Abstract
Radon is a radioactive hazardous and ubiquitous gas. It has been recognized to be one of the major contributors to natural radiation even causing lung cancer if present at enhanced levels. There are large variations in data available in the literature for radium content and radon exhalation rates of various materials. It is a well-documented fact that radon exhalation from the ground surface depends upon a number of parameters such as soil grain size, soil porosity and radium content. For this purpose, in this study the so-called can technique has been used to measure radium content and exhalation rates of radon in soil samples collected from different places of Aligarh, Etah and Mathura districts of Uttar Pradesh—a province in northern India. These districts lie within the subtropical region of the Indo-Gangetic plains. The values of effective radium content are found to vary from 8.11 to 112.83 Bq kg−1 with a mean value of 33.21 Bq kg−1 and a standard deviation of 28.15. The values of mass exhalation rates of radon vary from 0.76 × 10−6 to 15.80 × 10−6 Bq kg−1 day−1 with a mean value of 4.21 × 10−6 Bq kg−1 day−1, while the surface exhalation rates vary from 1.97 × 10−5 to 41.03 × 10−5 Bq m−2 day−1 with a mean value of 10.93 × 10−5 Bq m−2 day−1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Radium (226Ra) and radon (222Rn) mainly come from naturally occurring uranium (238U), which is present in all types of rocks, building materials and soils in parts per million (ppm). The global average of uranium content in the earth’s crust is about 4 ppm. Radium, being a member of uranium radioactive series, is present everywhere in the earth’s crust; therefore, radon, which is the daughter product of radium, is also found everywhere in varying levels. Radium mainly enters the body through the food chain. Radium, being chemically similar to calcium, tends to follow it in metabolic processes and becomes concentrated in bones. The alpha particles given off by radium and radon bombard the bone marrow and destroy tissues that produce red blood cells. It may cause bone cancer. The radium content of a sample also contributes to the level of environmental radon as radon is produced from 226Ra through α-decay. Higher values of 226Ra in soil contribute significantly to the enhancement of indoor radon. The main contributors to indoor radon concentrations are soil gas emanating from the ground beneath a dwelling (Åkerblom et al. 1984), and the materials from which the dwelling is constructed (Bossew 2003; Denman et al. 2007). If a uranium-rich material lies close to the surface of the earth, there can be higher radon exposed hazards (Archer et al. 1973; Sevc et al. 1976; UNSCEAR 1993). Thus, radon level in dwellings depends upon the radium content of the ground and building materials, also on how many radon atoms it contains. Texture and size of the grains, ambient temperature and pressure affects the indoor radon too (Fleischer 1980, 1982; Tanner 1980; Anderson et al. 1983).
Radon gas continuously undergoes radioactive decay spontaneously into four solid short-lived radionuclides, such as, 218Po, 214Pb, 214Bi and 214Po, out of which polonium isotopes are α-emitters. Entry of radon into a dwelling from the soil gas is influenced by a number of parameters (Arvela and Winqvist 1989). These include the concentration of radon in the soil gas itself, soil moisture, soil permeability, environmental and meteorological conditions in the vicinity of the dwelling (Singh et al. 2005), together with the nature of the physical entry routes into the dwelling. In addition to the natural daily cycle, longer periodicities are evidently related to other causes such as external temperature (Miles 2001; Karpińska et al. 2005), barometric pressure, wind direction (Miles 2001), rainfall (Mose et al. 1991), occupancy patterns (Paridaens et al. 2005), seismic events (Crockett et al. 2006) and tidal effects, including ocean tidal loading and earth tides (Groves-Kirkby et al. 2006b). The underlying geology (Gillmore et al. 2005; Miles and Appleton 2005) has also been identified as introducing additional temporal modulation to the soil gas radon flux. In general, the ultimate driving influences are the absolute pressure difference between the radium source and the earth’s surface, and the under-pressure caused by temperature differences between the dwelling’s interior and the external atmosphere, the ‘stack effect’ (Eaton and Scott 1984). The indoor radon level depends on the intrinsic radium activity of the materials in question (Bossew 2003) and their bulk permeability to radon gas (Keller et al. 2001), with the most significant parameters being the effective diffusion range, the effective surface area for emission (Abu-Jarad et al. 1980) and the superficial structure (Carrera et al. 1997).
As with soil gas, concentration of radon levels attributable to building materials also vary significantly from dwelling to dwelling and from area to area. In extreme cases, they have been identified as contributing up to 85% of the total radon input. Unlike soil gas, however, radon emanating from walls and ceilings cannot be significantly reduced by sub-slab depressurization. These studies indicated that the total indoor radon concentration within a dwelling could be represented by two main components, radon entering the house entrained in the soil gas and radon generated by the building materials of the dwelling. Although building materials can contribute a significant component of the overall radon levels, the nature of the sub-slab depressurization and impermeable barrier mitigation approaches ensure that they only effectively attenuate radon originating from the ground (Naismith et al. 1998; Groves-Kirkby et al. 2006a). The influence of building materials on indoor radon concentrations is thus well recognized.
Radon exhalation from building materials varies with their type and origin. It is well known that granite exhales more radon than other building materials due to presence of relatively large uranium content in its natural formation (Durrani and Ilic 1997). The knowledge of radon exhalation rate from building materials may enable the estimation of indoor radon levels. Such measurements have been the subject of many studies (Abu-Jarad et al. 1980; Mustonen 1984; Savidou et al. 1996; Durrani and Ilic 1997; Al-Jarallah 2001; Al-Jarallah et al. 2001).
Inhalation of 222Rn and its α-emitting progeny 218Po and 214Po present health hazards that are believed (Darby et al. 2001) to provide the major part of radiation dose received by the respiratory system and deposited within the lungs (NBS 1980; Durrani and Ilic 1997). This α-radiation damages the sensitive inner cells of the lungs, increasing the risk of lung cancer. A direct consequence of the trapping of radon and its progeny in the lungs has been the association between enhanced levels of indoor radon and increased risk of lung cancer (BEIR 1999; Darby et al. 2005; Krewski et al. 2005; Bayyson et al. 2004). It is estimated that the annual mortality from radon exposure in the dwellings in Europe represents 9% of all deaths from lung cancer, and 2% of all cancer deaths (Darby et al. 2005). The dose due to inhaled radon progeny accounts for more than 50% of the total radiation dose to the public from natural sources (UNSCEAR 1994). Several studies have demonstrated a link between high radon levels in indoors and a raised incidence of lung cancer among dwellers (Lubin and Boice 1997; Denman and Phillips 1998; Kreienbrock et al. 2001; Darby et al. 2001; Krewski et al. 2005).
Numerous studies have been carried out to determine the radium equivalent activity of soil samples in many countries (Ibrahiem et al. 1993; Ibrahim 1999; Sroor et al. 2001; Singh et al. 2003; Al-jundi et al. 2003; Mireles et al. 2003) and quite a number of reports on radionuclides content in building materials are available (Todorovic et al. 1994, 2000; Popovic et al. 1996, 2000). However, comparatively much less work has been done on radon exhalation from ground surface in tropical (Ferry et al. 2002; Guo et al. 2004; Böllhöfer et al. 2006) and particularly subtropical regions.
Radon exhalation is a complex phenomenon depending upon a number of parameters such as radium content in soil, soil morphology, soil moisture, soil grain size, temperature, atmospheric pressure, rainfall and vegetation (Tanner 1964, 1980). Such studies can be useful for the assessment of public radiation dose and performance of epidemiology as well as for keeping reference data records, to ascertain changes in the environmental radioactivity due to nuclear, industrial and other human activities (Singh et al. 2009).
With the above important points in mind, a study was undertaken for the assessment of natural distribution of radium content and radon exhalation rates (both surface exhalation and mass exhalation rate) of the soil samples collected from the northwestern part of the subtropical regions of the Indo-Gangetic plains. These regions are located in the districts of Aligarh, Mathura and Etah situated in northwest Uttar Pradesh. These regions have relatively recent alluvial soils. Practically no data are available for radium contents of soils from these regions.
Experimental methods
Geology of the study area
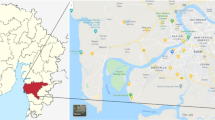
The area under study is a part of the Indo-Gangetic or Ganga plain and lies in the sub-tropical climate zone (Umar and Ahmad 2000). The details of the geology of the region are also reported elsewhere (Khan et al. 2011b). The Aligarh, Etah and Mathura districts of the state of Uttar Pradesh in the northern part of India (Fig. 1) are, respectively, situated between 27°53′24″N latitudes and 78°3′36″E longitudes, 27°18′ to 28°2′N latitudes and 78°11′ to 79°17′E longitudes, and 27°41′N latitudes and 77°41′E longitudes.
The State of Uttar Pradesh has an area of about 240,928 km2. It extends from 23°52′15″ to 30°25′5″N latitude and 77°5′36″ to 84°38′10″E longitude and is characterized by rock formations ranging in age from the Archean (the Bundelkhand Graniticgneisses) to the recent (the Ganga alluvium).
The Gangetic Plain, which dominates the landscape and nearly covers three-fourths of the geographical area of the state, lies between the rocky Himalayan belt in the northern and southern hilly tract comprising mainly Pre-Cambrian rocks. Flexing of the Indian lithosphere in response to the compressive forces due to collision and thrust fold loading produced the Gangetic Plain foreland basin. It is filled with recent alluvial sediments, which are at places more than 1,000 m thick and contain an amalgam of sand, silt and clay in varying proportions.
The southern hilly tract is roughly parallel to the Ganga–Yamuna lineament. The tract is underlain by granitic complex in the Bundelkhand region and Sonbhadra. It is overlain by rocks of Mahakoshal (Bijawar) and Vindhyan Supergroup. The younger rocks comprise coal-bearing Gondwana rocks in the southwestern area of Sonbhadra and basaltic rocks in the southern part of Lalitpur. The granitic complex is considered to be a potential for the search of metallic minerals such as copper, lead, zinc, molybdenum, gold and nickel, Uranium and Platinum group of elements. The overlying sediments of Mahakoshal (Bijawar) and associated iron formation show a potential for the search of copper, uranium and gold in Lalitpur, and andalusite, sillimanite, gold, calcite, marble and clay in Sonbhadra. The lower Vindhyan sediments of Sonbhadra contain deposits of cement-grade limestone, flux-grade dolomites and building stone, and are also a potential for the search of placer gold and other metals, while the Upper Vindhyan sandstones are suitable for making decorative slab/tiles. Deposits of silica sands and bauxite are available in Allahabad and Chitrakoot districts.
Sample collection and processing
In the present investigations, “sealed can technique” or “accumulation chamber” technique (Tuccimei et al. 2006; Sharma et al. 2003; Petropoulos et al. 2001; Chau et al. 2005; Hafez et al. 2001; Righi and Bruzzi 2006) was used to study the radium content and radon exhalation rates. The material of interest was enclosed in a sealed cylindrical can (Abu-Jarad et al. 1980; Jonassen 1983; Samuelsson and Pettersson 1984; Abu-Jarad 1988; Somogyi 1990; Ramola and Chaubey 2003). The samples were collected by the grab sampling method from different places of the districts of Aligarh, Etah and Mathura of Uttar Pradesh province in northern India. The experimental arrangement is shown in Fig. 2. A dried and sieved sample (100 g) was placed at the bottom of a cylindrical sealed can of 10-cm height and 7-cm diameter. The mouth of the cylindrical can was sealed with a cover and fitted with LR-115 type-II plastic track detectors at the top inner surface (Abu-Jarad et al. 1980; Khan et al. 1992; Mahur et al. 2008). The detector records the tracks of α-particles emitted by radon gas produced through the α-decay of radium contents of the samples. The detectors were exposed for a period of about 3 months. After exposure, the detectors were retrieved and etched for 2 h in 2.5 N NaOH solution maintained at a temperature of (60 ± 1)°C in a constant temperature water bath to reveal the tracks. The etching was carried out to reduce the thickness of the LR-115 detectors to about 5 μm (Somogyi 1986). Subsequently, α-tracks were counted using an optical microscope at a magnification of 100×.
Theoretical considerations
The track density ρ (in track cm−2) is related to the radon activity concentration C Rn (in Bq m−3) and the exposure time T by the formula:
where K (=1/30 tracks cm−2 day−1/Bq m−3) is the sensitivity factor of LR-115 detector with an uncertainty of about ±15% (Somogyi 1986). The value of K depends on the height and radius of the measuring cylindrical can (Somogyi 1986). An effective equilibrium (about 98%) for radium–radon members of the decay series is reached in about 30 days (>7 half-life of 222Rn and 220Rn) so that the daughter products of 226Ra up to 210Pb and of 228Th up to 208Pb achieve equilibrium with their respective parent radionuclides (Singh et al. 2003). Once the radioactive equilibrium is established, one may use the radon alpha analysis for the determination of steady-state activity of radium. The activity of radon will increase with time T after closing of the cylindrical can, according to the relation:
where C Ra is the effective radium content of the sample. Since a plastic track detector measures the time-integrated value of the above expression, i.e., the total number of alpha disintegrations in unit volume of the cylindrical can with a sensitivity K during the exposure time T, hence the track density observed is given by:
where T e denotes the effective exposure time given by:
Effective radium content and radon exhalation rates
The effective radium content of the solid sample can be calculated by the formula (Azam et al. 1995; Mahur et al. 2008; Khan et al. 2011a):
where M is the mass of the solid sample in kg, A is the area of cross section of the cylindrical can in m2 and h is the distance between the detector and top of the solid sample in meters.
The mass exhalation rate of the sample for release of the radon can be calculated by using the expression (Khan et al. 2011a):
The surface exhalation rate of the sample for release of radon can be calculated by using the expression (Khan et al. 2011a):
Alpha index
Several indexes dealing with the assessment of the excess α-radiation due to radon inhalation originating from building materials (called ‘‘alpha indexes’’ or ‘‘internal indexes’’) have been developed (Stoulos et al. 2003). The alpha indexes were determined using the following formula:
where C Ra is the effective 226Ra content (Bq kg−1) in the building material. When its value exceeds 200 Bq kg−1, it is possible that the radon exhalation from this material could cause indoor radon concentrations exceeding 200 Bq m−3. On the contrary, when its value is below 100 Bq kg−1, it is unlikely that the radon exhalation from the building materials could cause indoor radon concentrations exceeding 200 Bq m−3 (Nordic 2000).
Results and discussion
Table 1 depicts the values of effective radium content and the mass and surface exhalation rates of radon for soil samples collected from different regions of Aligarh, Etah and Mathura districts of Uttar Pradesh State in northern India. It is seen that the value of effective radium content in collected samples varies from 8.11 to 112.83 Bq kg−1 with a mean value of 33.21 Bq kg−1 and a standard deviation of 28.15.
Radon flux density (i.e., 222Rn exhalation rate) depends upon a number of parameters that behave in a stochastic and independent fashion, such as the radioactive disintegration of 226Ra to produce radon, the direction of recoil of radon in the grain, the interstitial soil moisture condition in the vicinity of the ejected radon atom and its diffusion in the pore space (Lawrence et al. 2009). A series of radon exhalation measurements, including the present one, have demonstrated that radon flux density distribution is better represented by a log-normal distribution than by a normal distribution (Böllhöfer et al. 2006). This behavior indicates that the random variables involved in radon exhalation interact in a multiplicative rather than an additive manner.
The radon exhalation rates measured in terms of mass and area of soil samples are found to vary from 0.76 × 10−6 to 15.80 × 10−6 Bq kg−1 day−1 with a mean value of 4.21 × 10−6 Bq kg−1 day−1 and 1.97 × 10−5 to 41.03 × 10−5 Bq m−2 day−1 with a mean value of 10.93 × 10−5 Bq m−2 day−1.
It is noteworthy from Table 1 and also Fig. 3 that the effective radium content of soil samples is least in Etah District (8.11 Bq kg−1) but highest in Aligarh District (112.83 Bq kg−1). The values of radium content in soils are higher in the regions of Aligarh (which is a part of the Indo-Gangetic Plain and situated in the Doab of Ganga and Yamuna rivers) in comparison with those from Etah and Mathura districts. The soil in this region is laterite that is a fine-grained soil created from weathering of rocks. Such soils contain high concentrations of iron oxides, iron hydroxides and high uranium content (Lawrence et al. 2009). Its high uranium content is reflected by the high radium content, and the soil porosity plays an important role in radon exhalation. Thus, it is possible to analyze the present data to obtain a relation between radium content and radon exhalation rates.
A relative comparison of variation of mass exhalation and surface exhalation rates of radon with radium content of soils has been shown in Figs. 4 and 5. It is seen that there is a good correlation between them. It may be observed that the radon exhalation rates correspond to the estimated values of radium content.
Table 2 shows the values of alpha index for soil samples from the various regions. There are a number of indexes dealing with the estimation of alpha-radiation due to the inhalation of radon and its short-lived decay products present in the natural environment. Since all the values of alpha indexes are much less than unity, it means that the soils from these regions cannot produce dangerous levels of indoor radon when used as building materials.
The values of effective radium content presented in this paper for soil samples are much lower than those reported for sand samples by Mahur et al. 2008. The maximum values of effective radium content in the soil samples studied are also below the maximum permissible value of 370 Bq kg−1 recommended by the Organization for Economic Cooperation and Development (OECD 1979). The radium contents in these soil samples do correspond with the values for soils from Kangra District (Himachal Pradesh) reported by other researchers (Singh et al. 2002; Sharma et al. 2003).
Conclusions
Radon exhalation rates (both the mass and surface exhalation rates) have been measured successfully using LR-115 plastic track detectors by the sealed can technique. The regions under study fall in the sub-tropical climate zone of the Indo-Gangetic Plains where the soil has younger alluvial sediments and contains an amalgam of sand, silt and clay in varying proportions.
The radium distribution is found to be heterogeneous as the radium content in the soil samples varies from region to region in the same district. Its value is larger in samples from Aligarh, lesser in samples from Mathura and least in the samples from Etah District. The overall average value of radium content in the soil samples from these districts of the Gangetic Plains is found to be 33.21 Bq kg−1, while the global average value of radium content in the earth crust is known to be 40 Bq kg−1.
The surface exhalation and mass exhalation rates for radon from these soil samples correspond to their radium contents. These exhalation rates are also larger for samples from Aligarh, lesser in samples from Mathura and least for the samples from Etah. The overall average of mass exhalation rate for the samples from the area under study is 4.21 × 10−6 Bq kg−1 day−1 and the average value for surface exhalation rate for radon in the soil samples is 10.93 × 10−5 Bq m−2 day−1.
The calculated values of alpha indexes for these samples are much less than unity in each case. Therefore, it is concluded that the soil samples of this region are quite safe to be used as building materials. They will not produce dangerous radon levels in dwellings.
References
Abu-Jarad FA (1988) Application of nuclear track detectors for radon related measurements. Nucl Tracks Radiat Meas 15(1–4):525–534
Abu-Jarad F, Fremlin JH, Bull R (1980) A study of radon emitted from building materials using plastic track detectors. Phys Med Biol 25:683–694
Åkerblom G, Andersson P, Clavensjö B (1984) Soil gas radon: a source for indoor radon daughters. Radiat Prot Dosim 7(1–4):49–54
Al-Jarallah M (2001) Radon exhalation from granite used in Saudi Arabia. J Environ Radioact 53(1):91–98
Al-Jarallah MI, Abu-Jarad F, Fazal-ur-Rehman (2001) Determination of radon exhalation rates from tiles using active and passive techniques. Radiat Meas 34(1–6):491–495
Al-Jundi J, Al-Bataina BA, Abu-Rukah Y, Shehadeh HM (2003) Natural radioactivity concentrations in soil samples along the Amman Aqaba highway, Jordan. Radiat Meas 36(1–6):555–560
Anderson P, Clavensjo B, Akerblom G (1983) The effect of the ground on the concentration of radon and gamma radiation indoors. Swedish Council for Building Research, Report R9, pp 1–442
Archer VE, Wagoner JK, Lundin FE (1973) Lung cancer among uranium miners in the United States. Health Phys 25(4):351–371
Arvela H, Winqvist K (1989) A model for indoor radon variations. Environ Intern 15(1–6):239–249
Azam A, Naqvi AH, Srivastava DS (1995) Radium concentration and radon exhalation measurements using LR-115 type II plastic track detectors. Nucl Geophys 9(6):653–657
Bayyson H, Tirmarche M, Tymen G, Gouva S, Caillaud D, Artus JC, Vergnenegre A, Ducloy F, Laurier D (2004) Indoor radon and lung cancer in France. Epidemiol 15(6):709–716
BEIR IV (1999) Report of the Committee on the Biological Effects of Ionizing Radiation. The health effects of exposure to indoor radon. National Research Council, National Academy of Science, Washington, DC
Böllhöfer A, Storm J, Martin P, Tims S (2006) Geographic variability in radon exhalation at a rehabilitated uranium mine in the Northern Territory, Australia. Environ Mon Assess 114:313–330
Bossew P (2003) The radon emanation power of building materials, soils and rocks. Appl Radiat Isot 59:389–392
Carrera G, Garavaglia M, Magnoni S, Valli G, Vecchi R (1997) Natural radioactivity and radon exhalation in stony materials. J Environ Radioact 34(2):149–159
Chau ND, Chrusciel E, Prokolski L (2005) Factors controlling measurements of radon mass exhalation rate. J Environ Radioact 82:363–369
Crockett RGM, Gillmore GK, Phillips PS, Denman AR, Groves-Kirkby CJ (2006) Radon anomalies preceding earthquakes which occurred in the UK, in summer and autumn 2002. Sci Total Environ 364:138–148
Darby S, Hill D, Doll R (2001) Radon: a likely carcinogen at all exposures. Ann Oncol 12:1341–1351
Darby S, Hill D, Auvinen A, Barros-Dios JM, Baysson H, Bochicchio F, Deo H, Falk R, Forastiere F, Hakama M, Heid I, Kreienbrock L, Kreuzer M, Lagarde F, Mäkeläinen I, Muirhead C, Oberaigner W, Pershagen G, Ruano-Ravina A, Ruosteenoja E, Schaffrath RA, Tirmarche M, TomáBek L, Whitley E, Wichmann HE, Doll R (2005) Radon in homes and risk of lung cancer: Collaborative analysis of individual data from 13 European case–control studies. Br Med J 330:223–227
Denman AR, Phillips PS (1998) A review of the cost effectiveness of radon mitigation in domestic properties in Northamptonshire. J Radiol Prot 18:119–214
Denman AR, Crockett RGM, Groves-Kirkby CJ, Phillips PS, Gillmore GK, Woolridge AC (2007) The value of Seasonal Correction Factors in assessing the health risk from domestic radon—a case study in Northamptonshire, UK. Environ Intern 33:34–44
Durrani SA, Ilic R (eds) (1997) Radon measurements by etched track detectors: Applications in radiation protection, earth sciences and the environment. World Sci Publ Co., Ltd., London
Eaton RS, Scott AG (1984) Understanding radon transport into houses. Radiat Prot Dosim 7(1–4):251–253
Ferry C, Richon P, Beneito A, Robe M (2002) Evaluation of the effect of a cover layer on radon exhalation from uranium mill tailings: transient radon flux analysis. J Environ Radioact 63:49–64
Fleischer RL (1980) Isotopic disequilibrium of uranium: alpha-recoil damage and preferential solution effects. Science 207:979–981
Fleischer RL (1982) Alpha-recoil damage and solution effects in minerals: uranium isotopic disequilibrium and radon release. Geochim Cosmochim Acta 46(11):2191–2201
Gillmore GK, Phillips PS, Denman AR (2005) The effects of geology and the impact of seasonal correction factors on indoor radon levels: a case study approach. J Environ Radioact 84:469–479
Groves-Kirkby CJ, Denman AR, Phillips PS, Crockett RGM, Woolridge AC, Tornberg R (2006a) Radon mitigation in domestic properties and its health implications: a comparison between during-construction and post-construction radon reduction. Environ Intern 32:435–443
Groves-Kirkby CJ, Denman AR, Crockett RGM, Phillips PS, Gillmore GK (2006b) Identification of tidal and climatic influences within domestic radon time-series from Northamptonshire, UK. Sci Total Environ 367:191–202
Guo Q, Sun K, Cheng J (2004) Methodology study on evaluation of radon flux from soil in China. Radiat Prot Dosim 112:291–296
Hafez AF, Hussein AS, Rasheed NM (2001) A study of radon and thoron release from Egyptian building materials using polymeric nuclear track detectors. Appl Radiat Isot 54:291–298
Ibrahiem NM, Abdel-Ghani AH, Shawky SM, Ashraf EM, Farouk MA (1993) Measurement of radioactivity levels in soil in the Nile Delta and Middle Egypt. Health Phys 64(6):620–627
Ibrahim N (1999) Natural activities of 238U, 232Th and 40K in building materials. J Environ Radioact 43(3):255–258
Jonassen N (1983) The determination of exhalation rates. Health Phys 45(2):369–372
Karpińska M, Mnich Z, Kapala J, Antonowicz K, Przestalski M (2005) Time changeability in radon concentration in one-family dwelling houses in the northeastern region of Poland. Radiat Prot Dosim 113:300–307
Keller G, Hoffmann B, Feigenspan T (2001) Radon permeability and radon exhalation of building materials. Sci Total Environ 272:85–89
Khan AJ, Prasad R, Tyagi RK (1992) Measurement of radon exhalation rate from some building materials. Nucl Tracks Radiat Meas 20(4):609–610
Khan MS, Zubair M, Verma D, Naqvi AH, Azam A, Bhardwaj MK (2011a) The study of indoor radon in the urban dwellings using plastic track detectors. Environ Earth Sci 63:279–282
Khan MS, Naqvi AH, Azam A, Srivastava DS (2011b) Radium and radon exhalation studies of soil. Iran J Radiat Res 8(4):207–210
Kreienbrock L, Kreuzer M, Gerken M, Dingerkus G, Wellmann J, Keller G, Wichmann HE (2001) Case–control study on lung-cancer and residential radon in Western Germany. Am J Epidemiol 153:42–52
Krewski D, Lubin JH, Zielinski JM, Alavanja M, Catalan VS, Field RW, Klotz JB, Létourneau EG, Lynch CF, Lyon JI, Sandler DP, Schoenberg JB, Steck DJ, Stolwijk JA, Weinberg C, Wilcox HB (2005) Residential radon and risk of lung cancer: a combined analysis of 7 North American case–control studies. Epidemiol 16(2):137–145
Lawrence CE, Akber RA, Böllhöfer A, Martin P (2009) Radon-222 exhalation from open ground on and around a uranium mine in the wet–dry tropics. J Environ Radioact 100:1–8
Lubin JH, Boice JD Jr (1997) Lung cancer risk from residential radon: meta-analysis of eight epidemiological studies. J Natl Cancer Inst 89:49–57
Mahur AK, Khan MS, Naqvi AH, Prasad R, Azam A (2008) Measurement of effective radium content of sand samples collected from Chhatrapur beach, Orissa, India using track etch technique. Radiat Meas 43(Supplement1):S520–S522
Miles JCH (2001) Temporal variation of radon levels in houses and implications for radon measurement strategies. Radiat Prot Dosim 93:369–375
Miles JCH, Appleton JD (2005) Mapping variation in radon potential both between and within geological units. J Radiol Prot 25:257–276
Mireles F, Davila JI, Quirino LL, Lugo JF, Pinedo JL, Rios C (2003) Natural soil gamma radioactivity levels and resultant population dose in the cities of Zacatecas and Guadalupe, Zacatecas, Mexico. Health Phys 84(3):368–372
Mose DG, Mushrush GW, Crosniak CE, Morgan WF (1991) Seasonal indoor radon variations related to precipitation. J Environ Mol Mutagen 17(4):223–230
Mustonen R (1984) Natural radioactivity in and radon exhalation from Finnish building materials. Health Phys 46(6):1195–1203
Naismith SP, Miles JCH, Scivyer CR (1998) The influence of house characteristics on the effectiveness of radon remedial measures. Health Phys 75(4):410–416
NBS (National Bureau of Standards) (1980) Radon in buildings, vol 581. NBS special publ, Washington, DC
Nordic (2000) Naturally occurring radiation in the Nordic Countries—recommendations. In: The flag-book series. The Radiation Protection Authorities in Denmark, Finland, Iceland, Norway and Sweden, Reykjavik
OECD (1979) Organization for economic cooperation and development. In: Exposure to radiation from natural radioactivity in building materials. Report by a group of Experts of the OECD Nuclear Energy Agency, OECD, Paris
Paridaens J, de Saint-Georges L, Vanmarcke H (2005) Mitigation of a radon-rich Belgian dwelling using active subslab depressurization. J Environ Radioact 79:25–37
Petropoulos NP, Anagnostakis MJ, Simopoulos SE (2001) Building materials radon exhalation rate: ERRICCA intercomparison exercise results. Sci Tot Environ 272:109–118
Popovic D, Djuric G, Todorovic D (1996) Radionuclides in building materials and radon indoor concentrations. Radiat Prot Dosim 63(3):223–225
Popovic D, Djuric G, Todorovi D, Spasic-Jokic V (2000) Radionuclides of building materials and radon concentrations in Belgrade dwellings. Cent Eur J Occupat Environ Med 6(2–3):129–133
Ramola RC, Chaubey VM (2003) Measurement of radon exhalation rate from soil samples of Garhwal Himalaya, India. J Radioanal Nucl Chem 256(2):219–223
Righi S, Bruzzi L (2006) Natural radioactivity and radon exhalation in building materials used in Italian dwellings. J Environ Radioact 88:158–170
Samuelsson C, Pettersson H (1984) Exhalation of 222Rn from porous materials. Radiat Prot Dosim 7(1–4):95–100
Savidou A, Raptis C, Kritidis P (1996) Study of natural radionuclides and radon emanation in bricks used in the Attica region, Greece. J Environ Radioact 31(1):21–28
Sevc J, Kunz E, Placek V (1976) Lung cancer in uranium mines and long-term exposure to radon daughter products. Health Phys 30(6):433–437
Sharma DK, Kumar A, Kumar M, Singh S (2003) Study of uranium, radium and radon exhalation rate in soil samples from some areas of Kangra District, Himachal Pradesh, India using solid-state nuclear track detectors. Radiat Meas 36(1–6):363–366
Singh S, Kumar A, Singh B (2002) Radon level in dwellings and its correlation with uranium and radium content in some areas of Himachal Pradesh, India. Environ Intern 28:97–101
Singh S, Singh B, Kumar A (2003) Natural radioactivity measurements in soil samples from Hamirpur District, Himachal Pradesh, India. Radiat Meas 36(1–6):547–549
Singh K, Singh M, Singh S, Sahota HS, Papp Z (2005) Variation of radon (222Rn) progeny concentrations in outdoor air as a function of time, temperature and relative humidity. Radiat Meas 39(2):213–217
Singh J, Singh H, Singh S, Bajwa BS, Sonkawade RG (2009) Comparative study of natural radioactivity levels in soil samples from the upper Siwaliks and Punjab, India using gamma-ray spectrometry. J Environ Radioact 100:94–98
Somogyi G (1986) Track detection methods of radium measurements. ATOMKI preprint E/25
Somogyi G (1990) The environmental behavior of radium, vol 1. Technical Reports Series No. 310, IAEA, Vienna, pp 229–256
Sroor A, El-Bahi SM, Ahmed F, Abdel-Haleem AS (2001) Natural radioactivity and radon exhalation rate of soil in southern Egypt. Appl Radiat Isot 55:873–879
Stoulos S, Manolopoulou M, Papastefanou C (2003) Assessment of natural radiation exposure and radon exhalation from building materials in Greece. J Environ Radioact 69:225–240
Tanner AB (1964) Radon migration in the ground: a review. In: Adams JAS, Lowder WM (eds) The natural radiation environment. University of Chicago Press, Chicago, pp 61–190
Tanner AB (1980) Radon migration in the ground: a supplementary review. In: Gesell TF, Lowder WM (eds) The natural environment III. National Technical Information Service, Springfield, VA, USDOE Report CONF-780422, pp 5–56
Todorovic D, Popovic D, Djuric G (1994) Natural radionuclides in building materials. In: Natural radiation in the environment. Inst Nucl Sci Vinca, Belgrade, pp 249–257
Todorovic D, Popovic D, Djuric G, Radenkovic M (2000) 210Pb in ground-level air in Belgrade city area. Atms Environ 34(19):3245–3248
Tuccimei P, Moroni M, Norcia D (2006) Simultaneous determination of 222Rn and 220Rn exhalation rates from building materials used in Central Italy with accumulation chambers and a continuous solid state alpha detector: Influence of particle size, humidity and precursors concentration. Appl Radiat Isot 64:254–263
Umar R, Ahmad MS (2000) Groundwater quality in parts of Central Ganga Basin, India. Environ Geol 39(6):673–678
UNSCEAR (1993) United Nations Scientific Committee on the effects of atomic radiation. Sources, effects and risks of ionizing radiation. United Nations, New York
UNSCEAR (1994) United Nations Scientific Committee on the effects of atomic radiation. Ionizing radiation: Sources and biological effects, United Nations, New York
Acknowledgments
The authors are thankful to the Chairman, Department of Applied Physics, Z. H. College of Engineering and Technology, Aligarh Muslim University, Aligarh, for providing necessary facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khan, M.S., Srivastava, D.S. & Azam, A. Study of radium content and radon exhalation rates in soil samples of northern India. Environ Earth Sci 67, 1363–1371 (2012). https://doi.org/10.1007/s12665-012-1581-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-012-1581-7