Abstract
Tracing techniques are commonly used to investigate groundwater quality and dynamics, as well as to measure the hydrogeological parameters of aquifers. The last decade has seen a growing interest in environmentally friendly tracers, including single-stranded DNA molecules. In this study, an electrolytic tracer and a synthetic DNA tracer are comparatively evaluated in laboratory scale tests to assess their potential application in field studies aimed at investigating groundwater environments. A real-time quantitative Polymerase Chain Reaction assay was developed and optimized to detect and quantify the DNA tracer, while tracer column tests were performed to investigate the DNA tracer behavior and to compare it to the electrolytic tracer. The results show that the DNA tracer has an almost pure convective flow, while the KCl tracer experiences dispersive behavior. The tracing method proposed can be applied in hydrogeological field studies involving calcareous fractured rock systems, with the DNA tracer particularly suitable in tracing karst systems, which are often characterized by several conduits of flow. To test the DNA tracer in operation, a preliminary test was conducted in the field.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tracing techniques are good, proven tools for investigating groundwater quality and dynamics and measuring the hydrogeological parameters of aquifers, and tracing tests have been widely used by hydrogeologists for aquifer characterization (Sutton et al. 2000) and determining principal hydrogeological parameters. Traditional hydrogeological tracing methods utilize fluorescent dyes, salts and radioactive isotopes, however, in the last decade, increasing concern has emerged about the application of these tracers in aquatic ecosystems due to their potentially negative impact on the environment (Field et al. 1995; Field 2005; Rossi 1992), especially at high concentrations. This concern underlines the ever growing demand for more environmentally friendly tracers, commonly referred to as biotracers, and including bacterial strains, endospores, bacteriophages, and even oligonucleotides. The latter being a versatile alternative to traditional tracers, with potential applications in various fields (Sabir et al. 2001).
The main advantages of using DNA molecules for groundwater tracing are briefly summarized as follows: DNA molecules can be transported by groundwater flow through porous media and complex fractured rock systems, do not affect water viscosity or density, are not harmful to human beings or the environment, can be detected at very low concentration, and are resistant to degradation (Sabir et al. 1999, 2000, 2001). However, DNA tracers are potentially limited by their low persistence in water environments with either a low pH or high microbial activity, although in situation with high microbial activity, DNA stability can be enhanced through chemical modification or through adsorption and binding with clay minerals and other particles (Sabir et al. 2001). First proposed by Aleström (1995), water DNA tracing techniques have been successfully used in a number of field experiments (Foppen et al. 2011; Ptak et al. 2004; Sabir et al. 1999, 2000). In their tracing tests, Sabir et al. (1999) demonstrated the value of a synthetic DNA tracer for evaluating water flow patterns and velocities in a number of complex fractured hard rock aquifers. In these experiments, the presence of the DNA tracer in groundwater samples collected at different sampling points was determined using Polymerase Chain Reaction (PCR) analysis, which is a molecular technique developed by Kary Mullis in 1983 (Bartlett and Stirling 2003) to amplify as little as one molecule of a given DNA sequence across several orders of magnitude, thus leading to the production of thousands to millions of copies of the initial DNA sequence (referred to as the DNA template or target DNA). The reaction relies on repeated cycles of heating and cooling for DNA melting, namely the denaturation of double-stranded DNA, the hybridization of short DNA primers to the target sequence and the further elongation of primers by thermostable DNA polymerases. PCR is currently used in medical and biological laboratories for a multitude of applications, including DNA cloning and sequencing, genetic fingerprinting, forensic tests, diagnostics, etc. Although this technique is characterized by extremely high sensitivity, it does not have the potential to quantify specific DNA sequences, thus explaining its use as a qualitative tracing method by Sabir et al. (1999, 2000). This limitation, which is particularly troublesome for specialists in hydrogeology who routinely need to quantify processes, has recently been overcome by the introduction of the real-time quantitative PCR (qPCR or qrtPCR). This technique allows a targeted DNA molecule to be detected and simultaneously quantified as the amplification reaction progresses in real time. DNA molecules are detected using fluorescent dyes, such as SYBR Green I, which intercalate on double-stranded DNA molecules, or alternatively modified oligonucleotides, commonly referred to as probes, which emit a fluorescent signal after their hybridization with the target sequence. As the reaction proceeds and the number of cycles increases, the number of newly synthesized molecules increases as well, increasing the fluorescent signal. The cycle at which this fluorescent signal starts to rapidly increase is defined as the “threshold cycle” (C t). To quantify a specific DNA target molecule in a given sample, a calibration curve must first be created by amplifying serial dilutions of known quantities of the template DNA and by plotting the initial amounts of the template DNA against the threshold cycles of the amplification curves on a Cartesian graph. The efficiency of the amplification reaction is calculated based on the slope of the calibration curve, whereas the specificity is evaluated at the end of the reaction by analyzing the melting curve of the amplicons. Melting curve analysis is an assessment of the dissociation characteristics of double-stranded DNA during heating. As the temperature is raised, the double strand begins to dissociate leading to a decrease in the fluorescent intensity.
qPCR was first used by Ptak et al. (2004) to detect a DNA tracer injected into an unconsolidated alluvial aquifer and was later used by Foppen et al. (2011) to investigate groundwater–surface water interactions. Foppen et al. simultaneously injected six synthetic DNA tracers differing in the nucleotide sequence into two small streams in the Netherlands along with NaCl, which was used as a control. Although DNA tracers and qPCR have the potential to spatially trace the distributed groundwater and surface water interactions, a downstream reduction in DNA mass was recorded, possibly due to adsorption, attachment, decay and/or biological uptake processes occurring in the two streams, thus suggesting the need for further research on the chemical and biological stability of DNA tracers.
Based on these premises, this study aims at comparing traditional hydrogeological tracers (KCl and fluorescein) with a synthetic DNA tracer for application in filed studies of groundwater environments. To this end, tracing tests were preliminary performed at a laboratory scale to evaluate whether the tracers were suitable for investigating mineral analytes that form an important aquifer in a mountain landscape in the Central Apennines (Marche region, Italy). The usefulness of laboratory experiments in studying the behavior of solutes has been discussed previously (e.g., Silliman et al. 1998; Tazioli 2011). In fact, breakthrough curves gathered from laboratory column test results have often been used to estimate the transport parameters for numerical flow models (Mascioli et al. 2005; Zengguang et al. 2012), while column experiments carried out with Polyethylene (PE) columns and KCl tracers (Rango et al. 2010) highlight the importance of this methodology in understanding tracer behavior in porous media. A recent investigation (Tazioli and Palpacelli 2013) underlined the importance of laboratory tests performed under the same conditions and with the same materials as tracer field tests.
In this study, tracer comparisons were run in the laboratory using the same lithological type, but with a porous pattern (i.e., a different porosity compared to the in site rock), owing to the scale required by the column test. The test equipment allowed the behavior of different tracers to be investigated under conditions similar to those of aquifers. A first application on a field scale was performed to verify test operations and possible interference of DNA tracer with the field environment.
Materials and methods
In a previous study (Tazioli and Palpacelli 2013), batch tests with several laboratory columns were performed using more than ten different lithotypes found in Central Italy to evaluate the potential and the behavior of different tracers and the effectiveness of these tracers in different aquifers under natural conditions. In particular, the tracer column tests were performed using two different compositions of mineral analytes that form aquifers in the above-mentioned area; in the present study only one analyte composition was investigated. The tested analyte geotechnical and geological properties, determined from specific laboratory tests, are reported in Fig. 1 and Table 1.
Mineral analyte samples were obtained by crushing the limestone formation of “Calcare Massiccio”, which is the basal limit of the hydrogeological Apenninic system in Central Italy. This lithotype mainly consists of calcite (98 %) and contains a permeable carbonate aquifer with a maximum thickness of 500 m. The sediment is 21.3 % gravel, 74.7 % sand and 4 % silt, and the mean diameter of the particles is 0.51 mm (Table 1). Total porosity (n), determined in the laboratory, was equal to 0.31. The mineral analyte, a commercial building material, was derived from crushing the above-mentioned geological formation. The differences with limestone aquifer were obvious in terms of specific surface and porosity; this choice was driven by the need for a material with a lithological composition identical to the in site rock and suitable for laboratory testing.
Tracers utilized in the tests
Potassium chloride
A solution prepared with KCl salt (Sigma-Aldrich Co. LLC) BioXtra powder, assay ≥99 %, was prepared using distilled water for laboratory purposes. The final concentration of the solution was 200 g L−1. This solution was used as a tracer solution without further dilution.
Fluorescein
A solution prepared with fluorescein sodium C20H10Na2O5 (Merck) extra pure powder, was prepared using distilled water for laboratory purposes. The final concentration of the solution was 10 mg L−1. This solution was used in the batch tests both as an undiluted tracer solution as well as with different levels of dilution.
Synthetic DNA tracer
A single-stranded DNA (ssDNA) molecule of 72 nucleotides with the following nucleic acid sequence was used as a biotracer: 5′-ATC ACA TTC GAG GTG TCC ACT AGA TCC CGC GTT TTG TAC CAT CAG TCA TTG CGC AGG TCG GTT GGT GGT AAG-3′. The ssDNA was synthesized, purified by High Performance Liquid Chromatography (HPLC) and lyophilized by MWG–Biotech (MMedical, Milan, Italy), with a final yield of 254.4 nmol, corresponding to ~1015 DNA molecules (~4 μg). The biotracer was suspended in 100 μL of molecular biology grade (Eppendorf, Milan, Italy) water to reach a final concentration of 1013 DNA molecules μL−1, and the solution was kept at −20 °C until use.
qPCR optimization and the conditions for DNA tracer detection
Primer design
Oligonucleotide primers R1c (5′-ATCACATTCGAGGTGTCCAC-3′) and L1c (5′-CTTACCACCAACCGACCT-3′) were designed using the PRIMER3 online interface (Rozen and Skaletsky 2000) provided by PREMIER Biosoft (http://frodo.wi.mit.edu/primer3/). The free web based tool Netprimer, which is also provided by PREMIER Biosoft (www.premierbiosoft.com/netprimer/), was used to predict the primer melting temperature (T m) and to evaluate secondary primer structures (hairpins, dimers, cross dimers, palindromes, repeats, and runs). The desired specificity was verified by submitting the primer sequences to the FASTA3 database search program (Pearson and Lipman 1988) provided by the European Bioinformatics Institute (www.ebi.ac.uk). The two oligonucleotides were synthesized, subjected to High Purity Salt Free (HPSF) purification, and lyophilized by MWG–Biotech (MMedical).
Amplification conditions
The performance and optimal annealing temperature of the primer pair were preliminarily determined as follows. Aliquots (5 μL) of tenfold dilutions of the DNA tracer suspension were amplified in a total volume of 25 μL containing 7 μL of molecular biology grade water (Eppendorf), 12.5 μL of 2X DyNAmo Flash SYBR Green qPCR Kit (MMedical) and increasing concentrations (50, 100, 200, or 400 nM) of each primer. Negative controls, consisting of samples prepared with molecular biology grade water instead of template DNA, were incorporated in each run. The PCR experiments were carried out in triplicate with gradient PCR (Mastercycler ep realplex, Eppendorf) and the following amplification program: polymerase activation and DNA denaturation at 95 °C for 7 min followed by 40 cycles of denaturation at 95 °C for 10 s, primer annealing at 50–55 °C for 15 s, primer extension at 72 °C for 2 min, and a final extension step at 72 °C for 2 min. The temperature transition rate between PCR target temperatures was programmed at 20 °C s−1. Fluorescence was measured at 520 nm at the end of the annealing period of each cycle to monitor the progress of amplification. The C t for each sample was determined via regression analysis of the amplification profile with the built-in ep realplex software (Eppendorf).
Control analysis of amplification products
After completion, the specificity of the PCR reactions was determined through melting curve analysis; melting curves were recorded by heating to 95 °C at 20 °C s−1 for 15 s, holding at 60 °C for 15 s, and then heating at 0.4 °C s−1 until 95 °C was reached, and holding for 15 s. Fluorescence was measured continuously during the slow temperature rise to monitor the dissociation of the SYBR Green I dye. Fluorescent signals were plotted automatically in real time against temperature (T) to produce melting curves, which were then converted, again automatically, into melting peaks by plotting the negative derivative of fluorescence versus T (dF1 dT−1 versus T).
To check the size of the amplification products, 10 μL of each sample was analyzed by electrophoresis in 1.5 % (wv−1) agarose gel and trisborate-ethylenediaminetetraacetic acid (Tris–EDTA buffer: 45 mM Tris–borate, 1 mM EDTA, pH 8.0), followed by staining with ethidium bromide (1 μg mL−1). PCR products were visualized on a UV transilluminator. A molecular size marker (O’GeneRuler DNA Ladder Mix, Fermentas, Thermo Fisher Scientific, Illkirch Cedex, France) was loaded on the gels to allow inter-gel normalization and comparison, and gel images were saved as digital photographs in JPEG format.
Batch test
Sorption between the investigated tracer and the mineral analytes was preliminarily evaluated by performing a batch equilibrium test. One-liter PE bottles containing 25 g of the minerals with KCl tracer solutions at different known concentrations ranging from 0.01 to 10 g L−1 (mineral to liquid ratio equal to 1:4) were agitated for 24 h using an overhead mixer with a frequency of 10 rpm to allow equilibrium adsorption to be reached. As reported in the available literature, the equilibrium adsorption of molecules is generally reached after 24 h (ASTM 1993; Hadi 1997; Leite et al. 2003; Roy et al. 1991). The samples were separated by centrifugation after 7 days of sedimentation, and the leachates were analyzed. The same procedure was followed for the fluorescein batch test, at different known concentrations ranging from 0.001 to 1 g L−1. The fluorescein samples were separated by centrifugation and kept in the dark until the leachates analysis.
Sorption between the DNA tracer and the same mineral analytes was tested as follows. A 250-mL PE bottle was filled with 25 mL of molecular biology grade water, 25 g of the minerals, and 1 μL of the concentrated DNA tracer solution containing 1013 DNA molecules μL−1. The bottle was agitated for 24 h using an overhead mixer with a frequency of 10 rpm to allow the equilibrium adsorption to be reached. One day later, the mixture was centrifuged and the target DNA that was freely suspended in the water fraction or adsorbed in the limestone granules was quantified by amplifying a 50-μL aliquot of the supernatant or the DNA eluate, respectively. The latter was obtained using the desorption procedure previously described by Mahler et al. (1998) with slight modifications. Briefly, the DNA–adsorbed mineral particles were washed five times with molecular biology grade water to remove any unabsorbed DNA. The ultimate wash was retained and subjected to real-time PCR to verify the absence of the target DNA. An aliquot (0.2 g) of the washed mineral particles was added to 80 μL of 10 mM EDTA (pH 8.0) and agitated overnight. The particle–EDTA mixture was then centrifuged, and 15 μL of the supernatant was removed, placed into a new tube, and added to 48 μL of 10 mM CaCl2.
Column test
Two sets of columns of varying diameters and lengths, made of plexiglass to facilitate visual inspection of the saturation process were utilized during the research (Table 2). The first set was 20-cm long with an internal diameter of 1.8 cm (referred to as B2), while the second set (referred to as ST1) was approximately 45-cm long with an internal diameter of 5.2 cm.
The columns were filled with the aquifer material analyte (previously washed with hydrogen peroxide and distilled water) arranged in several layers, each packed with a cylindrical plastic hammer. Filters were arranged at the top and the bottom of the columns and a natural gradient was imposed using a Polyvinyl Chloride (PVC) reservoir filled with tap water at a constant head, supplying the columns with an upward flow of water through silicon tubes to minimize air bubble formation (Fig. 2). The flow rate was adjusted to obtain a water transport velocity of 2.1 m d−1, which is similar to the rate expected in field experiments. Column saturation, which was normally reached after at least 10 PVs (1 pore volume is equal to the product of the column volume times the porosity), occurred when the measured outflow discharge was constant. After column saturation was achieved, the tracer test began.
In each column, instantaneous injections of both the electrolytic tracer and the DNA tracer were performed after saturation was reached (Table 3). This methodology was applied as tracers are commonly injected instantaneously in field experiments.
Five milliliters of a KCl solution containing 0.4 g of tracer were injected at the inlet of both the columns using a 5-mL syringe (Hamilton 1005 AD SYR). Water samples were collected at the outflow in 5-mL glass vials and were stored at 4 °C until analysis. Continuous measurements of electrical conductivity were performed at the outflow using an EC probe. The DNA tracer (1013 molecules; 394 ng) was suspended in 1 mL of molecular biology grade water and injected at the inlet of both the columns using a 5-μL syringe (Hamilton, 65 RN SYR). Water samples for DNA detection were collected at the outflow in 2-mL DNase–free plastic tubes, buffered with Tris–HCl pH 8.0 to a final concentration of 10 mM, and stored at −20 °C until qPCR analysis.
Data interpretation
The main purpose of the column tests was to identify the optimal conditions for field tracer experiments as well as the hydraulic parameters of the investigated aquifer material, the latter being particularly useful for comparing the behavior of different tracers relative to a carbonate material. The best-fit method (based on the Least Squares Method, Leibundgut et al. 2009) was used to calculate mean transit time (t t) and the dispersion coefficient (D) by fitting a theoretical breakthrough curve (BTC) to the measured BTC obtained from laboratory experiments. The BTCs were normalized to the maximum observed concentration (Cm). The Advection Dispersion Equation (ADE, Freeze and Cherry 1979) and the Convection Dispersion Equation (CDE, Parker and van Genuchten 1984; Leibundgut et al. 2009) usually describe solute transport in a porous media. In the literature (Freeze and Cherry 1979; Parker and van Genuchten 1984; Leibundgut et al. 2009), reference to both ADE and CDE are reported, sometimes meaning and describing the same process. Correctly, convection is the sum of transport by diffusion and advection. The analytical solution, for the 1D case, has the following equation (Lenda and Zuber 1970):
where M is the mass of the tracer injected, Q is the volumetric flow rate, v is the pore water velocity, and D L is the longitudinal dispersion coefficient.
The results of the column tests were assessed using this 1D dispersion model. The measured BTCs were iteratively fitted using either MS Excel (Microsoft Corporation, Redmond, Washington, USA) or STANMOD (PC-PROGRESS, s.r.o., Prague, Czech Republic) software (Simunek et al. 1999). The fitting parameters were found using the Least Squares Method.
The CDE with a deterministic equilibrium solution model was applied. Flux-averaged concentrations (Parker and van Genuchten 1984) were measured at the outlet and inputted into the software. As a boundary condition, the third type was applied (Toride et al. 1999) using the Dirac delta input function and a zero initial concentration. The fitting results allowed the transport velocity (u) and the longitudinal dispersion coefficient (D L ) to be obtained. From these results, dispersivity (α), mean transit time of the tracer (t t) and effective porosity (n e) can be determined.
DNA tracer detection
Five-microliter aliquots of each water sample were amplified in a total volume of 25 μL under the optimized conditions described previously (primer concentration: 200 nM; annealing temperature: 53 °C). Two types of negative control, consisting of samples prepared with either water collected from the column before injection or pure molecular biology grade water, were incorporated in each run. Amplification products from randomly selected water samples were subjected to sequence analysis with primers R1c and L1c. The DNA was preliminarily purified and concentrated using the GFX PCR DNA and Gel Band Purification Kit (GE Healthcare, Milan, Italy) to remove excess primers and nucleotides and was then sent to MWG Biotech (MMedical) for sequencing.
Electrolytic tracer detection
Normally, salt tracer detection is performed using Ion Chromatography; this method allows very low concentrations of the tracer to be detected. However, sample collection using this method is time-sensitive, requiring exact peak time to be determined and a large number of samples to be collected and analyzed. As an alternative option, electrical conductivity can be continuously measured using a probe placed at the outflow, and therefore, only a few samples need to be collected. The latter method was adopted for this study.
Fluorescent tracer detection
Fluorescent tracer detection was performed using a Perkin Elmer LS-2 lab filter fluorometer; the LS-2 fluorometer allows low concentrations of the tracer to be detected. Three standard solutions (1, 10, 100 μg L−1) were prepared to calibrate the fluorometer. All samples were filtered using a 0.45-μm membrane filter before the analysis.
First application at field scale
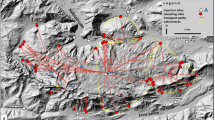
The tracers behavior observed in the laboratory has been then verified in a field study at a site that is representative of the hydrogeological features of limestone aquifers in the central Apennines (Marche Region, Central Italy). The study area is characterized by aquifers of Calcare Massiccio, Maiolica and Scaglia, divided by the aquiclude formations of Marne a Fucoidi and Rosso Ammonitico. The geological structure is very complex and characterized by extended overthrusts and small faults, which bring the different aquifers into hydraulic contact with each other. These aquifers are characterized by rocks of differing porosity forming a very complex structure. As a consequence, the hydrogeological system is particularly complicated (Fig. 3) and the degree to which different groundwater mix is unknown. Moreover, limestone aquifers (often containing important springs), are extensively fissured and fractured, and sometimes affected by karstification. In certain areas, the fissure/fracture system is so extensive that the rocks may be considered as porous media. Karstification in these areas is, however, not well known as there is little karstification extension in surface figures; streams, and in general, surface water poorly contributes to groundwater recharge. Karst plains (with a maximum areal extension of a few square kilometers) are located at different elevations in the Apennines carbonate ridge. In such plains, rain water and snowmelt infiltration often occur in morphological hollows in which swallow holes and sinkholes of modest extension are present. These aquifers recharge several important springs, often utilized for drinking water. The site chosen for the field application, named “Monte Lago”, is characterized by a sinkhole, located in a small plain, and some springs emerging at a lower altitude. In current literature, only a few examples of DNA tracer application in the field have been reported, with no applications to groundwater. Karst aquifers act as a very large reservoir for potable water. Therefore, the application of DNA tracer in such field investigations is innovative and very important for testing the suitability of the DNA tracer in field studies, especially those concerning karst hydrogeology, these aquifers being very interesting for water supply purposes. This preparatory application in the field was performed to verify DNA tracer operations for later real-world investigations, with the main goal being to verify whether the amount of DNA tracer was similar to the amount of traditional tracers and to evaluate the tracer behavior in the subsurface environment. In extreme synthesis, this test could be defined as a preliminary test having a qualitative aim, mainly to determine whether the DNA tracer arrived at the sampling points and how it behaved. Further applications, currently being implemented at other field sites, and to be performed under different hydraulic conditions, will have a quantitative aim for hydrogeology parameter determination. They will be reported in a future publication.
Hydrogeological map, location of the Montelago sinkhole, the sampling points and fluorescein traps. 1 Scaglia limestone and marly limestone formation (aquifer), 2 Marne a Fucoidi formation (aquiclude), 3 Maiolica limestone formation (aquifer), 4 Jurassic sequence formation (aquiclude), 5 Massiccio limestone (aquifer), 6 Laga formation (aquiclude for limestone aquifers), 7 thrusts faults, 8 “Montelago plain” karst area
Practically, the tracer tests employed injection of fluorescein and the DNA tracer into the Montelago sinkhole and the measurement of samples collected by an automatic sampler located at the S. Giovanni spring (Fig. 3). In addition, as a first qualitative step, fluorescein traps were placed downstream at different surface water and groundwater monitoring points around the carbonate structure (thought to be in hydraulic contact with the Montelago plain). The aim of this preliminary survey was first to detect any potential connection to the Montelago sinkhole, and secondly to gain an idea of the tracer arrival time (Fig. 3). As the tracing ability and strength of DNA tracer were not yet known, the tracer tests were performed when the discharge of the S. Giovanni spring was close to minimum flow, approximately 8 L/s, and injection of the DNA tracer was done 1 week after that of fluorescein, to avoid possible interference between the tracers.
Results
Optimization of the qPCR assay
Optimization of the reaction components and the thermal cycling protocol is fundamental for establishing a successful and well-performing real-time PCR assay. Accordingly, increasing concentrations of primers and annealing temperatures were assayed; a primer concentration of 200 nM and an annealing temperature of 53 °C proved to be optimal for the amplification of the DNA tracer in terms of amplification efficiency and specificity (data not shown).
Sensitivity, linearity, and efficiency of the optimized qPCR assay
The detection limit of the optimized qPCR assay, assessed by amplifying tenfold serial dilutions of the DNA tracer, was 10 DNA molecules per qPCR well containing 20 μL of the reaction mixture and 5 μL of the sample. This translated into a minimum DNA sample concentration of 0.33 aM (attomolar: 10−18 mol L−1). The linearity of the calibration curve, obtained by plotting the threshold cycle (C t ) values against the starting quantity of DNA tracer, was determined to be between 1010 and 10 molecules per qPCR (Fig. 4). For negative controls, no signals above the detection limit were obtained in any of the assays.
When background correlated data were brought down to the baseline, the standard curve had a correlation coefficient of 0.991 (Fig. 4), whereas the amplification efficiency calculated using the formula E = [10 (−1 slope)−1] was 100 %. For the melting curve analysis, the melting temperatures (Tm) of the desired amplicons varied between 81.2 and 80.0 °C, whereas significantly lower Tm values were observed for the negative controls (Fig. 5).
Batch test
The DNA tracer was detected in both the DNA–mineral particles supernatant and the eluate. The real-time quantification of the DNA in water and solid fractions revealed that almost all the molecules (1013) initially introduced into the water and mineral granules mixture were freely suspended in the water fraction after reaching the adsorption equilibrium, with just one-hundredth of these molecules (~1011) adsorbed by the mineral granules. No DNA was detected in the last wash of the particulate fraction, thus confirming the absence of unadsorbed target DNA.
The KCl tracer was only detected in the eluate. In all cases, the concentrations found in the eluate were close to the initial concentrations, indicating approximately zero adsorption in the mineral grain surface. Fluorescein tracer was detected in the eluate, however, with no significant adsorption either.
Column test
Direct DNA sequencing of the PCR products randomly selected among those obtained from the water samples collected during the column tracing tests confirmed the amplification of the injected 72-nucleotide-long oligonucleotide.
The results of the column tests are reported in Table 4, including the calculated values of the pore water velocity, the calculated effective porosity (obtained using the solution of 1D dispersion model), the measured effective porosity (obtained by weighing the saturated column at the end of the test), the dispersion coefficient, the mean transit time and the Peclet column number (i.e., the ratio of transport by advection to the rate of transport by diffusion in a column test). The results obtained in column B2 (1.8-cm diameter and 20-cm length) are shown in Fig. 6, whereas those of the tracer tests in column ST1 (5.2-cm diameter and 44.6-cm long) are shown in Fig. 7.
In these figures, the DNA tracer breakthrough curve and the KCl breakthrough curve are shown. The tracer peaks are well defined in all the column tests, although occurring at different times.
Field test
The breakthrough curves obtained from the DNA and fluorescein tests performed at the Montelago site are shown in Fig. 8. The fluorescein tracer peak was recorded 94 h after injection, whereas the DNA tracer peak was recorded 80 h after injection. The DNA tracer BTC showed multiple peaks occurring at different times (before and after the main peak). The different tracer peak arrival times were only observed in the laboratory column tests; in the field, a more marked behavior in the DNA tracer BTC was noted.
Discussion
In this study, an optimized qPCR assay using SYBR Green I chemistry was designed and optimized for the detection and accurate quantification of the DNA tracer used for the column tests. The qPCR of serially diluted suspensions of the DNA tracer demonstrated a dynamic range, defined as the ratio between the highest and lowest measurable concentration of target DNA without any sample dilution or pre-treatment, of 9 logs (from 1010 to 10 molecules per well). This finding is similar to the dynamic range of 8–9 log units reported by Foppen et al. (2011) for qPCR assays. In contrast, a higher minimum number of detectable DNA molecules, corresponding to 10 per sample, was measured with our optimized qPCR assay with respect to that measured by Foppen et al. (2011) using different DNA tracers. Accurate quantification of low concentrations of target DNA is often impacted by the amplification of primer–dimers, consisting of primer molecules that hybridize with themselves or with each other because of strings of complementary bases in their sequences. As a result, the amplification of primer–dimers leads to a competition for PCR reagents among templates, thus potentially inhibiting the amplification of the target DNA sequence.
Thereafter, a preliminary investigation of adsorption of KCl, fluorescein and DNA tracers in the studied aquifer material was conducted by performing batch equilibrium tests consisting of the agitation of mineral analyte samples in different bottles containing different concentrations of relatively large volumes of water solution containing the tracer under study. These tests are known to simulate a completely dispersed particle system (Tanchuling et al. 2003), thus allowing the kinetics of sorption, i.e., adsorption isotherm curves, to be investigated. Batch tests performed with KCl, fluorescein and single-stranded DNA molecules revealed no significant adsorption of these tracers on limestone, confirming their suitability for tracing in fractured calcareous rock systems.
Column test studies were carried out using different equipment and applying several test conditions and configurations, to optimize the test and to evaluate the laboratory conditions (with the aim also being to compare laboratory and field conditions). In fact, the duration of the test is a basic element to be accurately investigated and taken into account both in laboratory and field tests, to plan the operations and to determine the effect of sorption on the flow properly. Contact time between aquifer material and water is known to play a significant role on sorption amount; therefore, to be more representative, the time duration of a column test should be similar (or at least of the same order of magnitude) to a field test. Moreover, the sample time span has to be taken into account (especially in preparing a field test), so as to provide the necessary equipment and plan the execution of the test.
Different equipment and test configurations were, therefore, assayed to select a test setup that was as similar as possible to the actual situation of the investigated aquifers (i.e., limestone fractured and/or karst aquifers, often containing important springs). Such aquifers are in fact extensively fissured and fractured, with intensive (but not well known) karstification in some zones. For these reasons, it is very important to perform laboratory studies of the tracer behavior in such aquifer materials, to evaluate potential sorption problems and to plan the time duration of in field tracer test. As a first step, test duration consistent with the sampling conditions both on site and in the laboratory is essential. The test equipment was, therefore, chosen to guarantee a transit time and a test duration similar to those occurring in the investigated aquifers. For these reasons, a minimum hydraulic gradient represented the best choice to ensure an appropriate duration of the test.
In the shorter column (B2), the breakthrough curve showed a main peak that was sharp and close to the time of injection; this was most likely due to the pressure transfer caused by the injection (Fig. 6) because of the short column length and the Dirac pulse of the tracer. In general, the occurrence of secondary peaks in tracer experiments was also related to the flow restriction and different channel length (Field and Leij 2012; Leij et al. 2012), typical of karst aquifers and fissured/fractured aquifers, where water has different velocities. The occurrence of flow regions, in which groundwater flow was slow or practically immobile, was probably responsible for the long tail and skewness in most measured BTCs (Field and Leij 2012). A secondary peak might be observed, depending on the balance of the hydrodynamic conditions after the injection and in the presence of preferential pathways for flow. The peak in the DNA tracer BTC preceded the peak in the KCl BTC by one hundred seconds. As stressed in a previous investigation (Tazioli and Palpacelli 2013), the presence of secondary peaks disappears when the column length is increased. The breakthrough curves of tests performed in columns with different lengths and diameters are shown in Fig. 9; the presence of secondary peaks has been shown to be associated with column length and transport velocity. In addition, BTCs exhibiting multiple peaks were present, highlighting the presence of preferential flow paths and flow restrictions zones. In the small column, the calculated effective porosity was significantly underestimated, and without any physical meaning, probably as a result of the column length and injection mode.
Comparison among different column tests. BTCs reported by Tazioli and Palpacelli (2013)
In the longer column (ST1), the DNA breakthrough curve showed a peak, which preceded the peak of the KCl breakthrough curve despite almost identical flow conditions. As clearly demonstrated by Ptak et al. (2004), such behavior is due to size exclusion effects and selective flow paths detected by the DNA tracer. The electrolytic tracer, on the other hand, covered almost the entire effective flow section. These findings suggest that in porous media, the DNA molecules travel more rapidly than ionic tracers, which are characterized by a more widely distributed flow. The rate of migration of linear DNA through porous media mainly depends on either the pore size or the length of the DNA molecules. During their transport, these molecules orient properly to “snake” through the pores. As the pore size decreases, the movement of the DNA molecules is impeded, thus explaining the preferential advancement of these molecules through the larger pores.
It has previously been demonstrated that the size of the DNA molecules largely affects their transport behavior, even through fractured rock systems (Sabir et al. 2000). Smaller DNA molecules are subjected to greater losses due to a more rapid diffusion into the relatively immobile water occurring within fine pores and fractures, whereas larger molecules enhance the retention processes in porous materials (Sabir et al. 2000).
In terms of water flow and solute transport through the column, the difference between the KCl and the DNA tracer behavior is that the former shows a strong dispersive process.
The calculated pore water velocity is higher in the case of the DNA tracer (33.6 m/d); consequently, the effective porosity is very low (0.08). The KCl tracer gave an effective porosity close to actual porosity, measured by weighing the saturated column at the beginning and at the end of the tracing test (Table 4).
The longitudinal dispersion coefficient obtained from the DNA tracer tests was one order of magnitude lower than that from the KCl tracer test, which corresponded to a dispersivity of 0.23 cm against a value of 26.3 cm (calculated from the KCl tracer test). The latter value confirms that the DNA tracer shows a longitudinal dispersion of approximately zero.
Finally, the preliminary field test (performed with the aim of verifying the test operations, the suitability of DNA tracers in groundwater and tracer behavior in the subsurface environment) had a qualitative aim, as a preparatory action to determine whether the DNA tracer arrived at the sampling points and how it behaved. As shown in Fig. 8, there was a slight difference in the peak times of fluorescein and the DNA tracer observed in the column tests. Different peak times between DNA and KCl tracer were previously observed in the column tests and also reported by Ptak et al. (2004). This was probably also due to slight differences in the hydraulic and piezometric conditions between the two field tests. This investigation was the first application of the DNA tracer for hydrogeological studies observing a real scale. Further tests will be necessary to take a deeper look into DNA tracer behavior, especially in relation to the multiple peaks showed in the BTC, probably due to the occurrence of several flow channels and conduits in the carbonate system. Such tests will be performed with simultaneous injection of several tracers during and after the recharge period, to fully demonstrate the suitability of DNA tracer in groundwater surveys.
Conclusions
The tracer tests performed in the column filled with natural aquifer material using either electrolytic or DNA tracers allowed for the following conclusions to be drawn.
The laboratory test conditions need to be similar to the site conditions, particularly regarding the method of tracer injection, flow velocity, aquifer material and tracer to be used. Column testing is very useful as a preliminary study of tracer behavior and field test features. The DNA tracer is useful for investigating aquifers characterized by karst conduits or double porosity, but is less suitable in porous media if the basic aim of the field study is effective porosity determination. In column tests performed using calcareous aquifer material, the biotracer showed an almost pure convective flow; the KCl tracer showed definite dispersive behavior, highlighted by a higher longitudinal dispersion coefficient. The DNA adsorption on calcareous rock is negligible; so the biotracer behaves as a conservative tracer with respect to carbonate materials.
The overall results of this study indicate that the tracing method proposed can effectively be applied in hydrogeological field studies investigating calcareous fractured rock systems and that the DNA tracer used is particularly suitable to trace such karst systems, which are often characterized by several conduits of flow. The preliminary results of the field tracer tests support the qualitative trend observed in the laboratory column tests, thus stressing the applicability of the DNA tracer in hydrogeological surveys. Further field investigations are in progress to validate these methods for the study of groundwater circulation in karst and fissured rocks.
References
Aleström P (1995) Novel method for chemical labeling of objects. Int Patent Applic. no. PCT/IB95/01 144 and Publ. no. W096/1 7954
ASTM (1993) Standard test method for 24-h batch-type measurement of contaminant sorption by soils and sediments. vol 04.04
Bartlett JMS, Stirling D (2003) A short history of the polymerase chain reaction. PCR Protoc 226:3–6. doi:10.1385/1-59259-384-4:3
Field MS (2005) Assessing aquatic ecotoxicological risks associated with fluorescent dyes used for water-tracing studies. Environ Eng Geosci XI:295–308
Field MS, Leij FJ (2012) Solute transport in solution conduits exhibiting multi-peaked breakthrough curves. J Hydrol 440–441:26–35
Field MS, Wilhelm RG, Quilan JF, Aley TJ (1995) An assessment of the potential adverse properties of fluorescent dyes used for groundwater tracing. Environ Monit Assess 38:75–96
Foppen JW, Orup C, Adell R, Poulalion V, Uhlenbrook S (2011) Using multiple artificial DNA tracers in hydrology. Hydrol Process 25(19):3101–3106
Freeze RA, Cherry JA (1979) Groundwater. Prentice Hall, Englewood Cliffs, NJ 07632
Hadi S (1997) New hydrological tracers synthesis and investigation. Druckerei & Verlag Gaggstatter, Freiburg
Leibundgut C, Maloszewski P, Kulls C (2009) Tracers in hydrology. WILEYBLACKWELL, London
Leij FJ, Toride N, Field MS, Sciortino A (2012) Solute transport in dual permeability porous media. Water Resour Res 48:1–13
Leite AAD, Paraguassu AB, Rowe RK (2003) Sorption of Cd2+, K+, F−, and Cl− on some tropical soils. Can Geotech J 40(3):629–642
Lenda A, Zuber A (1970) Tracer dispersion in groundwater experiments. In: IAEA (ed) Isotope hydrology, Proceedings series. IAEA, Vienna, Austria, 9–13 March 1970 pp 619–641
Mahler BJ, Winkler M, Bennett P, Hillis DM (1998) DNA-labeled clay: a sensitive new method for tracing particle transport. Geol 26(9):831–834
Mascioli S, Benavente M, Martinez D (2005) Estimation of transport hydraulic parameters in loessic sediment, Argentina: application of column tests. Hydrogeol J 13(5–6):849–857
Parker JC, van Genuchten MT (1984) Flux-averaged and volume-averaged concentrations in continuum approaches to solute transport. Water Resour Res 20(7):866–872
Pearson WR, Lipman DJ (1988) Improved tools for biological sequence comparison. Proc Natl Acad Sci USA 85(8):2444–2448
Ptak T, Piepenbrink M, Martac E (2004) Tracer tests for the investigation of heterogeneous porous media and stochastic modelling of flow and transport-a review of some recent developments. J Hydrol 294(1–3):122–163
Rango T, Colombani N, Mastrocicco M, Bianchini G, Beccaluva L (2010) Column elution experiments on volcanic ash: geochemical implications for the main Ethiopian rift waters. Water Air Soil Pollut 208(1–4):221–233
Rossi P (1992) Use of bacteriophages as groundwater tracers: decay rate and adsorption, In: Hötzl H, Werner A (eds) Tracer hydrology 92, Proceedings of the 6th international symposium on water tracing, September 21–26, Karlsruhe, Balkema, Rotterdam, 65–70
Roy WR, Krapac IG, Chou SFJ, Griffin RA (1991) Batch type procedures for estimating soil adsorption of chemicals. vol EPA 530/SW-87-006
Rozen S, Skaletsky H (2000) Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132:365–386
Sabir IH, Torgersen J, Haldorsen S, Aleström P (1999) DNA tracers with information capacity and high detection sensitivity tested in groundwater studies. Hydrogeol J 7(3):264–272
Sabir IH, Haldorsen S, Torgersen J, Aleström P, Gaut S, Colleuille H, Pedersen TS (2000) Synthetic DNA tracers: examples of their application in water related studies. In: A D (ed) Tracers and Modelling in Hydrogeology. TraM’2000 Conference Belgium. IAHS International Association of Hydrological Sciences:159–165
Sabir IH, Aleström P, Haldorsen S (2001) Use of synthetic DNA as new tracers for tracing groundwater flow and multiple contaminants. J Appl Sci 1(3):233–238
Silliman SE, Zheng L, Conwell P (1998) The use of laboratory experiments for the study of conservative solute transport in heterogeneous porous media. Hydrogeol J 6(1):166–177
Simunek J, van Genuchten MT, Sejna M, Toride N, Leij FJ (1999) The STANMOD computer software for evaluating solute transport in porous media using analytical solutions of convection-dispersion equation. Versions 1.0 and 2.0 edn. IGWMC-TPS-71, International Ground Water Modeling Center, Colorado School of Mines, Golden, Colorado, Golden, Colorado
Sutton DJ, Kabala ZJ, Schaad DE, Ruud NC (2000) The dipole-flow test with a tracer: a new single-borehole tracer test for aquifer characterization. J Contam Hydrol 44(1):71–101
Tanchuling MA, Khan MRA, Kusakabe O (2003) Zinc sorption in clay using batch equilibrium and column leaching tests. RMZ Mater Geoenviron 50(1):4
Tazioli A (2011) Experimental methods for river discharge measurements: comparison among tracers and current meter. Hydrol Sci J 56(7):1314–1324
Tazioli A, Palpacelli S (2013) Scelta del tracciante ideale per indagini idrogeologiche: risultati preliminari da test in laboratorio. Acque Sotterranee Italian Journal of Groundwater, accepted for publication
Toride N, Leij F J, van Genuchten M T (1999) The CXTFIT code for estimating transport parameters from laboratory or field tracer experiments. Version 2.1 edn. Research Report No. 137, US Salinity Laboratory, USDA, ARS, Riverside, CA
Zengguang X, Yanqing W, Hui X (2012) Optimization of a PRB structure with modified chitosan restoring Cr(VI)-contaminated groundwater. Environ Earth Sci. doi:10.1007/s12665-012-1902-x
Acknowledgments
The authors thank the two reviewers and the associate editor for their suggestions and constructive reviews that surely improved the previous version of the manuscript. Thanks to Mirco Marcellini of Università Politecnica delle Marche for the assistance in the field test. Many thanks to Azienda San Severino Marche SpA for funding support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aquilanti, L., Clementi, F., Landolfo, S. et al. A DNA tracer used in column tests for hydrogeology applications. Environ Earth Sci 70, 3143–3154 (2013). https://doi.org/10.1007/s12665-013-2379-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-013-2379-y