Abstract
The integrated use of isotopic and hydrochemical tracers is an effective approach for investigating complex hydrological processes of groundwater. The stable isotope composition and hydrochemistry of the groundwater around Qinghai Lake were investigated to study the sources and recharge areas. Most of the groundwater points lie close to the local meteoric water line, indicating that the ground waters were recharged primarily from precipitation in the basin, though it had undergone varying degrees of evaporation. The hydrochemical analysis showed that the groundwater was mainly freshwater and that the hydrochemical type was Ca–Mg–HCO3; the results of the boomerang envelope model and solutes calculated indicated that the groundwater chemistry was mainly controlled by carbonate dissolution around Qinghai Lake. The recharge altitudes of groundwater were relatively low (at 3,400 m.a.s.l) on the northern shore of Qinghai Lake (locations G1 and G5), relatively high (above 3,900 m.a.s.l) on the southern shore (locations G3 and G4), and approximately 3,700 m.a.s.l on the western shore (location G2). Furthermore, groundwater samples from the fault zone (e.g., G3) would be recharged in part from fissure or inter-basin water. High salinity of groundwater on the western shore (location G2) was related with the evaporite dissolution, the groundwater is unsuitable for drinking, and the drinking water should be improved and enhanced in this area. Knowledge of our research can promote effective management of water resources in this cold and semiarid region and add new data to global groundwater database.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater, as a component of total water resources, is important for irrigation, industry, mining and domestic use. Especially in arid and semiarid regions with shortages of surface water, groundwater plays a dominant role in shaping the economy and ecological environment because of its stable quality and quantity. Therefore, comprehensive studies of the recharge source, refresh rate, residence time and flow path of groundwater are essential and contribute to the planning strategies and recommendations for the rational use of groundwater resources (Adams et al. 2001; Edmunds et al. 2006; Chang and Wang 2010). The integrated use of isotopic and hydrochemical tracers is an effective approach for investigating the complex hydrological processes of groundwater over a range of spatial and temporal scales (Clark and Frjtz 1997; Alyamani 2001; Gibson et al. 2005; Song et al. 2006; Carol et al. 2009; Wassenaar et al. 2011; Kanduč et al. 2012). For example, Weyhenmeyer et al. (2002) applied the altitude effect of the isotope composition in rainfall under stable climate conditions to locate the most important recharge areas and groundwater flow paths and to evaluate the groundwater recharge rates from different altitudes. Based on hydrochemical and isotope data, Carol et al. (2009) studied the salinization process and the hydrological dynamics of groundwater in the southern part of Samborombón Bay. Wassenaar et al. (2011) used stable isotopes to evaluate the sources of water in shallow and deep groundwater systems in the southern Okanagan. Kanduč et al. (2012) used hydrochemical and isotopic tracers to characterize the recharge area and flow paths of some of the most important Alpine aquifers in northern Slovenia. All of these studies demonstrate the applicability and value of isotopes and ions as conservative tracers for hydrologic research, particularly for groundwater systems with complex hydrological processes in arid regions.
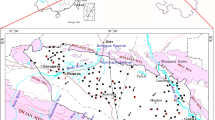
The Qinghai Lake Basin, a closed basin with an area of 29,661 km2, lies in the cold and semiarid region of the NE Qinghai-Tibet Plateau, China (Fig. 1). Qinghai Lake is the largest inland lake in China, with surface area 4,400 km2 within the basin. This lake is one of China’s national nature reserves and an important water body for the ecological security of the NE Qinghai-Tibet Plateau (Tang et al. 1992). The region around the lake is a center of ecological tourism and animal husbandry for the Qinghai Province (Tang et al. 1992). However, Qinghai Lake is saline, and the water is not suitable for consumption by humans or animals; thus, the groundwater around the lake is an important freshwater source for irrigation and drinking. Therefore, studies of the recharge areas, ion concentrations and flow path of the groundwater around the lake are crucial. Such research contributes to knowledge of groundwater quality and to planning water strategies in the basin. Previously published studies on the groundwater in the Qinghai Lake Basin mainly focused on investigating the hydrochemical composition (Jin et al. 2010; Xu et al. 2010; Xiao et al. 2012a), but very few studies have used chemical and isotopic information to investigate recharge areas of the groundwater around the lake.
The objectives of this study were: (1) to investigate the characteristics of stable isotopes and hydrochemistry of the groundwater around Qinghai Lake and (2) to study the recharge areas of the groundwater for knowledge of groundwater quality and planning water strategies in the basin. Meanwhile, it is expected that the results will provide insight into the hydrological and geochemical processes of cold and alpine groundwaters in addition to water resource management in the basin and the northeastern Qinghai-Tibet Plateau.
Background of Qinghai Lake
Qinghai Lake (36°32′–37°15′N, 99°36′–100°47′E), is the largest inland lake in China with a surface area 4,400 km2 that lies at an altitude of 3,194 m above sea level. It lies in the cold and semiarid region of China’s NE Qinghai-Tibet Plateau (Fig. 1). Qinghai Lake has a water volume of 71.6 × 109 m3 and has a watershed area of approximately 29,661 km2. The lake water has a salinity of 15.5 g/L and pH of 9.06. Its water chemistry is characterized by Na+ > Mg2+ > K+ > Ca2+ and Cl− > SO4 2− ≈ CO3 2− > NO3 − (Sun et al. 1991). Around the lake, the average annual air temperature is ~1.2 °C. The average annual precipitation is 357 mm, but evaporation is 3 to 4 times higher than precipitation (Li et al. 2007).
There are more than 50 rivers or streams flowing into Qinghai Lake (LZBCAS 1994). The main rivers are the Buha River, Shaliu River, Haergai River, Quanji River and Heima River, which together produce a total discharge of 1.34 × 109 m3/year. The discharge from these rivers accounts for over 80.3 % of the total volume of the inflowing surface water. Almost all of the rivers are seasonal rivers; 85 % of the total discharge occurs between June and September. The average total dissolved solids (TDS) of the river water is 300.66 mg/L (Xu et al. 2010), which is higher than the global average (115 mg/L; Hu et al. 1982). The rivers are mainly located north and northwest of the lake; thus, their distribution is asymmetric in the Qinghai Lake Basin (Fig. 1; Yan and Jia 2003). In recent decades, 50 % of the rivers flowing into Qinghai Lake have dried up due to climate change and human activity (LZBCAS 1994; Li et al. 2007). The groundwater table around Qinghai Lake usually varies between 4 and 7 m. In the sparsely populated region, the groundwater table is approximately 3 or 4 m, which is shallower than the table in the densely populated region (Xiao et al. 2012a).
The Qinghai Lake Basin, situated on the southern edge of the Qilian Fold Belt, is a closed piggyback basin surrounded by several mountain ranges (Bian et al. 2000). These mountains, generally with elevations above 4,000 m (Fig. 1), are predominantly overlain by late Paleozoic marine limestone and sandstones, Silurian sandstone and schist, Triassic granite, Mesozoic diorite and granodiorite, and Quaternary alluvial and lacustrine sediments, with a minor contribution of late Cambrian phyllite and gneiss (Fig. 2). The distribution of these rocks is associated closely with NWW, NNW, and N–S trending faults (Bian et al. 2000). Fault escarpments and lacustrine sediment terraces have been extensively developed along the southern shore (Fig. 2). The groundwater aquifers in the regions of late Paleozoic marine limestone and sandstones, Silurian sandstone and schist, Triassic granite, and Quaternary are primarily carbonate aquifer, clastic rocks aquifer, intrusive rock aquifer and alluvial aquifer, respectively. The north-western part (Buha River Catchment) of the basin is predominantly covered by late Paleozoic marine limestone, sandstone, Silurian sandstone and schist (Figs. 1, 2). Triassic granites are spread over the northern part of the basin near the shores of Qinghai Lake. Mesozoic diorite and granodiorite are spread over the southern part of the basin. The areas surrounding the lake and rivers are overlain by alluvial and lacustrine sediments whose age ranges from Quaternary to recent times (Fig. 2). The mountain runoff in the diluvial and alluvial plains recharges the groundwater via infiltration. Groundwater discharge largely occurs in the lacustrine plain surrounding the lake (Xiao et al. 2012a).
Sampling and analytical methods
Precipitation samples were collected around the lake monthly from July 2009 to June 2010 at 14 locations that were evenly distributed throughout the basin, and groundwater samples were collected at 5 locations (Fig. 1). Information about location was acquired by global positioning system (GPS). A total of 135 samples, 75 of precipitation and 60 of groundwater (see online supplemental material), were collected during the observation period. The precipitation samples were measured and stored in 100 ml high-density polyethylene square bottles for isotopic analyses. The groundwater samples were stored in 500 ml bottles for isotopic and chemical analyses. The pH and EC of the groundwater were measured in situ using a handheld meter with a probe.
The isotopic compositions of each sample was determined, and the major hydrochemical ions in the groundwater samples collected in July 2009 were determined and are listed in Table 1. The stable isotopes were analyzed using the Picarro L1102-i water isotope analyzer in the Stable Isotope Laboratory of the Institute of Geology and Geophysics at the Chinese Academy of Sciences. The isotopic values were reported using the standard δ notion relative to the V-SMOW (Vienna Standard Mean Ocean Water) standard; the precisions were ±0.5 and ±0.1 ‰ for δ2H and δ18O, respectively. The major cations of the samples were determined using a Dionex-600 ion chromatograph, and the anions were measured using a Dionex-500 ion chromatograph in the Analytical Laboratory of the Beijing Research Institute of Uranium Geology.
The recharge areas and flow paths of groundwater were ascertained using the altitude effect of the oxygen isotope in precipitation (Payne et al. 1978; Bortolami et al. 1979; Weyhenmeyer et al. 2002; Song et al. 2006). The equation is described below:
where H is the altitude of the recharge area of the groundwater (m.a.s.l); h the altitude of the sampling site of the groundwater (m.a.s.l), δ G and δ P the δ18O values of the groundwater and the local precipitation at the sampling site (‰), respectively, and K is the gradient of the altitude effect of δ18O in precipitation (‰/m).
Results and discussion
Stable isotope in precipitation
According to the precipitation samples collected around the lake monthly from July 2009 to June 2010, the δ18O and δ2H values of precipitation range from −16.69 to −2.80 ‰ and from −123.28 to −11.54 ‰, respectively. The ranges were within that previously reported for China: δ18O and δ2H values ranging from −35.5 to +2.5 ‰ and from −280.0 to +24.0 ‰, respectively (Tian et al. 2001). The local meteoric water line (LMWL) was simulated by the δ18O and δ2H content of precipitation in the Qinghai Lake Basin (Fig. 3):
The LMWL deviated slightly from the Global Meteoric Water Line (GMWL) because of the local variation in moisture sources and evaporation modes (Craig 1961; Clark and Frjtz 1997).
In successive precipitation samples, the altitude effect was a temperature-dependent phenomenon and explained by the rainout from adiabatic cooling (Coplen 1993). The altitude effect of δ18O was significant in the annual precipitation, and the best-fit equation was:
The equation indicates that the δ18O was depleted at a rate of −0.18 ‰/100 m elevation (Fig. 4). The calculated value of the altitude effect for δ18O was similar to that of the Tianshan Mountains, which are high-altitude (Pang et al. 2011), and was relatively smaller than the effect calculated in other regions (Niewodniczanski et al. 1981; Stowhas and Moyano 1993; Saravana et al. 2010).
Stable isotopes of the groundwater
The δ18O and δ2H values of the groundwater range from −10.20 to −6.45 ‰ and from −66.79 to −39.61 ‰, respectively. The ranges were smaller than the ranges of precipitation (Fig. 3). Comparing the groundwater samples with the LMWL was useful for determining the water source in the investigation of the regional hydrology (Clark and Frjtz 1997). The relationship between groundwater samples and the LMWL is displayed by a bivariate plot of δ18O versus δ2H in Fig. 5. Most of the isotope data points lie close to the LMWL, and the slope of local evaporation line (LEL: δ2H = 6.63 δ18O + 2.08) of groundwater samples (6.63) was smaller than the slope of LMWL (7.91), indicating that the groundwater around Qinghai Lake mainly come from the precipitation in the basin that had undergone variable degrees of evaporation before infiltration (Friedman et al. 1962; Paul and Wanielista 2000).
As in Fig. 6, the δ18O of the lake water was much higher than the δ18O of the groundwater, and range from 0.77 to 2.02 ‰ with an average of 1.35 ‰, indicating that the lake water underwent high degree of evaporation. Meanwhile, the δ18O of the groundwater was in the order: δG1 > δG5 > δG2 > δG3 > δG4, with average δ18O values of −6.59, −7.28, −8.62, −8.95 and −8.99 ‰, respectively. The δ18O of the groundwater, excluding G3, fluctuated only slightly within the year (Fig. 6), indicating that the sources of groundwater were stable and weakly influenced by the external environment. The fluctuation of the δ18O in location G3, ranging from −10.20 ‰ in October to −8.25 ‰ in May, was larger than that of the other locations, indicating that the groundwater sources in location G3 were relatively complex. According to the map of geological structure around the lake (Fig. 2), G3 and G4 are located on the fault zone of Zongwulongshan–Qinghai Nanshan; therefore, the groundwater in location G3 and G4 would be recharged by the fissure water. Overall, the groundwater around the lake was mainly recharged by precipitation, and the δ18O values of the different locations suggest that the altitude of the recharge areas varied.
Hydrochemistry of the groundwater
The pH values of the water samples range from 7.05 to 7.61 with a median value of 7.35 (Table 1), indicating that the groundwaters were slightly alkaline. These pH values were relatively lower than those of the river waters (7.60–8.55) in the Qinghai Lake Basin (Jin et al. 2010). The TDS and electrical conductivity (EC) values of most of the groundwater samples (G1, G3, G4 and G5) range from 438.11 to 621.27 mg/L and from 0.219 to 0.461 mS/cm (Table 1), respectively, with averages of 544.06 mg/L and 0.356 mS/cm, respectively. Compared with the river water (the average TDS and EC were 377.78 mg/L and 0.20 mS/cm, respectively) (Cui 2011), the TDS and EC of the groundwater were higher, indicating that the interaction between water and rocks was stronger in the groundwater than in the river water. The hydrochemical type of groundwater, excluding G2, was Ca–Mg–HCO3 (Fig. 7). The concentrations of Ca2+ and Mg2+ were relatively high, accounting for more than 60 % of the cations. This finding indicates that the groundwater chemistry may be mainly controlled by carbonate dissolution around Qinghai Lake.
The TDS and EC values of groundwater at G2 were 2,264.33 mg/L and 2.4 mS/cm, respectively, the hydrochemical type was Na–Cl-HCO3 (Fig. 7), and the groundwater was brackish (TDS > 1,000 mg/L; Table 1). There could be two reasons for the high TDS and high concentrations of Na+, Mg2+, Cl− and SO4 2− in G2. One possibility is that the longer flow path increased the interaction time between water and rocks and raised the dissolved solids; the second possibility is that the groundwater in location G2 was recharged by the lake water. G2 is near (3.6 km) Qinghai Lake and at a similar altitude, 3,196 m.a.s.l in July 2009 compared to the lake’s 3,193.38 m.a.s.l. Compared with the lake water, the δ18O of G2 was much lower than the δ18O of lake water and range from 0.77 to 2.02 ‰ with an average of 1.35 ‰ (Fig. 6), indicating there was no significant hydraulic contact between G2 and the lake. High TDS and high concentrations may be dominated by heavy evaporation or dissolved solids.
The boomerang envelope model developed by Gibbs (1970) describes three mechanisms that control the composition of the dissolved salts of the world’s waters: atmospheric precipitation, rock dominance, and the evaporation–crystallization. As shown in Fig. 8, all of the samples fell within the evolutionary path from “Rock dominance” to “Ocean” in the Gibbs boomerang envelope, suggesting that the chemical composition of the groundwater was dominated by rock-weathering. The results were supported by that rock-weathering, ion exchange and precipitation which are the major geochemical processes responsible for the solutes in the groundwater within the Qinghai Lake Basin (Xiao et al. 2012a).
Using the forward method, different sources of the groundwater solutes (atmospheric input, carbonate weathering, silicate weathering, and evaporite dissolution) were calculated (Xiao et al. 2012b) and are shown in Fig. 9. The results show that carbonate weathering, evaporite dissolution, silicate weathering, and atmospheric input contributed 41.8, 24.5, 22.4, and 11.3 % of the total dissolved cations for the groundwaters. Carbonate weathering dominated in G1, G3, G4 and G5, contributing 37.5, 38.7, 55 and 39 % of the total dissolved cations, respectively. This finding indicates that the groundwater chemistry is mainly controlled by carbonate dissolution around Qinghai Lake. Evaporite dissolution dominated in G2, contributing 48 % of the total dissolved cations. Therefore, the high salinity of G2 was related to the evaporite dissolution in this area.
In drinking water, high SO4 2−concentrations can cause diarrhea, dehydration and weight loss (World Health Organization 2008), and high NO3 − concentrations can result in birth defects, hypertension and high-Fe hemoglobin (Carpenter et al. 1998). According to the Chinese State Standard of drinking water, the highest acceptable limits of TDS, total hardness (TH), pH and the concentrations of Na+, Cl−, NO3 − and SO4 2− are 1,000, 450, 8.5, 200, 250, 20 and 250 mg/L, respectively (Ministry of Health 2006). As shown in Table 1, most of the groundwaters (G1, G3, G4 and G5) were slightly and moderately hard freshwater (TH ≤ 210.75 mg/L) and fell within the standards for drinking water in all indices. The TDS, Na+, Cl−, NO3 − and SO4 2− of the groundwater from location G2 were all higher than the highest acceptable limits set by the standards, indicating that the groundwater in location G2 was not suitable for drinking and that the drinking water should be improved and enhanced in these areas.
Recharge sources of groundwater
Most of the isotope data points plot close to the LMWL (Fig. 5), indicating that the groundwater around Qinghai Lake came primarily from the precipitation in the basin and had undergone variable degrees of evaporation (Friedman et al. 1962; Paul and Wanielista 2000). Previous researchers have successfully used the altitude effect of oxygen isotope to ascertain the main recharge areas and flow paths of groundwater (Payne et al. 1978; Bortolami et al. 1979; Weyhenmeyer et al. 2002; Song et al. 2006). A clear altitude effect of −0.18 ‰/100 m (Eq. 3, Fig. 4) was revealed in the oxygen isotope of precipitation in the Qinghai Lake Basin. The relationship plot of δ2H–δ18O for groundwater (LEL) in each location is listed in Table 2. The slopes of LELs, ranging from 3.0 to 7.32, were smaller than the slope of LMWL (7.91), indicating that all groundwater samples had undergone evaporation before infiltration (Weyhenmeyer et al. 2002). To eliminate the influence of evaporation on the groundwater, the intersections between the LELs of groundwater in each location and the LMWL of precipitation were calculated (Table 2). The values of δ2H and δ18O on the intersections were the recharge (initial) isotope values from precipitation to the groundwater. Then, the recharge altitude for each groundwater site was calculated from the altitude effect of precipitation and Eq. (1) (Table 2). The recharge altitudes of groundwater in locations G1 and G5 were relatively low (at 3,400 m.a.s.l), and the recharge altitudes in locations G3 and G4 were relatively high (more than 3,900 m.a.s.l). The recharge altitude for location G2 was approximately 3,700 m.a.s.l.
According to the contour lines in the Qinghai Lake Basin (Fig. 1), the altitude is approximately 3,400 m.a.s.l just to the north of locations G1 and G5, indicating that local precipitation was the source of G1 and G5. Because G2 lies in the Buha River Catchment, the source for G2 would be precipitation from the middle reaches (3,500–4,300 m.a.s.l) of the catchment (Fig. 1). The recharge area of the groundwater in location G4 should be above 3,900 m.a.s.l on the mountain Qinghai Nanshan. That site is in the south of the Qinghai Lake Basin and its flow path is relatively short, as indicated by the lower TDS and lower EC (Table 1). The fluctuation of the δ18O in G3 (Fig. 6) would indicate that the recharge altitudes range from 3,500 m.a.s.l (May) to 4,300 m.a.s.l (October), but there is very little area exceeding 4,000 m.a.s.l above the G3 site (Fig. 1). Therefore, the groundwater source in location G3 must be more complex, with groundwater recharged by fissure water or inter-basin water.
Conclusions
The chemical composition of the groundwater around Qinghai Lake was dominated by rock-weathering (mainly carbonate dissolution). Almost all groundwaters were Ca–Mg–HCO3 type; few were Na–Cl–HCO3 type on the west shore and were not suitable for drinking. The groundwater came primarily from precipitation in the basin. The recharge altitudes of groundwater were relatively low (at 3,400 m.a.s.l) on the northern shore of Qinghai Lake (locations G1 and G5), relatively high (above 3,900 m.a.s.l) on the southern shore (locations G3 and G4), and approximately 3,700 m.a.s.l on the western shore (location G2).
The groundwater source for location G3 was complex, possibly including fissure water or inter-basin water. The high salinity of G2 was related with the evaporite dissolution. Overall, the results indicated that the sources of groundwater around Qinghai Lake are very complex. Future studies should extend the period of observation and use multiple methods, e.g., tritium (3H) and radiocarbon (14C), to determine the groundwater residence times and to assess additional groundwater recharge sources along selected groundwater flow paths.
References
Adams S, Titus R, Pietersen K, Tredoux G, Harris C (2001) Hydrochemical characteristics of aquifers near Sutherland in the Western Karoo, South Africa. J Hydrol 24:191–203
Alyamani MS (2001) Isotopic composition of rainfall and ground-water recharge in the western province of Saudi Arabia. J Arid Environ 49:751–760
Bian QT, Liu JQ, Luo XQ, Xiao JL (2000) Geotectonic setting, formation and evolution of the Qinghai Lake. Seismol Geol 22(1):20–26 (in Chinese)
Bortolami GC, Ricci B, Susella GF (1979) Isotope hydrology of the Val Corsaglia, Maritime Alps, Piedmont, Italy. In: Isotope Hydrology, Neuherberg, Germany, pp 327–350
Carol E, Kruse E, Mas-Pla J (2009) Hydrochemical and isotopical evidence of ground water salinization processes on the coastal plain of Samborombón Bay, Argentina. J Hydrol 365:335–345
Carpenter SR, Caraco NF, Correll DL, Howarth RW, Sharpley AN, Smith VH (1998) Non-point pollution of surface waters with phosphorus and nitrogen. Ecol Appl 8:559–568
Chang J, Wang GX (2010) Major ions chemistry of groundwater in the arid region of Zhangye Basin, northwestern China. Environ Earth Sci 61:539–547
Clark ID, Frjtz P (1997) Environmental isotopes in hydrogeology. Lewis Publishers, New York
Coplen TB (1993) Uses of environmental isotopes. In: Alley WM (ed) Regional groundwater quality. Van Nostrand Reinhold, New York, pp 227–254
Craig H (1961) Isotopic variation in meteoric waters. Science 133:1702–1703
Cui BL (2011) Water cycle and transformation relations among precipitation, river water, groundwater and lake water in the Qinghai Lake Basin based on the stable isotope. PhD Dissertation, Beijing Normal University (in Chinese)
Edmunds WM, Ma JZ, Aeschbach-Hertig W, Kipferd R, Darbyshire DPF (2006) Groundwater recharge history and hydrogeochemical evolution in the Minqin Basin, North West China. Appl Geochem 21:2148–2170
Friedman I, Machta L, Soller R (1962) Water vapour exchange between a water droplet and its environment. J Geophys Res 67:2761–2766
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170:1088–1090
Gibson J, Edwards T, Birks S, Amour N, Buhay W, McEachern P, Wolfe B, Peters D (2005) Progress in isotope tracer hydrology in Canada. Hydrol Process 19:303–327
Hu MH, Stallard RF, Edmond JM (1982) Major ion chemistry of some large Chinese rivers. Nature 298:550–553
Jin ZD, Wang SM, Zhang F, Shi YW (2010) Weathering, Sr fluxes, and controls on water chemistry in the Lake Qinghai catchment, NE Tibetan Plateau. Earth Surf Proc Land 35:1057–1070
Kanduč T, Mori N, Kocman D, Stibilj V, Grassa F (2012) Hydrogeochemistry of Alpine springs from North Slovenia: insights from stable isotopes. Chem Geol 300–301:40–54
Li XY, Xu HY, Sun YL, Zhang DS, Yang ZP (2007) Lake-level change and water balance analysis at Lake Qinghai, west China during recent decades. Water Resour Manage 21:1505–1516
LZBCAS (Lanzhou Branch of Chinese Academy of Sciences) (1994) Evolution of recent environment in Qinghai Lake and its prediction. Science Press, Beijing (in Chinese)
Ministry of Health (2006) Standards for drinking water quality, GB5749-2006. Ministry of Health of the People’s Republic of China, Beijing (in Chinese)
Niewodniczanski J, Grabczak J, Baranski L, Rzepka J (1981) The altitude effect on the isotopic composition of snow in high mountains. J Glaciol 27:99–111
Pang Z, Kong Y, Froehlich K, Huang T, Yuan L, Li Z, Wang F (2011) Processes affecting isotopes in precipitation of an arid region. Tellus B 63(3):352–359
Paul G, Wanielista M (2000) Effects of evaporative enrichment on the stable isotope hydrology of a central Florida (USA) river. Hydrol Process 14:1465–1484
Payne BR, Leontiadis J, Dimitroulas C (1978) A study of the Kalamos springs in Greece with environmental isotopes. Water Resour Res 14(4):653–658
Saravana KU, Bhishm K, Rai SP, Sharma S (2010) Stable isotope ratios in precipitation and their relationship with meteorological conditions in the Kumaon Himalayas, India. J Hydrol 391:1–8
Song X, Liu X, Xia J, Yu J (2006) A study of interaction between surface water and ground-water using environmental isotope in Huaisha River basin. Sci China 49(12):1299–1310
Stowhas L, Moyano JC (1993) Simulation of the isotopic content of precipitation. Atmos Environ 27(3):327–333
Sun DP, Tang Y, Xu ZQ, Han Z (1991) A preliminary investigation on chemical evolution of the Lake Qinghai water. Chin Sci Bull 15:1172–1174 (in Chinese)
Tang RC, Gao XQ, Zhang J (1992) The annual changes of the water level of the Lake Qinghai in the recent thirty years. Chin Sci Bull 37(6):524 (in Chinese)
Tian LD, Yao TD, Sun WZ, Stievenard M, Jouzel J (2001) Relationship between δD and δ18O in precipitation from north to south of the Tibetan Plateau and moisture cycling. Sci China 44(9):789–796
Wassenaar LI, Athanasopoulos P, Hendry MJ (2011) Isotope hydrology of precipitation, surface and ground waters in the Okanagan Valley, British Columbia, Canada. J Hydrol 411:37–48
Weyhenmeyer CE, Burns SJ, Waber HN (2002) Isotope study of moisture sources, recharge areas, and groundwater flow paths within the eastern Batinah coastal plain, Sultanate of Oman. Water Resour Res 38(10):1184–1206
World Health Organization (2008) Guidelines for drinking-water quality, incorporating 1st and 2nd addenda, vol. 1, Recommendations, Geneva
Xiao J, Jin ZD, Zhang F, Wang J (2012a) Solute geochemistry and its sources of the groundwater’s in the Qinghai Lake Catchment, NW China. J Asian Earth Sci 52:21–30
Xiao J, Jin ZD, Ding H, Wang J, Zhang F (2012b) Geochemistry and solute sources of surface waters of the Tarim River Basin in the extreme arid region, NW Tibetan Plateau. J Asian Earth Sci 54–55:162–173
Xu H, Hou Z, An Z, Liu X, Dong J (2010) Major ion chemistry of waters in Lake Qinghai catchments, NE Qinghai-Tibet Plateau, China. Quatern Int 212:35–43
Yan HY, Jia SF (2003) Water balance and water resources allocation of Qinghai Lake. J Lake Sci 15(1):35–40 (in Chinese)
Acknowledgments
The authors would like to thank anonymous reviewers for their valuable and constructive comments. This study was supported by the National Science Foundation of China (NSFC41130640, NSFC41201039), the Fundamental Research Funds for the Central Universities, PCSIRT (No. IRT1108), and the Project supported by State Key Laboratory of Earth Surface Processes and Resource Ecology.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cui, BL., Li, XY. Characteristics of stable isotope and hydrochemistry of the groundwater around Qinghai Lake, NE Qinghai-Tibet Plateau, China. Environ Earth Sci 71, 1159–1167 (2014). https://doi.org/10.1007/s12665-013-2520-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-013-2520-y