Abstract
This study investigates the origin and chemical composition of the thermal waters of Platystomo and Smokovo areas in Central Greece as well as any possible relationships of them to the neighboring geothermal fields located in the south-eastern part of Sperchios basin. The correlations between different dissolved salts and the temperature indicate that the chemical composition of thermal waters are controlled by, the mineral dissolution and the temperature, the reactions due to CO2 that originates possibly by diffusion from the geothermal fields of Sperchios basin and the mixing of thermal waters with fresh groundwater from karst or shallow aquifers. Two major groups of waters are recognized on the basis of their chemistry: thermal waters of Na–HCO3–Cl type and thermal waters mixed with fresh groundwater of Ca–Mg–Na–HCO3 type. All thermal waters of the study area are considered as modified by water–rock interaction rainwater, heated in depth and mixed in some cases with fresh groundwater when arriving to the surface. Trace elements present low concentrations. Lithium content suggests discrimination between the above two groups of waters. Boron geochemistry confirms all the above remarks. Boron concentration ranges from 60 μg L−1 to 10 mg L−1, while all samples’ constant isotopic composition (δ11B ≈ 10 ‰) indicates leaching from rocks. The positive correlation between the chemical elements and the temperature clearly indicates that much of the dissolved salts are derived from water–rock interactions. The application of geothermometers suggests that the reservoir temperature is around 100–110 °C. Chalcedony temperatures are similar to the emergent temperatures and this is typical of convective waters in fault systems in normal thermal gradient areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The study area of Platystomo belongs to the graben structure of Sperchios basin (Central Greece), which owes its formation to the N–S extensive movement of the Aegean plate, due to both the subduction of the African plate under the Eurasian plate and the extrusion of the Anatolian block (Taymaz et al. 1991), while the study area of Smokovo is located at the north margin of the Sperchios Graben. The Basin of Sperchios is an E–W valley ~50 km long by 3–12 km wide, which is drained by the Sperchios river that flows eastward toward to Maliakos gulf (Aegean Sea). The south part of Sperchios basin, between the mid-basin and the Maliakos gulf, hosts several thermal springs, those of Platystomo area and those located at the southern edge such as “Hypati” located 2 km apart from the first relieves of Iti mountain as well as “Kamena Vourla”, “Psoroneria”, and “Thermopylae” thermal springs located in the south–eastern part of the basin near the coast of Maliakos gulf (Fig. 1). Many scientists studied the thermal springs of Sperchios basin, especially those located in the south-eastern edge. However, the geochemical patterns and the origin of the thermal waters of Platystomo and Smokovo areas remain poorly documented.
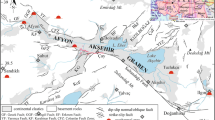
A simplified geological map of the study area (Bornovas and Rondogianni-Tsiambaou (1983) modified by Lambrakis; 1 alluvial loose deposits; 2 oligocene molassic formations in the Mesohellenic Trench: clays, conglomerates, sandstones and marls; 3 neogene sediments (undivided): lacustrine deposits: conglomerates, sandstones, marls and glays; 4 flysch sediments of Pindos zone; 5 flysch sediments of Giona–Parnas zone; 6 flysch sediments of Pelagonian zone; 7 limestones of Upper Cretaceous; 8 limestones of Triassic-Lower Jurassic of the Parnassos-Ghiona zone; 9 limestones Upper Jurassic; 10 jurassic shale and cherts formation: radiolarites, sandstones, pelites with white limestone, lenses and enclosed ophiolitic bodies; 11 ophiolitic complex; 12 fault; 13 overthrust; 14: geological boundary; 15 thermal spring
According to Stahl et al. (1974), the high salinity of Sperchios basin thermal water is due to the intrusion of seawater into the karst system fresh water bounding the geothermal field. The geothermal field is located in the hanging wall of the graben and it is associated to tectonic breccias (Gartzos and Stamatis 1996). Dotsika (1991) compared different geothermometers to assess the temperature of reservoirs and she concluded that Sperchios thermal fields are low-enthalpy hydrothermal systems. Psomiadis et al. (2008) showed that most of the thermal waters come from a mixing process between seawater and various fresh waters from different locations and altitudes. According to Duriez et al. (2008), the distribution of the salinity is independent from the distance to the shoreline in the south-eastern part of Sperchios basin.
The thermal springs of Platystomo and Smokovo areas are known for their curative properties especially for stomach and liver diseases. For some of the thermal springs, chemical analyses comprising major elements, gases and isotopes, together with field studies undertaken by the Greek Institute of Geological and Mineral Exploration (Papastamataki and Leonis 1982; Barnes et al. 1986; Kakavas 1986; Papadeas 1992) gave interesting indications on water properties and origin.
The main objective of this study is the investigation of the origin and chemical composition of the alkaline springs of Platystomo and Smokovo areas. The study is mainly based on the evaluation of major trace elements and environmental isotopes. The chemical characteristics of “Hypati” thermal spring located in the south edge of Sperchios basin are also examined for comparison with the alkaline springs of the study areas. “Hypati” spring exhibits both thermal (32.6 °C) and mineral (TDS > 1,000 mg L−1) character.
Geological and hydrogeological setting
The study areas belong to the north surrounding mountainous region of the Sperchios Graben and are built up by rocks of (a) Subpelagonian zone, (b) Parnassos–Ghiona zone, and (c) Pindos zone (Fig. 1) (Bornovas and Rondogianni-Tsiambaou 1983). The Subpelagonian zone lying in the eastern part of the area consists of Cretaceous age flysh, Mesozoic limestone of Upper Cretaceous and Upper Jurassic age, shale, and cherts. Ophiolitic thrust sheets cover a significant part of the zone. They consist of basic and ultrabasic igneous rocks, such as diabases, peridotites, pyroxenites, gabbros, serpentinites, laves, and tuffs. The peridotites are usually serpentinized. The Parnassos–Ghiona zone in the southernmost part consists of Paleocene flysch as well as limestone and dolomite of Triassic to Lower Jurassic. The Pindos zone covers the western part of the study area. This zone consists mainly of Paleocene age pelitic and psammitic flysch, which is characterized by the intense presence of organic material, as well as limestones and radiolarites of Jurassic to Cretaceous age. The lower part of the Sperchios Graben is covered by unconsolidated sediments of Pleistocene–Holocene age (Fig. 1).
Tectonically the Sperchios Graben is related with the Anatolia fault that passes through the north Turkey enters the Aegean Sea and ends to the Sperchios Graben. The micro-plate of the eastern Mediterranean region proposed by McKenzie (1972) is northwards delimitated by an important extension zone. This zone coincides with the Anatolian fault. Hence, the Platystomo area is characterized by intense neo-tectonic activity with large faults and rift structures. Generally, the deep extensional faults enhance the formation of geothermal activity.
The dominant rift structure of the study area is the E–W large normal faults. Another major group of faults are those of NW–SE direction that represent the tectonic contacts of the different stratigraphic sequenced geological formations (Fig. 1). Thermal spring emergencies are facilitated by faults. Electrical resistivity prospecting suggests that the Thermi spring (PL-T-5) rises through a fault with an altitude of at least 1,000–1,100 m (Kakavas 1986).
The thermal springs of Paleovracha (PA-10), Platystomo (PL-T-5); (PL-K-6); (PL-M-7), Smokovo (SM-2), Soulada (SO-1) issue from recent alluvial deposits that consist of sands and clays with pebbles and gravels. This formation, presenting limited extension and thickness, overlay the eastern Pindos flysch, Danian to Eocene age, with a total thickness of 2,000 m (Kallergis et al. 1970). Pindos flysch consists of fractured thick-bedded sandstones and greywacke with conglomerate, clayey and silty intercalations. Shallow fresh water aquifers have been developed in the conglomerate intercalations. Ekkara (EK-4) and Archani (AR-9) spring issue from peridotites, which belong to the ophiolitic complex and host less productive aquifer. Kaitsa spring (KA-3) issues from limestones, which host a local aquifer.
Analytical procedures and methods
Sampling was undertaken on September 2005. Samples were collected at the outlets of spring, except for PL-T-5 samples that were collected some meters away from the spring using a pump. They were kept in two polyethylene bottles. The first bottle of 0.5 L volume was filtered on-site through 0.45 μm pore size Millipore filters. It was then acidified to pH about 2 with 65 % ultra pure HNO3 and used for major cation analysis, trace element determination, and boron isotopes. The second non-acidified aliquot (1 L) was retained to determine major anions and cations. Temperature (T), electrical conductivity (E.C.), Redox potential (Eh), pH, and sulfites hydrogen (H2S) were measured from untreated samples in situ. Since dissolved oxygen could not be measured, Eh was used as the main tool to identify aerobic and anaerobic conditions. The major cations Ca2+, Mg2+, Na+, and K+ were determined by atomic absorption spectroscopy (GBC Avanta). Trace element determinations and boron isotope analysis were conducted using ICP-MS Perkin Elmer, ELAN 6100. For major cation and trace element determinations, accuracy was controlled using appropriate laboratory standards. For boron isotopes, a standard deviation of less than 1.5 % was determined by repeat analysis of NIST SRM-951 standard (11B/10B = 4.043 ± 0.005). Isotope ratios are reported as δ11Β = [(11Β/10Β)sample/(11B/10B)SRM 951r − 1] × 1,000.
Alkalinity was measured by volumetric titration using bromocresol green-methyl red indicator by Hach® titration kits. Similarly, chlorides (Cl−) were measured using the AgNO3 method by Hach® titration kits while sulfates (SO4 2−), nitrates (NO3 −), and ammonium (NH4 +) were determined using a spectrophotometer (Hach®, DR/4000). Temperature (T), pH, electrical conductivity (E.C.), and redox potential (E h) were determined using an ion/E.C. meter (Consort® C533) with combined electrodes. All analyses were conducted at the Laboratory of Hydrogeology, University of Patras immediately after collection. The charge balance error for all chemical analyses is within a highly acceptance range (±5 %). All chemical calculations, including those for saturation indices and geothermometers, were undertaken using the PHREEQC software package (Parkhurst and Appelo 1999). A sample from Hypati thermal spring (YP-11), which issues from the Pleistocene–Holocene unconsolidated sediments of the south part of Sperchios Graben, was taken for comparison purposes. Additionally, a fresh water sample from flysch conglomerates (PL-X-8) was also taken for comparison purposes.
Groundwater chemistry
Classification of thermal waters
Chemical data for all samples are provided in Tables 1 and 2. As it is shown in Table 1, all water samples of Platystomo and Smokovo areas are alkaline with pH ranging between 7.4 and 11.2. They are characterized by negative values of redox or strong reducing conditions. Some of the samples present high amounts of ammonia (apart from PL-X-8, PL-K-6, EK-4 and KA-3), low concentrations of NO3, high concentration of H2S (apart from PL-X-8, EK-4 and AR-9), and low salt concentrations. Hypati spring shows different chemical characteristics with pH value 6.41, low concentration of ammonia, and high salt concentrations similar to that of all thermal springs in the eastern edge of Sperchios valley (Stahl et al. 1974; Psomiades et al. 2008).
Gas analyses from Platystomo thermal waters (Papastamataki and Leonis 1982; Minissale et al. 1989) show that the N2/O2 ratio values range between 4.3 and 13.1. These values are higher than those of the atmosphere (3.71) indicating the presence of non-atmospheric nitrogen in the thermal water circuits. Radon (222Rn) and Thorium (Th) content is low and varies from 0.5 to 6 Bq kg−1 and from 0.1 to 0.8 Bq kg−1, respectively. Kamena Vourla thermal spring, which is situated at the eastern part of Sperchios Basin, exhibits much higher Radon content (up to 100 Bq kg−1) than Platystomo thermal springs. Hence, a relationship based on Radon and Thorium content of waters of the thermal springs of Platystomo area and Kamena Vourla seems not to exist.
Classification of thermal water was made using Piper diagram. As shown in Fig. 2, thermal springs can be mainly classified in three groups (two major groups and a third one). The first one includes all spring waters hosted in flysch formation, collected from Thermi (PL-T-5), Morfoneri (PL-M-7), Palaiovracha (PA-10), Soulada (SO-1), and Smokovo (SM-2) springs presenting Na–HCO3–Cl water type. This group is characterized by low concentrations in calcium and magnesium about 2.5 and 0.3 mg L−1, respectively. The alignment of the samples on a line (Fig. 2) between (PT-T-5) and (SM-2) depends on their Cl− content. Concerning the water samples, PA-10, PL-T-5, and PL-M-7 the increased chloride concentration (app. 120 mg L−1) in relation to the other samples (app. 20 mg L−1) could possibly be attributed to leachate from local saline deposits in the sediments (see Sect. 5).
The second group includes the samples Klouvio (PL-K-6), Xenia (PL-X-8), and Kaitsa (KA-3) of Ca–Mg–Na–HCO3 water type. This water type could be considered as thermal and fresh water mixture, which is also indicated by the presence of nitrate ions. The PL-K-6 and PL-X-8 samples of the second group exhibit higher calcium and magnesium content related to the PL-T-5 and PL-M-7 samples of the first group, although all these samples originate from Platystomo area. The low Ca and Mg concentration of the samples of the first group could be probably explained by carbonate precipitation (see Sect. 4.3).
The third group includes the most alkaline samples of Archani (AR-9) and Ekkara (EK-4) springs that originated from the ophiolitic complex and presents no dominant cation.
Aqueous geochemistry
The solid line, in Fig. 3a–f, reflects the concentration–dilution characteristic line for seawater, while the dotted line is the linear regression line of the samples. These lines do not converge indicating that for all water samples of the study areas, the ratios of Cl to the elements Na, SO4, B, Ca, and Mg present different values from those of a diluted seawater. All thermal waters are enriched in Na in relation to seawater. This enrichment could be attributed to the weathering of silicate minerals according to the reactions showing in section 4.3. Exception is the YP-11 sample (Hypati spring), which lies on the concentration–dilution characteristic line and presents high salt content. As is shown in Fig. 3, the chemical species for this sample, sea and rain have a common origin indicating that the chemical composition of Hypati spring has influenced from seawater.
The thermal waters are even more enriched in sulfate (Fig. 3b) in relation to seawater. Sulfate could be probably produced by anoxic oxidation of S2− by oxidized forms of metallic cations as Fe3+, thus producing high Fe contents (Andrews et al. 1994). This could actually happen, since samples with higher Fe concentrations correspond to higher sulfate concentrations.
Additionally, sulfate concentrations may be resulted from the oxidation of H2S at, or close to, the thermal water output according to the reaction: H2S + 2O2 → SO4 2− + 2H+ (Valentino and Stanzione 2003). This reaction, which produces two moles of H+ for the oxidation of each mole of H2S, contributes to the lowering of the pH of solution. However, as the pH increases, the above-mentioned process becomes strongly limited.
Sulfate could also be probably produced from the dissolution of the rocks of molassic formations (conglomerates, sandstones). As is shown in Table 1, SO 2−4 concentration and H2S content (1 and 0.1 mg L−1 respectively) in PL-X-8 sample are low compared with the thermal springs PL-T-5 and PL-M-7, which exhibit higher sulfate concentration.
The B/Cl ratio of the samples is enriched compared with that of seawater (Fig. 3c). All samples are aligned between the two end members PA-10 and PL-K-6. The next Fig. 3d, e confirms that Ca/Cl and Mg/Cl ratios, which are completely different from those of seawater, range between the same end members, which belong to the first and second group of the classification of thermal waters. Hence, the chemical composition is a mixture between these end members so that some samples of the alkaline thermal waters of the study area include a fresh water component that masks their initial pure thermal character. This could also mean that some of the thermal springs are in the first stages of interaction with rocks.
The linear regression line of Fig. 3f shows that most of the samples have a constant B/Cl ratio, which corresponds to a value of δ 11B equal to about 10 ‰ and is perpendicular to the line connecting samples with a marine composition. This perfect divergence along with the precedents suggests that thermal water composition is only related with water–rock interaction.
The analyses of oxygen and hydrogen isotopes (Papastamataki and Leonis 1982), shown in Table 3, indicate that the thermal water of Platystomo is closely related to the rainfall in the region.
Additionally, available Tritium data with low to null Tritium content suggest that thermal water of some springs (PL-T-5, PL-M-7) were isolated from atmosphere for many years, while thermal water of other springs (PL-K-6, AR-9) for fewer. This could confirm that the springs PL-T-5 and PL-M-7 belong to the same group as it is already mentioned, while thermal waters are modified by rock–water interaction rainwater, heated in depth, and mixed with fresh water when arriving to the surface. The mixed waters are characterized by higher calcium and magnesium concentrations in relation to the non-mixed waters.
Carbonate system chemistry of the Platystomo-Smokovo areas
The low Ca and Mg content of thermal water imply that any Ca or Mg supply from plagioclase or calcite dissolution has to be removed from the solution.
Two mechanisms make this possible: cation exchange and removal of calcium due to calcite precipitation, and CO2-induced silicate hydrolysis.
Cation exchange processes are described by the equations below (Andrews et al. 1994):
where Χ− denotes the ion exchanger that may be clay minerals.
The above equations result in the continuous removal of calcium that leads to solutions under calcite saturation. Table 4 compares the saturation index values for the various spring waters. Some of the samples are undersaturated in respect to calcite, aragonite, and dolomite.
Carbon dioxide diffusion into the aquifer of thermal water is probably attributed to the groundwater content in CO2 (approximately 1.000 mg L−1) of Hypati spring (YP-11), which is located in the vicinity of the area. The presence of recently active and normal EW faults would assist this diffusion. Gartzos and Stamatis (1996), based on carbon stable isotope data, suggested that CO2 in the wider area is liberated from metamorphic reactions (decarbonisation) of carbonate at depth, as it is the case for many areas worldwide (Bailey 1989; Ceron and Pulido-Bosch 1999). Silicate hydrolysis of plagioclases (albite, anorthite and anaclite) claims consumption of CO2, according to the reactions below (Andrews et al. 1994):
In saturation conditions, the release of calcium from the hydrolysis of anorthite can lead to its removal from the solution in the form of calcite according to the follow equation:
Both mechanisms described above lead to waters poor in calcium and magnesium and rich in sodium, as is the case for the most springs of the study area. The most probable mechanism seems to be the last, although due to the presence of large amounts of calcite in the flysch sediments, the first mechanism of calcium removal could not be excluded.
Trace elements
Trace elements were determined to investigate the quality and origin of thermal waters (Table 2). All trace elements, except boron and strontium, present low concentrations, below the maximum admissible limits of potable water standards (E.U Council 1998, European Council Directive 98/83/EC on the quality of water intended for human consumption). Several redox sensitive trace elements including Mn, U, Cr, As, Se, and Pb exhibit null concentrations. However, in pure reducing conditions, as for example in PL-T-5 and PL-M-7 samples, Mn, U, Cr, As, Se, and Pb concentrations show higher values than those of PL-K-6 and PL-X-8, which comprise mixed waters. Iron concentration varies between 10 and 145.5 μg L−1 for thermal waters, while for fresh waters (PL-X-8) it is 28 μg L−1 approximately. In reduced environments, iron is found in the form of soluble Fe2+ and in oxidized environments the dominant form is the increased valence of insoluble form, Fe3+, as also for the previously mentioned metals (Hem 1988). Iron shows increased concentrations in water samples with low Eh.
Strontium and Barium elements are related with calcareous minerals. They are also found in K-feldspars and some aluminosilicate minerals, from where they pass to the waters after incongruent dissolution (Edmunds et al. 2002). These elements show increased concentrations in fresh water PL-X-8 and the mixed water PL-K-6. Rubidium and Lithium are also found in feldspars and aluminosilicate minerals and their dissolution in water is facilitated by temperature. In the Platystomo and Smokovo thermal waters, Lithium has higher concentrations in hot than in cold waters. Edmunds and Smedley (2000) used this element as a reliable indicator of groundwater residence time. It has been also used by Carrillo-Rivera et al. (1996) to discriminate between thermal and shallow groundwaters. According to this consideration, thermal waters of (PL-K-6), (PL-X-8), and (EK-4) samples with low Li concentration should follow a short circuit before emerging to the land surface. This is consistent for the first two samples that comprise fresh water and consequently may be valid for the third (EK-4). Copper, Lead, and Zinc concentrations do not show systematic trends and it appears that they are neither related to residence time nor geothermal influence.
The geochemistry of boron element
Chemical and isotopic studies suggested that most of the dissolved boron in natural waters derived from rocks dissolution depends on the nature of host rocks (Palmer 1991). Boron is one of the most mobile cations during hot fluid–rock interactions (Ellis and Mahon 1967). It exhibits high concentrations in seawaters, thermal waters and wastewaters due to the detergents and consequently all above may contribute to the groundwater composition of an aquatic system. In silicicate material groundwater, unaffected by seawater intrusion and wastewater, the boron content varies as happens in Platystomo and Smokovo areas. Pennisi et al. (2000) reported that the alkaline (pH 7.2–8) and cold (T 12–18.2 °C) groundwater from flysch-filled basement depressions of the western and south western flank of Mt. Etna have a boron concentration of about 0.5–1.45 mg L−1. Andrews et al. (1994) reported much higher concentration of boron that varies between 1.4 and 6.2 mg L−1 in the alkaline (pH 7.25–9.25) and thermal (T 27–39 °C) groundwater of the continental intercalary aquifer of the Irhzer Plain at Niger. In Platystomo and Smokovo areas, boron content of thermal waters varies between 60 μg L−1 and 10 mg L−1. High concentrations (2–10 mg L−1) present the samples of the first group (in flysh hosted thermal springs), while lower concentrations (60–1,000 μg L−1) exhibit the mixed flysh waters and the spring waters issue from ophiolitic complex. Figure 4a shows that the boron content of the thermal water of Platystomo and Smokovo areas is increases with the increase in temperature.
In most groundwaters, boron occurs in the forms of Β(ΟΗ)3 and Β(ΟΗ) −4 . In groundwater with pH values less than 8.8 (25 °C), the undisassociated boric acid Β(ΟΗ)3 dominates and boron behaves as an inert element. Boron isotope ratios vary by more than 80 ‰ in geological materials (Palmer and Swihart 1996). From sediments hosted basins δ11B varies between−10.1 and 23.3 ‰, (Palmer 1991). Siliciclastic sediments typically have low δ11Β values (–15 to +5 ‰), (Palmer and Swihart 1996). Pennisi et al. (2000) reported δ11Β values that vary between 4.3 and 9 ‰, corresponding to low salt content (Cl 95–140 mg L−1) alkaline cold groundwater from flysch-filled basement depressions of the western and south western flank of Mt. Etna. In similar materials at the Tecopa Hot Springs, waters contain 8–10 mg L−1 boron and have δ11Β values that vary between−4.5 and−5 ‰ (Larsen et al. 2001). The seawater δ11Β values approach 40 ‰ (Spivack and Edmond 1986), while the sea salt aerosols also, near ocean have a similar value. Thermal fluid of continental geothermal areas has low δ11B values (Yellowstone National Park, δ11B varies between−9.3 and 4.4 ‰, Palmer and Sturchio 1990) according to the isotopic composition of the source rocks. Marine carbonate is characterized by the dominance of the heavier isotope in relation to most igneous and sedimentary rocks, because boron is incorporated into marine water during carbonate precipitation. Between boron isotopes and under the condition that boron is in the form of Β(ΟΗ)3 and Β(ΟΗ) −4 , the latter is enriched in the lighter isotope. Kakihara et al. (1977) computed that at 25 °C the enrichment factor is 1.0194. Then during sorption reactions or precipitation that removes Β(ΟΗ) −4 from the water, B(OH)3 is enriched in the heavier isotope.
For the Platystomo and Smokovo areas, increase of temperature seems to contribute to the fractionation of boron isotopes resulting in the enrichment of the heavier isotope (Fig. 4b). Data processing showed that a similar process takes place, considering the evolution of the temperature versus the H2BO3/H3BO3 ratio. A general negative trend suggests clearly that H3BO3 specie enhanced relative to the H2BO3 − along with the temperature increases. The sample YP-11 is differentiated from this general trend. That could be attributed to the completely different nature of the rocks hosting the thermal waters and the fact that probably, YP-11 is influenced by volcanic phenomena as many authors propose (Marinos and Frangopoulos 1973; Garagunis 1978; Gartzos and Stamatis 1996; Fyticas et al. 1984). The pH strongly affects the H2BO3−/H3BO3 ratio of thermal waters. In Fig. 4d all samples, included rain, sea and YP-11, lie on the regression line that exhibits a high correlation coefficient, which is up to 98 %. The evolution of this ratio is independent from geological conditions, unlike boron isotope ratio, which is independent from thermal water pH, and H2BO3−/H3BO3 ratios (Fig. 4e, f).
Geothermometry
The geothermometers are important tools in estimating the temperature of the thermal water reservoir. They are based on the assumption that chemical equilibrium between minerals in the reservoir rocks and groundwater has been attained. After a good methodology followed by Minissale et al. (1997), the temperature is first calculated using geothermometers for known temperature reservoir. The coincidence of measured and calculated temperature reflects attainment of full equilibrium in the reservoir. In this case, the geothermometer could be used for estimation of the temperature of similar geothermal areas. The above-described methodology cannot be used for the estimation of the reservoir temperature of Platystomo and Smokovo areas and therefore, the state of the saturation of thermal water to different minerals was computed. Relationships between the measured temperature in the outlet of the springs and the ion composition of the waters have been also taken into consideration. Data processing (Table 4) showed that except calcite, aragonite, dolomite, chalcedony, and quartz with which thermal waters are almost in chemical equilibrium, for the rest of the minerals the saturation indices show undersaturated waters.
Figure 5 shows the dependence between temperature and the chemical species SiO2, and Na. These positive correlations clearly indicate that the dissolved salts of the non-marine thermal waters are derived from water–rock interactions (Vengosh et al. 2002) confirming the proposed suggestions of Sect 4.2 regarding the origin of thermal waters. Then, the reservoir temperature was estimated using the selected quartz, chalcedony, and Na, K, Ca geothermometers. For the sample EK-4 with negative saturation indices in Chalcedony and Quartz the corresponding geothermometers could not be applied.
Table 5 shows that using the 4, 5 and 6 geothermometers, the flysch hosted thermal waters exhibited similar calculated temperatures of about 100 °C. For the sample PL-K-6 of mixed water, the geothermometers 4 and 5 gave a shifted temperature, about 30–40 °C higher than the precedents. This different behavior can be explained by the poor sensitivity of the Na/K ratio to mixing process. A same shift is shown for samples AR-9, EK-4, and KA-3 originated from the ophiolitic complex. The geothermometer 6 showed more homogenized temperatures for all samples ranging from 100 to 110 °C. The Chalcedony and Quartz geothermometer showed the lowest temperatures of the thermal water reservoir ranging from 70 to 100 °C. However, the Quartz geothermometer cannot be considered as a reliable estimation because all samples are saturated to Quartz, while the chalcedony temperatures are similar to the emergent temperatures. This is typical of convective waters in fault systems in normal thermal gradient areas.
Conclusions
The alkaline thermal water of the Platystomo–Smokovo area are characterized by strong reducing conditions that are related with high amounts of ammonia, low concentrations of NO3, and high concentration of H2S. They can form two major groups: the first with thermal waters of a Na–Cl–HCO3 type from flysch formations correspond to the most pure ones. The second with samples of Ca–Mg–Na–HCO3 water type comprise mixed water from flysch as well as the samples from the ophiolitic complex. Scatter plot diagrams show that the thermal water composition is mainly developed by rock–water interactions. Rock–water interactions are enhanced under the presence of CO2 that is probably originates from metamorphic reactions (decarbonisation) of carbonate at depth, or through the recently active east–west fault systems from the Hypati (YP-11) spring located in the geothermal field of the south part of Sperchios Basin, where the groundwater content in CO2 is about 1.000 mg L−1. It is further suggested that the thermal waters of Platystomo and Smokovo areas are modified by rock–water interaction rainwater, heated in depth, and mixed with fresh water when arriving to the surface. A main characteristic of these waters is the significant low amounts in earth alkaline that may be explained by silicate hydrolysis of plagioclases. Although the concentrations of trace elements are strongly low until null, for the redox sensitive trace elements, this is related to the Eh conditions. Lithium content could be differentiated in thermal waters of a short circuit. Boron behavior depends on the temperature and the pH of the water. Although is not very clear, boron isotopes show enrichment in the lighter member when temperature increases. The decrease of the H2BO3 −/H3BO3 ratio relative to the temperature suggests enrichment of H3BO3 specie with the temperature increases. Divergences of the YP-11 sample from the above-described general trend confirm that this sample origins from different thermal water reservoirs. The temperature of the thermal water reservoir was computed with the use of selected geothermometers. The selection was based on the study of the element concentration relative to the temperature evolution as well as to the study of the saturation indices of minerals. The selected Chalcedony, Quartz, and Na, K and Ca geothermometers showed that the reservoir temperature ranges from 100 to 110 °C.
References
Andrews N, Fontes JC, Aranyossy JF, Dodo A, Edmunds MW, Joseph A, Travi Y (1994) The evolution of alkaline groundwaters in the continental intercalaire aquifer of the Irhazer Plain, Niger. Water Resour Res 30(1):45–61
Bailey DK (1989) Carbonate melt from the mantle in the volcanoes of South–East Zambia. Nature 338:415–418
Barnes I, Leonis C, Papastamataki A (1986) Stable-isotope tracing of the origin of CO2 discharges in Greece. In: Morfis A, Paraskevopoulou P (eds) Proceedings of the 5th international symposium on the underground water tracing, pp 29–43
Bornovas J, Rondogianni-Tsiambaou Th (1983) Geological map of Greece, 1:500 000. Institute of Geology and Mineral Exploration, (IGME), Athens
Carrillo-Rivera JJ, Cardona A, Moss D (1996) Importance of the vertical component of groundwater flow: a hydrogeochemical approach in the valley of San Luis Potosi. Mexico J Hydrol 185:23–44
Ceron JC, Pulido-Bosch A (1999) Geochemistry of thermomineral waters in the overexploited Alto Guadalentin aquifer (South–East Spain). Wat Res 33(1):295–300
Dotsika E (1991) Use of the SO4−H2O isotopic geothermometer for high temperature aquifers under potential marine influence: geothermal systems from Greece. Ph.D Thesis, Universite Paris-Sud, Orsay, p 184
Duriez A, Marlin C, Dotsika E, Massault M, Noret A, Morel JL (2008) Geochemical evidence of seawater intrusion into a coastal geothermal field of central Greece: example of the Thermopylae system. Environ Geol 54:551–564
Edmunds WM, Smedley PL (2000) Residence time indicators in groundwater: the East Midlands Triassic sandstone aquifer. Appl Geochem 15:737–752
Edmunds WM, Carrillo-Rivera JJ, Cardona A (2002) Geochemical evolution of groundwater beneath Mexico City. J Hydrol 258:1–24
Ellis AJ, Mahon WAJ (1967) Natural hydrothermal systems and experimental hot water/rock interactions (Part II). Geochim Cosmochim Acta 31:519–531
E.U Council (1998) European Council Directive 98/83 on the quality of water intended for human consumption. Official Paper of the European Communities
Fournier RO (1977) Chemical geothermometers and mixing models for geothermal systems. Geothermics 5:41–50
Fournier RO (1979) A revised equation for the Na–K geothermometer. Geoth Res Counc Trans 3:221–224
Fournier RO, Truesdell AH (1973) An empirical Na–K–Ca geothermometer for natural waters. Geochim Cosmochim Acta 37:1255–1275
Fyticas M, Innocenti F, Manetti P, Mazzuoli R, Peccerillo A, Vallari L (1984) Tertiary to Quaternary evolution of volcanism in the Aegean region. In The Geological Evolution of the Eastern Mediterranean, Geological Society of London Special Publication No 17:687–699
Garagunis CN (1978) Hydrogeologische Untersuchungen der Thermal und Mineralquellen im östlichen Mittelgriechenland. Steir Beitr Hydrogeol 30:5–82 Graz
Gartzos E, Stamatis G (1996) Genesis of the thermal springs of the Sperchios graben, Greece. N. Jb. Geol Paläont Mh. H. 2:91–115
Hem J (1988) Stydy and Interpretation of the Chemical Characteristics of Natural Water, 4th edn. Geological Survey Water-Supply Paper 1473, U.S.G.S., p264
Kakavas N (1986) Explanation of Platystomo thermomineral spring function mechanism. Geol. Geoph. Res., Spec. iss., 145–150, I.G.M.E., Athens (in Greek)
Kakihara H, Kotaka M, Satoh S, Nomura M, Okamoto M (1977) Fundamental studies on the ion exchange separation of boron isotopes. Bull Chem Soc Jap 50:158–163
Kallergis G, Koch K, Nicolaus J (1970) Geological map of Greece (scale 1:50000). Spercheias sheet, I.G.M.E., Athens
Larsen D, Swihart HG, Xiao Y (2001) Hydrochemistry and isotope composition of springs in the Tecopa basin, southeastern California. USA Chem Geol 179:17–35
Marinos P, Frangopoulos I (1973) La source thermominerale d’Hypati (Greece Central). Etude Hydrogeologique, Hydrodynamique, Geochimique et Geotechnique de la source et de sa region environmante, decouverte d’un nouveau foyer thermomineral. Ann Geolog des Pays Hell 25:105–214 (in Greek)
McKenzie D (1972) Active tectonics of the Mediterranean region. Geoph J R Astron Soc 30:109–185
Minissale A, Duchi V, Kolios N, Totaro G (1989) Geochemical characteristics of Greek thermal springs. J Volcanol Geoth Res 39:1–16
Minissale A, Duchi V, Kolios N, Nocenti M, Verrucchi C (1997) Chemical patterns of thermal aquifers in the volcanics isldands of the Aegean arc, Greece. Geothermics 26(4):501–518
Palmer MR (1991) Boron-isotope evidence of Helmahera arc (Inddonesia) lavas: Evidence for involvement of the subducted stab. Geology 19:215–217
Palmer MR, Sturchio NC (1990) The Boron isotope systematics of the Yellowstone National Park (Wyoming) hydrothermal system: A reconnaissance. Geochim Cosmochim Acta 54:2811–2815
Palmer MR, Swihart GH (1996) Boron isotope geochemistry: An overview. In: Grew ES, Anovitz LM (eds) Boron: Mineralogy, Petrology and Geochemistry. Rev Mineralogy 33:2811–2815
Papadeas G (1992) Geological-Tectonic-Hydrochemical-Geothermic investigation in the Spercheios Fthiotis basin Report, I.G.M.E, Athens (in Greek)
Papastamataki A, Leonis I (1982) Geochemical exploration of geothermal fields, No IV, Ypati area Report, I.G.M.E, Athens, 18p (in Greek)
Parkhurst DL, Appelo CAJ (1999) User’s guide to PHREEQC (version 2). US Geol Surv Water Resour Inv Rep 99–4259:312p
Pennisi M, Leeman PW, Tonarini S, Nabelek P (2000) Boron, Sr, O, and H isotope geochemistry of groundwaters from Mt. Etna (Sicily)–hydrologic implications. Geochim Cosmochim Acta 64(6):961–974
Psomiadis D, Dotsika E, Poutoukis D, Albanakis K, Karidakis G, Metaxas A, Raco B, Zisi N, Tzavidopoulos I (2008) Geochemical study of the geothermal field of the Sperchios area, Greece.In: Proceedings of 8th International Hydrogeological Congress of Greece, vol I, pp 361–370
Spivack A, Edmond JM (1986) Determination of boron isotope ratios by thermal ionization mass spectrometry of the dicesium metaborate cation. Anal Chem 58:31–35
Stahl W, Aust H, Dounas A (1974) Origin of artesian and thermal waters determined by oxygen, hydrogen and carbon isotope analyses of water samples from Sperkios valley, Greece. J I A E A 182(15):317–339
Taymaz T, Jackson J, McKenzie D (1991) Active tectonics of the north and central Aegean Sea. Geophys J Int 106:433–490
Truesdell AH (1975) Summary of section III. Geochemical techniques in exploration. Proceedings of the 2nd UN Symposium on the Development and Use of Geothermal Energy, San Francisco, CA, 20–29 May 1975, pp liii–lxxxix
Valentino MG, Stanzione D (2003) Source processes of the thermal waters from the Phlegraean Fields (Naples, Italy) by means of the study of selected minor and trace elements distribution. Chem Geol 194:245–274
Vengosh A, Helvaci C, Karamanderesi I (2002) Geochemical constraints for the origin of thermal waters from western Turkey. Appl Geochem 17:163–183
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lambrakis, N., Zagana, E. & Katsanou, K. Geochemical patterns and origin of alkaline thermal waters in Central Greece (Platystomo and Smokovo areas). Environ Earth Sci 69, 2475–2486 (2013). https://doi.org/10.1007/s12665-012-2073-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-012-2073-5