temporal and spatial scale. The processes such as mineralization–immobilization, nitrification, denitrification, and plant uptake (for the different pathways of nitrogen dynamics) are major soil transformation processes that greatly affect nitrate leaching (Addiscott et al. 1991; Jacks and Sharma 1983; Rajmohan and Elango 2005). These reactions depend on pH, temperature, soil water content, and soil biological characteristics. Release of N from biomass depends on oxic conditions, which is likely to occur in the unsaturated zone, whereas, in reducing environment, rich in denitrifying bacteria, denitrification takes place (Rao et al. 2013; Hamed et al. 2013).
All over the world, wherever nitrogenous fertilizers have been used extensively to increase the agricultural productivity, high nitrate level in groundwater is evident. Often, nitrate contamination of groundwater may be also associated with point sources such as domestic sewage, industrial waste, livestock feeding operations and septic tanks, etc. Various physical, chemical, and biological processes in the soil zone and ground water determine the nitrate level in ground water (Johnsson et al. 2002).
An elevated nitrate level in drinking water is dangerous to human health, and nitrate levels exceeding 45 mg/L cause methemoglobinemia in infants (WHO 1984). US Environmental Protection Agency has established a maximum permissible contaminant level of 10 mg/L NO3-N (US EPA 2000). It is possible that nitrate in drinking water may be converted in the human gastrointestinal tract to nitrosamines, some of which are carcinogenic and the effects of long-term exposures are potentially serious. Also, excess nitrate is harmful to many crops at critical stages of development (Datta et al. 1997).
Geographical Information System (GIS) has been proved to be useful in assessment of nitrate leaching risk indices, and designating areas of dissimilar vulnerability (Wylie et al. 1995; Nolan et al. 1997; Refsgaard et al. 1999; Lasserre et al. 1999). In this brief background, the present study attempts to identify the factors controlling the nitrate contamination in the study area using hydrochemistry in integration with GIS.
Physiography, climate and hydrogeology of the area
The study area covers the districts of Ahmedabad, Mehsana, Gandhinagar, Kheda and Anand in northern Gujarat with approximately 7,221 km2 (Fig. 1) geographical area. Sabarmati River and its tributaries is the main source of surface water in the area. In the north and northeast part it is bounded by the Aravalli hills; in the eastern part a ridge separates it from the Mahi Basin; in the south, by the Gulf of Cambay; and in the west, by a ridge separating it from the basins of minor streams draining into the Rann of Kutch. Geologically the area comprises crystalline rocks of Archean age in the northern and north eastern parts while recent alluvial deposits cover the central and southern parts (Datta et al. 1980), and large part is occupied by thick quaternary continental deposits of fluvio-marine origin. Pre-cambrian hard rocks, semi-unconsolidated mesozoic formations, tertiary formation and unconsolidated alluvial deposits forms the aquifers in this area.
The meta-sediments of Aravalli and Delhi super group, granites and basic intrusive of post Delhi period, Himmatnagar sandstones and Deccan traps form hard rock aquifer with low yield. The sandy layers forming aquifer horizons are seen to be laterally continuous and vertically interspersed with thin semi-permeable clay/silt layers that may not have lateral continuity over a large area. The recharge area is in the foothills of Aravalli Mountains and discharge area is in the Gulf of Cambay. Groundwater occur both in unconfined and confined conditions, and there is no hydraulic continuity of the phreatic aquifers. Due to high gradient and undulating topography, the runoff is high, which limits the aquifer storage. Climate in the study area varies from arid to semi-arid condition with three distinct seasons namely: summer (March–May), monsoon (June–September) and winter (October–February). The southwest monsoon during mid-June to mid-September contributes nearly 91–94 % of annual rainfall. Rainfall decreases from ~90 cm per annum in the east to ~65 cm annum in the region of the Cambay basin, further decreasing to <60 cm annum on the west flank (Agarwal et al. 2006). The coefficient of variation of rainfall in major part of the basin is between 40 and 55 % (Datta et al. 1980).
Sampling and analytical procedure
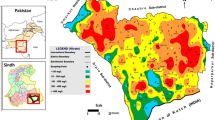
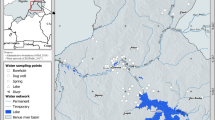
The study area was divided into grids of 5 km × 5 km, based on the topographic sheets and satellite image. The locations of the sampling sites were recorded using global positioning system (Garmin, GPSMAP60CS) shown in the Fig. 1. Representative groundwater samples from 56 locations (geographically equitably distributed) from the aquifers mentioned in the above districts adjacent to the Sabarmati River were collected in September 2008 end, representing post-monsoon period. Sixty-one samples from different locations were collected in May 2009, representing pre-monsoon period. The land use/land cover (LULC) map of the area (Fig. 1) was prepared using LISS image of IRS P6. An unsupervised classification approach based on ISODATA clustering algorithm using Erdas Imagine 9.3 was used for classification. It shows that most of the area is crop land interspersed with fallow land.
Inverse distance weighing (IDW) interpolation technique (Mueller et al. 2004; Singh et al. 2011) is employed to generate spatiotemporal variation of nitrate. IDW assigns weights by taking the inverse of the distance from an observation’s location to the location of the point being estimated (Burrough and McDonnell 1998). IDW interpolation was performed using ArcGIS 9.3.
Groundwater samples were collected from tube wells, bore wells and hand pumps. At each site water samples were collected in two separate clean polypropylene bottles (Tarsons; 250 and 125 mL), rinsed 2–3 times with groundwater to be sampled. One of the bottle (125 mL) containing samples was acidified using HNO3 to stabilize major cations and the other bottle (250 mL) containing un-acidified sample was used for anions analysis. The physico-chemical parameters were determined by following the standard protocol as given in American Public Health Association (APHA 2005). pH, electrical conductivity (EC) and total dissolved solids (TDS) were measured onsite using respective electrodes (Hanna). Subsequently, the samples were brought to laboratory in ice-containing Styrofoam boxes, vacuum filtered with 0.22μ Millipore filter paper, and stored at 4 °C for further analysis. Carbonate and bicarbonate were determined using titration method. Anions (F−, Cl−, SO4 2−, and NO3 −) were analysed using ion chromatography (ICS-90 Dionex) and major cations (Ca2+, Mg2+, Na+, K+) were analysed using atomic absorption spectrophotometer (Thermo Fisher, M series). Dissolved silica was analysed by molybdo-silicate method as given in American Public Health Association (APHA 2005).
Results and discussion
Table 1 gives the range, mean and standard deviation (SD) of different water quality parameters of both pre- and post-monsoon season. The groundwater of the study area is alkaline in nature. Na+, Mg2+ and Ca2+ contributed 99.18 % of total cation whereas Cl−, HCO3 − and SO4 2− contributed 83.78 % of total anions in pre-monsoon. In post-monsoon Cl−, HCO3 − and SO4 2− contributed 83.15 % of total anions where as Na+, Mg2+ and Ca2+ contributed 99.28 % of total cations. In both pre-monsoon and post-monsoon the abundance of major anions were in the order Cl− > HCO3 − > SO4 2− > SiO2 −, whereas dominant cations followed the order: Na+ > Ca2+ > Mg2+ > K+.
The statistical summary indicates that the nitrate concentration in groundwater in the pre-monsoon ranged from 1.6 to 630.7 mg/L with average concentration 50.1 mg/L, whereas in post-monsoon it ranged from 2.7 to 131.7 mg/L with average concentration 33.3 mg/L. Except for a few places, in the northern parts in pre-monsoon nitrate concentration was higher than the recommended limit of 45 mg/L for drinking water by the BIS (1991) and World Health Organization (2007).
Spatial variation of nitrate is shown in Fig. 2a, b. Very wide range and non-homogenous distribution of groundwater NO3 − concentration suggests contributions from both point and non-point sources.
Along with anthropogenic activities, potential sources of nitrate in the groundwater may be due to leaching of nitrogen present in manures, fertilizers, dry deposition. In the saturated zone of aquifer, concentration of nitrate at any location is governed by the nitrate content of the water recharging from the unsaturated zone above and by the additions from groundwater flowing from the surroundings. Vertical mixing in the saturated zone is likely to be slow and inputs of differing nitrate concentration along a flow-path may remain distinct, because the high-nitrate water masses migrate through aquifers as plume with little dilution relatively (Datta et al. 1997). After reaching the groundwater, nitrate migrates in the aquifer via advection and dispersion.
The LULC map indicates that the upper part of the study area is mostly occupied by crop land and nitrate concentration in groundwater in this region suggests the impact of agricultural activity. Lower part of the study area is occupied by both crop land and built-up (Fig. 1) which suggests the impact of both agricultural along with the anthropogenic inputs. Decreased nitrate content in the post-monsoon season seems to be due to dilution caused by infiltration of rain and/or irrigation water to aquifer. In pre-monsoon, evapotranspiration leading to accumulation of agrichemicals salts in groundwater and non-availability of sufficient recharge water could be some of the other reasons for higher nitrate content in the groundwater. In pre-monsoon, the lower most part of the study area which is near Gulf of Cambay shows high nitrate concentration although this region does not have much agricultural activity. Nitrate concentration in this region might have resulted also to some extent from seawater because of various algal and microbial activity of organism present in seawater (Rina et al. 2011).
Chloride and nitrate relationship in the study area
Generally, chloride is an inert tracer remaining conservative during passage through the unsaturated zone. The Cl−/NO3 − ratio in the study area varies from 1.14 to 179.91 with average value of 23.02 in pre-monsoon, whereas in post-monsoon it varies from 1.05 to 101.68 with average value of 16.93. Considering the average Cl−/NO3 − ratio (4.9) of major river water as reference point and comparing it with the values of Cl−/NO3 − for the aforesaid area it was found that 19.67 % samples in pre-monsoon and 26.78 % samples in post-monsoon shows Cl−/NO3 − ratio less than the reference (4.9) (Majumder et al. 2008). The samples showing Cl−/NO3 − ratio less than 4.9 indicates build up of nitrate in the respective groundwater samples. However, the samples showing higher Cl−/NO3 − ratio suggests removal of nitrate from the groundwater due to denitrification (Majumder et al. 2008).
HCO3 − vs NO3 − relationship in the study area
Chemically and biologically induced reaction favours the dentrification processes such as reduction of nitrate to N2 in the presence of organic carbon and denitrifying bacteria. Both heterotrophic and autotrophic bacteria are capable of denitrification, reduction of NO3 − to N2 via intermediates NO2 −, NO and N2O (Tiedje 1994). Reducing environment favours denitrifying bacteria for denitrification in the presence of labile organic carbon (Starr and Gillham 1993). Less NO3 in post-monsoon may be due to high HCO3 − concentration. High bicarbonate concentration and oxygen-scarce environment generate a strongly reducing environment within the shallow aquifers. In the presence of organic carbon, nitrate is reduced to nitrogen and it leads to high concentration of HCO3 − which can be expressed as
Thus higher concentration of HCO3 − shows a favourable environment of denitrification in the aquifer in post-monsoon season. Both in pre and post-monsoon with increase in reducing environment nitrate concentration decreases (Fig. 3a, b). Maximum nitrate concentration is up to 200 mg/L of HCO3 − concentration. Even at the same reducing environment, high nitrate level is present in agricultural region in the northern part of study area, which shows the impact of fertilizers and other agrichemicals. In southern part of the area, although, agricultural, urbanization and industrial activities exist, yet, higher reducing environment is possible due to contribution by dissolution of higher amount of CO2 released from industrial activity during monsoon rainfall, which has favoured denitrification process in this area. At sampling location 45, which has highest bicarbonate concentration nitrate concentration is low which shows the impact of reducing environment on denitrification.
In pre-monsoon also concentration of nitrate decreases with increase in reducing environment. At sampling locations 34 and 35 where nitrate contamination is very high, 630.73 and 301.23 mg/L, respectively, this may be due to point source contamination.
Nitrate reduction by organic carbon in the presence of microbes leading to high concentration of HCO3 − can be expressed as:
Four molecules of NO3 is reduced to two molecules of nitrogen and four molecules of HCO3 is produced, when organic carbon is the substrate. Thus higher concentration of HCO3 indicates that more denitrification has been taken place in the post-monsoon.
SO4 2− vs NO3 − relation in the area
In semi-arid environment, less organic matter is present in the aquifers. In the absence of organic carbon, denitrification is also takes place in the presence of chemolithotrophic denitrifiers which can use S or Fe in reduced minerals such as pyrite (FeS2) as an electron donor (Appelo and Postma 1994). Denitrification using pyrite takes place according to the following reaction:
From the scatter plot Fig. 4a, b it is clear that both in pre-monsoon and post-monsoon at higher sulphate concentration very less amount of nitrate is present which is indicative of denitrification in presence of pyrite.
However, if pyrite is the substrate for denitrification, 2.8 molecules of NO3 is lost for each molecule of electron donor, whereas only 0.8 molecule of NO3 is reduced when organic carbon is the donor.
SO4 2− vs Cl− relationship in the study area
In the present study, SO4 2−/Cl− ratio varied from 0.111 to 0.992 with average value of 0.319 in post-monsoon, whereas in pre-monsoon it varied from 0.077 to 0.609 with average value of 0.295. According to Galy and Frace-Lanord (1999), denitrification in presence of pyrite is favoured in presence of high SO4 2−/Cl− ratio. A high SO4 2−/Cl− ratio indicates a favourable condition of denitrification in post-monsoon; as a result, nitrate concentration is less in aquifers during post-monsoon.
A good correlation of Cl− and SO4 2− ions in the scatter plot (Fig. 5a, b) suggests common source of these ions. The possible source of Cl− and SO4 2− could be relict sulphide and sulphate-rich marine clay and silt deposits, secondary evaporite minerals dissolution as well as anthropogenic activities especially agricultural activities (Rajmohan and Elango 2006). Agricultural activity especially irrigation return flow, chemical fertilizers (urea, gypsum, ammonium sulphate, etc.) and farm manures may also be responsible for high SO4 and Cl concentration.
Mg vs NO3 relation in the study area
Magnesium is an important micronutrient which is essential for chlorophyll production and hence in photosynthesis. Magnesium nitrate is applied directly to the leaves (foliar feeding), or to the soil or in dip irrigation as fertilizer. Mg shows a good correlation with nitrate in pre-monsoon Fig. 6a, whereas in post-monsoon there is no correlation between Mg and NO3 as clear in scatter plot Fig. 6b. It may be due to vertical and lateral mixing of water, aquifers are not in balanced state in post-monsoon. There is no correlation between Mg and NO3. From the scatter plot different mixing lines can also be observed, whereas in pre-monsoon, due to absence of recharging water and high evaporation accumulation of salt takes place. Besides in agricultural activity MgNO3 is also used in the ceramics, printing, chemical and agricultural industries.
The movement of the ionic species through the soil is known to be concentration dependent and is limited by two opposite mechanisms: adsorption and dispersion. Hence, more than one solution of non-linear isotherm equations is expected (Datta et al. 1997). The non-linearity in the trend is due to combined effect of evaporation superimposed with mixing, leaching and desorption/adsorption (Datta et al. 1996b). Although, most of the irrigation water is commonly absorbed by plant roots, the remainder contains fertilizers and pesticides which may get concentrated during unsaturated zone evaporation. Because recharge includes irrigation water mixed with local rainfall, such effects are of paramount concern.
K and NO3 relationship in the study area
Nitrate and potassium mostly originate from agriculture and from industrial and domestic waste to some extent, so a relationship is expected between them. From the scatter plot (Fig. 7a) it is clear that no significant correlation exists between potassium and nitrate in pre-monsoon. After removing the point source, different mixing line is clearly visible in Fig. 7b. The scatter plot between K and NO3 (Fig. 7b, c) for both pre- and post-monsoon, depicts two positive type of correlations: one is only at very few sampling locations nitrate is correlated with potassium (NO3 = K), which indicates that such waters have a common source of both nitrate and potassium, whereas at most of locations high nitrate are generally associated with low potassium content (NO3 = 10K and 25K), suggest no common source of both nitrate and potassium content. This indicates that in addition to agricultural activity various others activities such as diary, agrichemical and food processing industry, present in the area, might be contributing to high nitrate levels in the groundwater in the area. Besides this, due to the very different chemistry of nitrate and potassium, even though the wastes and fertilizers are deposited or applied simultaneously on the land surface, there may be a considerable time lag between their rate of movement in the unsaturated zone and subsequent arrival at the saturated zone of the groundwater aquifer; this could results in anomalous (low/high) concentration levels (Datta et al. 1997).
Conclusion
The study indicates a significant influence of increasing intensive irrigated agriculture and other human land use activities on nitrate levels and its spatial and temporal variations in groundwater. Nitrate level was observed to be above the permissible limits set by WHO at many of the locations. The results suggest both point and non-point sources of nitrate contamination. The higher levels of nitrate in pre-monsoon was due to non-availability of sufficient recharge and high evaporation induced accumulation of agrichemicals salts in groundwater. Denitrification seems to be major process controlling the groundwater nitrate concentration. Relatively higher Cl−/NO3 − ratio and HCO3 − favour removal of nitrate from the aquifers. High SO4 2−/Cl− ratio in the post-monsoon period also contributed to denitrification processes in the area. The scatter plot of K vs NO3 suggests a common source of these ions only at very few sampling locations, when the concentration is <5 mg/L. Relict sulphide and sulphate-rich marine clay and silt deposits, secondary evaporite mineral dissolution as well as anthropogenic activities especially agricultural activities such as irrigation return flow, chemical fertilizers and farmyard manures govern the nitrate levels in the groundwater. Since, in future use of such groundwater for irrigation is likely to increase in this water-scarce region, to protect the groundwater from vulnerability to nitrate, it is desirable to monitor nitrate levels in relation to depth of water table and its seasonal variation, for better understanding of the pollutant sources.
References
Addiscott TM, Whitmore AP, Powlsen DS (1991) Farming. Fertilizers and the nitrate problem. CAB International, Wallingford, p 170
Agarwal M, Gupta SK, Deshpande RD, Yadava MG (2006) Helium, radon and radiocarbon studies on a regional aquifer system of the North Gujarat—Cambay region, India. Chem Geol 228:209–232. doi:10.1016/j.chemgeo.2005.10.007
American Public Health Association (APHA) (2005) Standard methods for the examination of water and waste water, 21st edn. American Public Health Association, Washington DC
Appelo CAJ, Postma D (1994) Geochemistry. Groundwater and pollution. Balkema, Rotterdam, p 536
Bureau of Indian Standards (BIS) (1991) Drinking water specification. IS 10500:1991
Burrough PA, McDonnell RA (1998) Principles of geographical information systems for land resources assessment. Oxford University Press, New York
Datta PS, Desai DI, Gupta SK (1980) Hydrological investigations in Sabarmati basin. I. Groundwater recharge estimation using tritium tagging method. Proc of the Indian Nat Sci Acad Part A 46(1):84–98
Datta PS, Deb DL, Tyagi SK (1996) Stable isotope (180) investigations on the processes controlling fluoride contamination of groundwater. J Contam Hydrol 24(1):85–96. doi:10.1016/0169-7722(96)00004-6
Datta PS, Deb DL, Tyagi SK (1997) Assessment of groundwater contamination from fertilizers in the Delhi area based on 180, NO3 and K+ composition. J Contam Hydrol 27:249–262. doi:10.1016/S0169-7722(96)00099-X
Fertilizer association of India (2009) http://www.indiastats.com
Galy A, Frace-Lanord C (1999) Weathering processes in the Ganges–Brahmaputra basin and the riverine alkalinity budget. Chem Geol 159:31–60
Hamed Y, Awad S, Saad AB (2013) Nitrate contamination in groundwater in the Sidi Aïch–Gafsa oases region, Southern Tunisia. Environ Earth Sci. doi:10.1007/s12665-013-2445-5
Jacks G, Sharma VP (1983) Nitrogen circulation and nitrate in groundwater in an agricultural catchment in southern India. Environ Geol 5:61–64
Johnsson HM, Larsson KM, Hoffmann M (2002) SOILNDB: a decision support tool for assessing nitrogen leaching losses from arable land. Environ Model Softw 17(6):505–517
Lasserre F, Razack M, Banton OA (1999) GIS-linked model for the assessment of nitrate contamination in groundwater. J Hydrol 224:81–90
Majumder RK, Hasnat MA, Hossain S, Ikeue K, Machida M (2008) An exploration of nitrate concentration in groundwater aquifers of central-west region of Bangladesh. J Hazard Mater 159:536–543. doi:10.1016/j.jhazmat.2008.02.110
Mueller TG, Pusuluri NB, Mathias KK, Cornelius PL, Barnhisel RI, Shearer SA (2004) Map quality for ordinary kriging and inverse distance weighted interpolation. Soil Sci Soc Am 68:2042–2047
Nolan BT, Ruddy BC, Hitt KJ, Helsel DR (1997) Risk of nitrate in groundwaters of the United States—a national perspective. Environ Sci Technol 31:2229–2236. doi:10.1021/es060911u
Rajmohan N, Elango L (2005) Nutrient chemistry of groundwater in an intensively irrigated region of southern India. Environ Geol 47:820–830. doi:10.1007/s00254-004-1212-z
Rajmohan N, Elango L (2006) Hydrogeochemistry and its relation to groundwater level fluctuation in the Palar and Cheyyar River basins, southern India. Hydrol Process 20:2415–2427. doi:10.1002/hyp.6052
Rao SM, Sekhar M, Rao PR (2013) Impact of pit-toilet leachate on groundwater chemistry and role of vadose zone in removal of nitrate and E. coli pollutants in Kolar District, Karnataka. India. Environ Earth Sci 68:927–938. doi:10.1007/s12665-012-1794-9
Refsgaard JC, Thorsen M, Jensen JB, Kleeschulte S, Hansen S (1999) Large scale modeling of groundwater contamination from nitrate leaching. J Hydrol 221:117–140
Rina K, Datta PS, Singh CK, Mukherjee S (2011) Characterization and evaluation of processes governing the groundwater quality in parts of the Sabarmati basin, Gujarat using hydrochemistry integrated with GIS. Hydrol Process 26:1538–1551. doi:10.1002/hyp.8284
Singh CK, Shashtri S, Mukherjee S (2011) Integrating multivariate statistical analysis with GIS for geochemical assessment of groundwater quality in Shiwaliks of Punjab, India. Environ Earth Sci 62:1387–1405. doi:10.1007/s12665-010-0625-0
Starr RC, Gillham RW (1993) Denitrification and organic carbon availability in two aquifers. Ground Water 31(6):934–948
Tiedje JM (1994) Denitrifiers. In: Weaver RW et al (eds) Methods of soil analysis: Part 2. Microbiological and biochemical properties. Soil Sci Soc of America, Madison, WI, pp 245–267
US Environmental Protection Agency (2000) Drinking water standards and health advisories. US Environmental Protection Agency, Office of Water, 822-B-00-001, p 12
WHO (1984) Guidelines for drinking water quality, vol vol 1. World Health Organisation, Geneva, pp 53–73
WHO (World Health Organization) (2007) Chemical safety of drinking water: Assessing priorities for risk management, WHO, Geneva, pp 160
Widory D, Kloppmann W, Chery L, Bonnin J, Rochdi H, Guinamant JL (2004) Nitrate in groundwater: an isotopic multi-tracer approach. J Contam Hydrol 72:165–188
Wylie BK, Shaffer MJ, Hall MD (1995) Regional assessment of NLEAP NO3-N leaching indices. Water Resour Bull 31(3):399–408
Acknowledgments
The author (Kumari Rina) thanks the University Grants Commission for providing the fellowship to carry out this research. The author also thanks Jawaharlal Nehru University for providing various instrument facilities to carry out research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rina, K., Datta, P.S., Singh, C.K. et al. Determining the genetic origin of nitrate contamination in aquifers of Northern Gujarat, India. Environ Earth Sci 71, 1711–1719 (2014). https://doi.org/10.1007/s12665-013-2575-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-013-2575-9