Abstract
The chemical speciation of potentially toxic elements (As, Cd, Cu, Pb, and Zn) in the contaminated soils and sulfides-rich tailings sediments of an abandoned tungsten mine in Korea was evaluated by conducting modified BCR sequential extraction tests. Kinetic and static batch leaching tests were also conducted to evaluate the potential release of As and other heavy metals by acidic rain water and the leaching behaviors of these heavy metals. The major sources of the elements were As-, Zn- and Pb-bearing sulfides, Pb carbonates (i.e., cerussite), and Pb sulfates (i.e., anglesite). The biggest pollutant fraction in these soil and tailing samples consists of metals bound to the oxidizable host phase, which can be released into the environment if conditions become oxidative, and/or to residual fractions. No significant difference in total element concentrations was observed between the tailings sediments and contaminated soils. For both sample types, almost no changes occurred in the mobility of As and the other heavy metals at 7 days, but the mobility increased afterwards until the end of the tests at 30 days, regardless of the initial pH. However, the mobility was approximately 5–10 times higher at initial pH 1.0 than at initial pHs of 3.0 and 5.0. The leached amounts of all the heavy metal contents were higher from tailings sediments than from contaminated soils at pH > 3.0, but were lower at pH < 3.0 except for As. Results of this study suggest that further dissolution of heavy metals from soil and tailing samples may occur during extended rainfall, resulting in a serious threat to surface and groundwater in the mine area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The oxidation of sulfides in mine tailings sediments has been a great concern due to its detrimental impact on the environment. When surface water infiltrates into mine waste rocks or tailings sediments, the oxidation of sulfides occurs in the presence of oxygen, resulting in acid rock drainage (ARD). ARD generally contains elevated concentrations of ions such as arsenic (As), other heavy metals, and sulfates, which can affect the quality of surface water and groundwater in mining areas (Johnson and Thornton 1987; Webster et al. 1994; Shaw et al. 1998).
The dissolved As and other heavy metals can be immobilized with precipitation as insoluble or less soluble Fe-(oxy)hydroxides, oxides, and sulfates (McGregor et al. 1998; Roussel et al. 2000; Lee et al. 2005), and by adsorption onto some materials such as clay minerals, oxides, and hydroxides (Masscheleyn et al. 1991; Karathanasis and Thompson 1995; Manning et al. 1998). In addition, newly formed secondary minerals from ARD have been considered to play an important role in immobilizing As and other heavy metals by co-precipitation and adsorption (Bigham et al. 1990; McCarty et al. 1998; Webster et al. 1998). However, heavy metals that are immobilized by the secondary minerals in the dry season can be released again during the wet season (Lottermoser and Ashley 1998).
The chemical partitioning of heavy metals must be taken into account in environmental studies because total heavy metal contents in soils can provide limited information on their mobility and bioavailability (Ma and Rao 1997) and overestimate potential risk to the environment (Rodríguez et al. 2009; Yang et al. 2009; Anju and Banerjee 2010). Hence, not only the determination of metal concentrations but also the identification of binding sites and phase associations is essential for assessing environmental quality and human health in mine areas. Sequential extraction procedures assume that mobility and bioavailability of metals in soils decrease in the order of extraction. Despite the lack of selectivity and re-adsorption problems (Kheboian and Bauer 1987; Belzile et al. 1989) for all presently available methods of solid phase fractionation (e.g., Tessier et al. 1979; Kersten and Förstner 1986; Ure et al. 1993; Sahuquillo et al. 1999), a sequential extraction method is nevertheless a useful tool for predicting long-term adverse effects of contaminated soils on the environment and for assessing mobility and bioavailability of metals in soils (Forsberg and Ledin 2006; Álvarez-Valero et al. 2009; Palumbo-Roe et al. 2009). Furthermore, chemical partitioning of metals in tailings sediments was determined to evaluate weathering behaviors of tailings sediments (Fanfani et al. 1997; Heikkinen and Räisänen 2009).
Several studies have investigated the leaching behaviors of heavy metals from sulfides (e.g., pyrite) or waste rocks at various conditions (Moses and Herman 1991; Williamson and Rimstidt 1994; Bonissel-Gissinger et al. 1998; Shaw et al. 1998; Strömberg and Banwart 1999; Lee et al. 2005; Yellishetty et al. 2009). Those studies suggested that assessment of the environmental impacts from the mine waste rocks or tailings sediments requires quantitative predictions of physicochemical processes that control contaminant release at a given site because the mobility of heavy metals can be affected by some factors such as their thermodynamic properties and the presence of secondary minerals. Thus, investigation of mobility of heavy metals at various conditions is essential to understand the potential and actual impact of elevated heavy metals released from mine waste rocks or tailings sediments. The objective of this study is to determine the chemical fractionation and to investigate the mobility of As and other heavy metals released from tailings sediments and soils of a former tungsten mine site in Korea in contact with water, applying a sequential extraction procedure as well as kinetic and static batch experiments.
Materials and methods
Mine site description
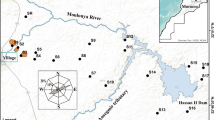
The sulfidic, As-rich, abandoned Cheongyang tungsten mine is situated approximately 100 km southwest of Seoul in Chungcheongnam-do province in Korea (Fig. 1). The mine area mainly consists of Precambrian biotite gneiss and younger Cretaceous granitic rocks. The deposit is composed of several tungsten-bearing hydrothermal quartz veins with a WO3 content ranging between 65 and 67 wt.%. The mine was operated actively from 1941 to 1945 and produced approximately 1,600 tons of tungsten, resulting in large amounts of mine wastes such as tailings sediments and waste rocks. The ore minerals are arsenopyrite (FeAsS), galena (PbS), sphalerite (ZnS), chalcopyrite (CuFeS2), wolframite ((Fe,Mn)WO4), scheelite (CaWO4), pyrrhotite (Fe(1−x)S), pyrite (FeS2), molybdenite (MoS2), bismuthinite (Bi2S3), gold (Au), and silver (Ag). Gangue minerals were quartz, beryl (Be3Al2Si6O8), rhodochrosite (MnCO3), calcite (CaCO3), and fluorite (CaF2) (So et al. 1993).
Geological map of the Cheongyang mine area showing the location of major ore bodies (after So et al. 1993)
Through ore extraction and processing, large amounts of tailings sediments having elevated contents of sulfide minerals were produced. These have been stacked on the ground of the mine area for more than 60 years. The toxic tailing slurries covered several hundred square meters of soil surrounding the mine area. The brownish and light blue colored mixtures of tailings sediments and soils (i.e., contaminated soils) have been slightly oxidized by exposure to the atmosphere. The sulfide-rich tailings sediments were totally dried and their surface color was light gray, in which a brownish material was sometimes present, indicating that tailings sediments are slightly oxidized. Indurate tailings sediments are mainly unaltered or unoxidized and have a circum-neutral pH. The particle size of the tailings sediments and contaminated soils varies from coarse sand to silt.
The mean monthly precipitations during the wet period from June to September and during the dry period from October to May are approximately 70 and 10 mm, respectively. Approximately 75% of the annual rainfall occurs during the wet period. The monthly average temperature seasonally varied from 25 to −3°C, with an annual mean temperature of 10°C. The mean annual relative humidity was 66% with a maximum of 75% in the summer and a minimum of 53% in the spring. The range of annual mean pH of rain calculated from volume-weighted mean concentrations of H+ at the study site ranged between 3.7 and 7.5 during 1996–2002 (KMA 2003).
Characterization of samples
Three tailing samples (T1, T2, and T3) and three soil samples (S1, S2, and S3) were collected from the Cheongyang tungsten mine area. Samples were placed in tightly closed plastic bags to prevent those from being oxidized before analysis. The samples were sieved through a 2-mm mesh to remove extraneous materials. After sieving, the collected samples were screened with a 150-μm sieve (No. 100 sieve). The screened samples were dried in an oven at 60°C for 2 days and then ground in an agate mortar. This drying process might cause the further oxidation of sulfides in the samples. The chemical compositions of the samples were determined by using the total digestion method according to the procedure suggested by the International Organization for Standardization (IOS 11466, 1995). Briefly, 3 g of sample was digested at room temperature with a 28 ml mixture of 37% HCl and 70% HNO3 (3:1) for 16 h. The suspension was then digested at 130°C for 2 h under reflux conditions. The suspension was filtered through 0.45-μm filter paper, stored in a polyethylene bottle, and acidified with nitric acid solution to pH < 2.0 prior to the chemical analyses.
Mineralogical identification of the primary and secondary minerals was performed by X-ray diffraction using a Philips X’PERT MPD diffractometer operating at 40 kV and 25 mA. Powdered specimens were prepared by grinding samples with an agate mortar and pestle and a micronizing mill until the particle size of the material was less than 10 μm. Randomly orientated specimens were continuously scanned in the range 3–70° 2θ at a rate of 2°/min, using Cu–Kα radiation (wavelength 1.5419 Å). Quantitative mineral components were determined using the Rietveld method with a quantitative XRD software program (Siroquant, version 3.0, Sietronics, Australia). Organic content was determined by using the loss-on-ignition method.
Batch kinetic and static leaching tests
A series of batch kinetic tests was conducted on both tailing and soil samples to determine the reaction time required to establish equilibrium conditions and the leaching kinetics for As and other heavy metals, using solutions at pHs of 1.0, 3.0, and 5.0. Five grams of sample was placed in a 100-ml polypropylene copolymer centrifuge tube. The tube was then agitated for various reaction times, ranging between 1 and 30 days, using a rotation shaker at 30 rpm, with the leachate collected at designated sampling times (i.e., 1, 2, 7, 14, 21, and 30 days). The pH of the suspension was measured using a pH meter immediately after shaking. The suspension was filtered through 0.45-μm filter paper, stored in polyethylene bottle, and acidified with nitric acid solution to pH < 2.0 prior to the chemical analysis.
Batch static tests were conducted on both tailing and soil samples in a 100-ml polypropylene copolymer centrifuge tube containing solutions with a specified initial pH ranging between 1.0 and 5.0 to evaluate the effect of initial pH on the leaching behaviors. The tube was then agitated for 7 days using a rotation shaker at 30 rpm, and this procedure was found to be generally sufficient for attaining equilibrium in the batch kinetic tests. The final pH of the suspension was measured using a pH meter immediately after tumbling. The suspension was filtered through 0.45-μm filter paper, stored in a polyethylene bottle, and acidified with nitric acid solution to pH < 2.0 prior to the chemical analysis.
Sequential extraction procedure (SEP)
The three-step sequential extraction procedure (SEP) proposed by the Standards, Measurements, and Testing program (formerly Community Bureau of Reference, BCR) of the European Union has generally been used to evaluate the mobility and potential bioavailability of metals, which might be affected by changes in environmental conditions (Quevauviller et al. 1993; Ure et al. 1993; Mossop and Davidson 2003). However, the modified three-step SEP proposed by Mossop and Davidson (2003) was used in this study to determine the partitioning of As, cadmium (Cd), copper (Cu), iron (Fe), lead (Pb), and Zinc (Zn) in tailings sediments and soils because of its improved reproducibility for the reducible trace element fraction in tailings sediments and contaminated soils.
One gram of an air dried sample (i.e., tailing or soil) was added to a Teflon centrifuge tube with 40 ml of 0.11 M acetic acid and shaken overnight. The mixture was then centrifuged and filtered through 0.45-μm filter paper to obtain the extract (step 1). The trace elements extracted obtained in step 1 corresponds to the exchangeable and water and acid soluble phases (F I). The residue obtained by centrifuging from step 1 was added to a Teflon centrifuge tube with 40 ml of 0.5 M NH2OH·HCl solution at pH 1.5 and shaken overnight. The mixture was then centrifuged to separate the extract (step 2). The trace elements extracted obtained in step 2 corresponds to the reducible phase (F II). The residue from step 2 was mixed with 40 ml of 8.8 M H2O2 and the mixture was then dried. After drying, the residue was added to a Teflon centrifuge tube with 40 ml of 3.2 M NH4OAc and shaken overnight. The mixture was then centrifuged to separate the extract (step 3). Elements dissolved in step 3 correspond to the fraction bound to oxidizable phase (F III). The residue from step 3 was digested in aqua regia (residual step). The chemical species from the residual step correspond to the residual phase (F IV).
Chemical analyses
Chemical analyses were performed with an ICP-AES (PerkinElmer Optima 3000XL) using an ultrasonic nebulizer. Standard solutions were prepared from 1,000 mg/L stock solutions (Merck). Blank assays with reagents were found to be negligible. For QA/QC, standards, including NIST Standard Reference Materials (2710 Montana Soil and 2709 San Joaquin Soil), were analyzed together with the tailings sediments and contaminated soils. Replicate analyses (at least 10 replicates) of the samples and standards showed a precision of typically <6.5% (coefficient of variation) for all the elements analyzed. The precision as represented by the relative deviations of the data was <3% for As and less than 5% for Cu, Pb, and Zn. The detection limit for analysis was 1 μg/L for Cu and Cd, 2 μg/L for As, 5 μg/L for Pb, and 50 μg/L for Fe and Zn.
Results and discussion
Characteristics of soils and tailings
The total elemental concentrations and mineralogical compositions of the six representative samples used in this study are summarized in Table 1. The mineralogical composition of the soil and tailing samples from Cheongyang mine has been obtained by X-ray diffraction (XRD).
XRD patterns showed that soils and tailings sediments consisted of quartz (SiO2), muscovite (KAl2(AlSi3)10(F,OH)2), microcline ((KAlSi3)8), arsenopyrite (FeAsS), sphalerite (ZnS), anglesite (PbSO4), chlorite ((Fe,Mg,Al)6(Si,Al)4O10(OH)8), talc (M g3Si4O10(OH)2), chalcopyrite (CuFeS2), cerussite (PbCO3), bassanite (CaSO4·0.5H2O), and galena (PbS) (Table 1). Only one soil sample (S3) contained a noticeable amount of a carbonate mineral (i.e., cerussite). A remarkable finding was the absence of pyrite and pyrrhotite and the minor sulfides in the ore vein (So et al. 1993). Furthermore, XRD analysis detected no Fe-(oxy)hydroxides or other Fe-precipitates probably because the amount of Fe hydroxides was too low to quantify it by XRD analysis or the Fe hydroxides existed as an amorphous form (e.g., ferrihydrite). Secondary minerals frequently might be formed in the soil and tailing impoundment of Cheongyang mine area (e.g., bassanite). Extensive bassanite could be formed a dense hardpan layer (i.e., a dense ferrous crust resulting from the secondary mineralization), and could prevent interaction between the mineral grains and the oxidized agents dissolved in solution, halting sulfide mineral oxidation. The development of these hardpans can favor the isolation of tailing materials from the weathering process resulting in reduced infiltration of water and a decreasing rate of sulfide oxidation.
The soil and tailing samples showed high concentrations for all metals indicating that almost all the samples can be classified to be very highly contaminated. For example, the average As concentrations for the soil and tailing samples were 213 and 188 mg/g, respectively. No significant difference in total element concentrations between tailing and soil samples was observed, whereas arsenopyrite was more enriched in soils than in tailings sediments, resulting in the higher average As concentrations in soils than in tailings sediments (Table 1).
Solid phase partitioning of metals: result of sequential extraction
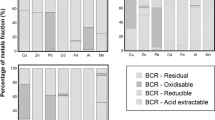
Selective sequential extraction tests were performed to evaluate the fractionation of metals within the soil and tailing samples. Results of the sequential extraction tests are summarized as leaching percentage, reflecting individual solid phase removal relative to the sum of all phases in Table 2.
Zn fractionation is dominated by the oxidizable (F III) phase (average 79.0 and 60.2% of total Zn concentrations for the soils and tailings sediments, respectively) and the residual (F IV) phase (average 15.8 and 36.4% of total Zn concentrations for the soils and tailings sediments, respectively), indicating that these two solid phases are of major importance as the Zn carriers in the soils and tailings sediments (Table 2). The oxidizable fraction for Zn extracted by H2O2 from tailings sediments is large even though the tailings sediments did not contain organic carbon. Taking into account the amounts of organic matter in tailings sediments, it seems reasonable to assume that most of the oxidizable metals are mainly associated with sulfide minerals (i.e., sphalerite, chalcopyrite, galena, and arsenopyrite, Table 1).
Cd existed primarily by the residual phase, accounting on average for 87.9% (soils) and 92.9% (tailings sediments) of total Cd concentrations (Table 2), with the oxidizable phase containing the majority of the remaining Cd (average 10.6 and 5.2% of total Cd concentrations for soils and tailings sediments, respectively). It is noteworthy that despite the similar geochemical characteristics of Zn and Cd particularly with respect to ionic structure, electro-negativities, and ionization energies (Fuge et al. 1993), their fractionation patterns are quite different.
Cu was primarily bound to the oxidizable phase (average 82.7 and 65.2% of total Cu concentrations for the soil and tailing, respectively), indicating the sulfide minerals acted as scavengers of Cu. The residual phase is also important, containing on average 15.9 and 33.7% of total Cu concentrations for soils and tailings sediments, respectively. The role of the reducible (F II) and exchangeable and acid soluble (F I) phases were not significant, accounting on average for only <1% of total Cu concentrations for both soils and tailings sediments (Table 2).
The residual phase might be a predominant sink for Pb, accounting for 84.5 and 80.8 % of total Pb concentrations for soils and tailings sediments, respectively, except for S3 soil sample. Pb in the reducible phase is also important, containing on average 10.2% (from 6.5 to 13.6 %) and 10.5% (from 4.9 to 13.3 %) of total Pb concentrations for soils and tailings sediments, respectively. The oxidizable phase contained on average 3.7% and 6.0% of the total Pb concentrations for soils and tailings sediments, respectively (Table 2). These results indicate that the residual, reducible, and oxidizable phases contain most of Pb in the soil and tailing in the Cheongyang mine area. Site-to-site variations were particularly large in the exchangeable and acid soluble phase (e.g., S3). Pb in the S3 sample was identified as potentially the most bioavailable element as it had the highest percentage bound to exchangeable and acid soluble sites, accounting for 23.4 % of total Pb concentration. This fraction includes weakly absorbed metals retained on the soil surface, metals that can be released by ion-exchange processes and metals that can be precipitated with carbonates. This fraction is considered to be the most bioavailable of the different metal forms (Chlopecka 1996). The XRD data confirmed that cerussite (PbCO3) occurred in the S3 soil sample. This finding is consistent with that of Li and Thornton (2001) and Clevenger (1990). The incoming Pb from polluting sources, such as sulfide oxidation, initially exists in unstable chemical forms and continued accumulation leads to the formation of precipitates, especially acid soluble phase or sulfate forms (Table 1). The majority of Pb in the sample is partitioned in the residual phase (59.7 %), while the proportion of Pb in the organic and sulfide phases was low (Table 2). It appears that the relative portion of Pb in the oxidizable and residual phases decreases significantly in the S3 sample compared to tailing samples. This shows that over a period, metal sulfide (i.e. galena) in S3 sample has been weathered from the contaminated soil.
As in the soils and tailings sediments is of low mobility, because exchangeable and acid soluble and reducible phases accounted for <2% of total As, and As is primarily bound to the residual phase, accounting on average for 93.7 and 95.6% of total As concentrations for soils and tailings sediments, respectively (Table 2). The residual phase should mainly contain primary minerals (e.g., arsenopyrite, Table 1), which may hold trace metals within their crystal structures. Craw et al. (2003) showed that arsenopyrite only can decompose under acidic conditions (pH < 4.0). Therefore, it does not seem reasonable to assume that these metals may be released under the conditions normally encountered in nature (Dang et al. 2002). High contents of As in the residual fraction have also been reported by Kim et al. (2003) and Wang and Mulligan (2009). On average, the oxidizable phase of As is only 5.2 and 2.4% of total As concentrations for soils and tailings sediments, respectively, and thus very low compared to those of Zn and Cu.
The large amounts of Fe were mainly associated with the residual fraction, ranging from 77.5 to 79.5% of total Fe concentrations for soils and from 84.3 to 88.8% of total iron concentrations for tailings sediments (Table 2). In this study, taken into account the absence of pyrite in the soil and tailing samples as determined by XRD (Table 1), it seems reasonable to assume that this highest percentage of total Fe contents in the residual fraction can be attributed mainly to the dissolution of arsenopyrite present in the soil and tailing samples. The Fe associated with the oxidizable fraction ranged from 17.3 to 18.4% of total Fe contents for soils and from 8.0 to 13.8% of total Fe contents for tailings sediments. The role of the reducible (F II) phase was not significant, ranging from 2.4 to 3.2% of the total metal contents for soils and from 1.1 to 2.6% of total metal contents. This observation indicates that most of the metal sulfides have not been weathered (oxidized) from tailings sediments within Cheongyang mine area. The first fraction from the BCR extraction procedure (F I) was the fraction with the lowest Fe content in all samples, ranging from 0.5 to 0.9% of the total Fe pool (Table 2).
In the uncovered tailings sediments, the area most prone to sulfide weathering is unsaturated layers in the tailing impoundments. However, the mineralogical and chemical speciation of soil and tailing samples of Cheongyang mine area indicates that the degree of sulfide oxidation and associated chemical reactions (i.e., formation of secondary minerals, trace metal release, and weathering of aluminosilicates) represent an early stage of weathering. The amounts of trace metals except for Pb associated with reducible fraction were very small (Table 2), indicating that tailings sediments were geochemically unaltered and resembled fresh tailings sediments. In addition, acid-producing pyrite is absent in the soil and tailing samples (Table 1) and thus the tailings sediments are not highly reactive. Even though oxidation of arsenopyrite can produce acid conditions, the oxidizable phase of As is only 5.2 and 2.4% of total As concentrations for soils and tailings sediments (Table 2).
Batch kinetic leaching tests
A series of batch kinetic tests was conducted on both tailing and soil samples to evaluate the leaching behaviors of As and other heavy metals over time, using solutions at pHs of 1.0, 3.0, and 5.0. The results of these leaching tests are shown in Figs. 2, 3, 4 and 5.
Kinetic characteristics of pH and Eh
The soil samples exhibited slight change in the final pH at initial pH 1.0, whereas the final pHs of the soil samples were increased by about 1–3 U at initial pH of 3.0 (Fig. 2) probably because soil samples contained carbonate mineral (i.e., cerussite) even though the carbonate mineral was found in only S3 by XRD analysis (Table 1). For the tailing samples, slight change in the final pH occurred at initial pH of 1.0, which is comparable to the results of the soil samples, whereas the final pH decreased by about 2 U at initial pHs of 5.0 because of oxidation of sulfides in the tailing samples (Table 1). However, almost no variation in the final pH was observed over time, regardless of the sample type and initial pH (Fig. 2).
No significant changes in Eh occurred over time, regardless of the sample type and initial pH. Eh varied between 32 and 256 mV for the soil samples and between 199 and 225 mV for the tailing samples at initial pHs of 3.0 and 5.0, but varied between 340 and 405 mV for both the tailing and soil samples at initial pH of 1.0 (Fig. 2). Eh affected the solubility of the elements. All element concentrations except for Pb significantly increased when Eh exceeded approximately 200 mV and tailings sediments released their metal load earlier than soils, as shown in Fig. 3. Figure 3 shows the relationship between Eh and element concentrations obtained from the batch kinetic tests at initial pHs of 1.0, 3.0, and 5.0. The increase in element concentrations with the increase in Eh was attributed to oxidation and subsequent dissolution of the sulfides (e.g., sphalerite and arsenopyrite).
Mobility of elements
In general, the mobility of elements (i.e., percentage of leached concentration at specific pH relative to total concentration) except for Pb increased with increasing reaction time for the tailing samples, whereas almost no changes occurred for the soil samples, regardless of initial pH (Fig. 4). However, the mobility was higher at initial pH of 1.0 than at initial pHs of 3.0 and 5.0, regardless of the sample type.
For both the tailing and soil samples, until day 7, the increase in element concentrations occurred generally more slowly than afterwards, but the increase was more significant after 7 days up to 30 days (i.e., by the end of the tests), regardless of initial pH. However, the mobility was approximately 2–100 times higher at initial pH of 1.0 than at initial pHs of 3.0 and 5.0.
For the tailing samples, at initial pHs of 3.0 and 5.0, the As and Fe contents in the leachate of the total element concentration were 0.3–0.6 and 0.2–0.5%, respectively, after 1 day and then gradually increased to 1.4–2.5 and 1.1–2.8%, respectively, after 7 days up to 30 days (Fig. 4). On the other hand, at initial pH 1.0, As and Fe contents were 3.5–8.3 and 4.3–10.1%, respectively, after 1 day and then gradually increased to 7.5–14.5 and 11.5–21.2%, respectively, at 30 days. For the soil samples, although no As, Fe, or Cu occurred at initial pHs of 3.0 and 5.0, As and Fe were leached from the soil samples at initial pH of 1.0 (As: 3.7–9.3%, Fe: 6.7–14.2%, Cu: 1.0–9.6%). These results were attributed to oxidation of residual arsenopyrite and dissolution of As-rich, poorly crystalline, Fe-hydroxides at pH 1.0, which may be confirmed by the correlation between As and Fe concentrations at pH range between 1.0 and 5.0, as shown in Fig. 5.
After 30 days, at initial pH 3.0 and 5.0, the mobility of Pb was almost similar in the tailing samples (0.2–0.4%) to that in the soil samples (0.1–0.5%), whereas at initial pH 1.0, Pb was more mobile in the soil samples (22.0–78.0%) than in the tailing samples (7.1–19.1%) (Fig. 4). These results support the presence of Pb as different phases in the tailing and soil samples (Table 1). In addition, for the tailing samples, most of the increase in the Pb content occurred before 2 days (12.3–19.1%) and then decreased significantly at 30 days (7.1–11.1%) (Fig. 4). However, an increase in Fe content with decreasing pH was not correlated with an increase in the Pb content (Fig. 5). These results suggest that the rapid dissolution of Pb-bearing minerals might be followed by the slow precipitation of a sparingly soluble mineral (i.e., anglesite).
On the other hand, at initial pHs 3.0 and 5.0, the mobilities of Zn and Cd were very low for the soil (Zn: 0.57–0.97%, Cd: 0.15–0.48%) and tailing (Zn: 0.72–1.71%, Cd: 0.34–0.89%) samples at 7 days. After 7 days, the increases in Zn and Cd mobility were more significant for the tailing samples (Zn: 5.86–10.50%, Cd: 2.65–4.68%) than for the soil samples (Zn: 1.40–2.51%, Cd: 0.53–1.24%) (Fig. 4). These results indicate that an increase in reaction time with moderate acid water can cause an increase in leached amounts of all elements except for Pb.
At initial pH of 1.0, a more significant increase in mobility of Zn occurred over time than that of Cd for both soil and tailings sediment samples and mobilities of Zn and Cd were higher at initial pH of 1.0 than at initial pHs of 3.0 and 5.0. In addition, Zn mobility was well correlated with Fe mobility at pH ranging between 1.0 and 5.0, probably due to oxidation of sphalerite (Fig. 5).
Batch static leaching tests
A series of batch static leaching tests was conducted on the soil and tailing samples at specific initial pH ranging between 1.0 and 6.2. The results are shown in Table 3 and Fig. 6.
For the soil sample, final pH increased by approximately 1–2 U at initial pH between 1.0 and 5.0, whereas for the tailings sediment sample the final pH decreased by about 1–2 U at initial pH between 3.5 and 6.2 and did not change at initial pH between 1.0 and 3.0. These results are comparable to those from the batch kinetic tests (Table 3).
In general, the contents of As and other heavy metals leached from the tailing sample were higher than those leached from the soil sample at pH > 3.0, but were lower at pH < 3.0 except for As. For example, at initial pH 5.0, the leached contents of As, Pb, Zn, Cd, Cu, and Fe from the tailing sediments sample (As: 0.38%, Pb: 0.19%, Zn: 1.62%, Cd: 0.22%, Cu: 0.19%, Fe: 0.43%) were higher than those from the soil sample (As: 0.00%, Pb: 0.07%, Zn: 0.42%, Cd: 0.09%, Cu: 0.00%, Fe: 0.00%), indicating the presence of water-soluble salts (i.e., Fe-sulfates) containing these elements in the tailing sediments sample. These results suggest that moderate acid rain may leach small amounts of As, Pb, and Zn from the tailings sediment sample, while these elements may remain fixed in the soil sample. At initial pH between 3.5 and 6.2, small amounts of Pb (0.06–0.08%), Zn (0.40–0.49%) and Cd (~0.09%) were leached from the soil sample (Table 3). As, Cu, and Fe were hardly observed in the leachates. These results suggest that the major portions of these metals were contained in the less soluble or insoluble secondary minerals.
At initial pH between 1.0 and 2.5 (i.e., final pH between 1.6 and 5.2), the Pb content leached from the soil sample (1.10–38.75%) was higher than that leached from the tailings sediment sample (0.30–5.64%) (Table 3). Pb was found to be most mobilized at initial pH < 3.0 in the Cheongyang mine. This variation in the leaching behavior of Pb according to the sample types can be related to the solubility of Pb-bearing minerals in these samples (Table 1). The increase in Pb content may be attributed to the increase in the dissolution of cerussite (PbCO3) at final pH lower than 5.2, which is confirmed by the results of the sequential extraction test. Pb occurred mainly as FI phase in the soil sample (23.4%) but not in the tailings sediment (2.3%) (Table 2). However, at very strong acid condition (final pH between 1.4 and 1.6), there was a significant increase in the Pb content leached from both the soil and tailing samples, which probably indicates dissolution of the anglesite (PbSO4) and oxidation of the residual galena. Thus, these results indicate that Pb existed as readily soluble mineral forms (mainly Pb carbonates) and as various less soluble forms (mainly Pb sulfates and sulfides) in the soil and tailing samples, respectively. Thus, a high content and fractionation of Pb in the soil sample (S3) and the high mobility of Pb at low pH suggest that the contaminated soils and tailings sediments might impose a high risk of Pb contamination to the surface- and groundwater quality under strong acidic conditions.
The As content leached from the soil sample (0.04–1.97%) and the tailing sample (0.68–3.88%) increased significantly at initial pH between 1.0 and 2.0 (final pH between 1.4 and 4.4) (Table 3). The dissolved As content correlates positively with Fe, especially for the tailings samples, absolute concentrations of both elements are comparably high (Fig. 5). As is found to be most mobilized at very strong acidic condition (<2.0), probably due to the dissolution of the components of the tailing such as arsenopyrite and/or metal oxides. This result is confirmed by the molar ratios of As to Fe (As/Fe) for soil (0.91) and tailing (0.87) samples. The molar ratios are close to unity and therefore similar to the molar As/Fe ratio in arsenopyrite. This result is also confirmed by the results of sequential extraction tests, which indicate that As in the soil and tailing samples is of relatively low mobility, because exchangeable and acid soluble As levels accounted for <1% of the total arsenic and the arsenic is concentrated (>98%) in the residual and oxidizable phases (i.e., arsenopyrite) (Table 2).
The Zn and Cd contents leached from the soil samples increased from 0.42–0.49 and 0.09–0.10% at a pH range between 3.5 and 5.0, to 1.10–8.16 and 0.18–1.27% at a pH range between 1.0 and 3.0, respectively (Table 3). However, for the tailing sample, the Zn (~1.63%) and Cd (0.45%) were less mobile than for the soil sample, even at a strong acid condition (pH = 1), probably due to the presence of less soluble mineral phases (e.g., sphalerite, ZnS) and/or incomplete dissolution. The XRD analyses confirmed the presence of substantial amounts of sphalerite within soil and tailing samples (Table 1). The large difference in Zn and Cd contents between the soil and tailing samples was probably because for Zn the F I phase was larger in the soil sample (i.e., F I: 6.5%, F IV: 14.6%) than the tailings sediment sample (i.e., F I: 1.9%, F IV: 47.7%), whereas a large fraction of Zn was bound to the residual phase in the tailing sample (Table 2). In addition, results of sequential extraction tests indicate that Cd in the soil and tailing samples is of relatively low mobility, because exchangeable, acid soluble, reducible, and oxidizable fractions of Cd accounted for <3% of their total contents and the Cd was concentrated (>87%) in the residual phase (Table 2).
The Cu content leached from the tailings sediment sample (0.57–1.12%) was lower than that leached from the soil sample (1.16–3.76%) at strong acid conditions (pH = 1.0–1.5) (Table 3), probably because exchangeable, acid soluble, reducible, and oxidizable fractions for the tailings sediment sample (51.5%) were lower than those for the soil sample (86.5%) and the Cu was more bound to the residual phase for the tailings sediment sample (48.5%) than for the soil sample (15.5%) (Table 2).
Conclusions
The soil and tailing samples from the Cheongyang tungsten mine of Korea contained various metals such as As, Pb, Zn, Cd, Fe, and Cu. In general, very high levels of heavy metals are found in the study area. The major sources of these elements in the soil and tailings sediment samples were As-, Zn- and Pb-bearing sulfides, Pb carbonates (i.e., cerussite), Pb sulfates (i.e., anglesite), and secondary Fe-(oxy)hydroxides.
The speciation data indicates that uniform fractionation pattern was observed for all the samples except for S3. As, Cd, Pb, and Fe are mainly associated with the residual fraction (average 94.7, 90.4, 78.5 and 82.4% of total concentrations for soils and tailings sediments), and Cu (73.9% of total Cu concentrations) and Zn (69.6% of total Zn concentrations) in the oxidizable fraction. Trace metals associated with both fractions can be attributed mainly to the dissolution of metal sulfides present in the solid matrix, and assumed to remain in the solid matrix for longer periods but may be mobilized by decomposition processes. The differences in fractionations of these metals are related to weathering behaviors of various sulfide minerals. The enhanced significance of exchangeable and acid soluble fraction in the S3 sample is probably a consequence of more alkaline and calcareous nature of the sample, resulting in being more mobile and potentially more dangerous for the environment of the study area.
The potential production of acidity deriving from the interaction between water and tailing samples was not buffered because of the absence of carbonates. At initial pH between 3.0–5.0, the release of larger amounts of As, Pb, Cd, and Zn from tailing samples can affect the quality of surface water and groundwater in the mine area, while these elements remain fixed in soil samples due to its acid buffering capacity, suggesting that the major portions of these metals are contained in the less soluble or insoluble secondary minerals in the soil samples.
On the other hand, at initial pH < 3.0, the As, Pb, Zn, and Cd contents tended to significantly increase for both the soil and tailing samples at an equilibrium pH lower than 3.5. The Pb, Zn, and Cd contents leached from the soil samples were higher than those leached from the tailing samples, whereas the As content was lower. This discrepancy was attributed to the different mineral forms of the metals in the samples.
In addition, the results of kinetic tests with the soil and tailing samples indicated that the mobility increased after 7 days up to 30 days (i.e., by the end of the tests) in both sample types, regardless of the initial pH. These results suggest that further dissolution of these heavy metals from soil and tailing samples may occur during extended rainfall, thereby posing a serious threat to surface and groundwater in the mine area. The applied methods for evaluation of tailing sediments and contaminated soils used in this study might be useful to assess the environmental impact of abandoned mine sites.
References
Álvarez-Valero A, Sáez R, Pérez-López R, Delgado J, Nieto JM (2009) Evaluation of heavy metal bio-availability from Almagrera pyrite-rich tailings sediments dam (Iberian Pyrite Belt, SW Spain) based on a sequential extraction procedure. J Geochem Explor 102:87–94
Anju M, Banerjee DK (2010) Comparison of two sequential extraction procedures for heavy metal partitioning in mine tailings sediments. Chemosphere 78:1393–1402
Belzile N, Lecomte P, Tessier A (1989) Testing readsorption of trace elements during partial chemical extraction of bottom sediments. Environ Sci Technol 23:1015–1020
Bigham JM, Schwertmann U, Carlson L, Murad E (1990) A poorly crystallized oxyhydroxysulfate of iron formed by bacterial oxidation of Fe(II) in acid mine waters. Geochim Cosmochim Ac 54:2743–2758
Bonissel-Gissinger P, Alnot M, Ehrhardt JJ, Behra P (1998) Surface oxidation of pyrite as a function of pH. Environ Sci Technol 32:2839–2845
Chlopecka A (1996) Assessment of form of Cd, Zn and Pb in contaminated calcareous and gleyed soils in southwest Poland. Environ Sci Technol 188:253–262
Clevenger TE (1990) Use of sequential extraction to evaluate the heavy metals in mining wastes. Water Air Soil Pollut 50:241–254
Craw D, Falconer D, Youngson JH (2003) Environmental arsenopyrite stability and dissolution: theory, experiment, and field observations. Chem Geol 199:71–82
Dang Z, Liu C, Haigh MJ (2002) Mobility of heavy metals associated with the natural weathering of coal mine spoils. Environ Pollut 118:419–426
Fanfani L, Zuddas P, Chessa A (1997) Heavy metals speciation analysis as a tool for studying mine tailings sediments weathering. J Geochem Explor 58:241–248
Forsberg LS, Ledin S (2006) Effects of sewage sludge on pH and plant availability of metals in oxidizing sulphide mine tailings sediments. Environ Sci Technol 358:21–35
Fuge R, Pearce FM, Pearce NJG, Perkins WT (1993) Geochemistry of Cd in the secondary environment near abandoned metalliferous mines, Wales. Appl Geochem 8:29–35
Heikkinen PM, Räisänen ML (2009) Trace metal and As solid-phase speciation in sulphide mine tailings sediments–Indicators of spatial distribution of sulphide oxidation in active tailings sediments impoundments. Appl Geochem 24:1224–1237
International Organization for Standardization (1995) International Standard. Soil quality: Extraction of trace elements soluble in aqua regia. ISO 11466
Johnson CA, Thornton I (1987) Hydrological and chemical factors controlling the concentrations of Fe, Cu, Zn and As in a river system contaminated by acid mine drainage. Water Res 21:359–365
Karathanasis AD, Thompson YL (1995) Mineralogy of iron precipitates in a constructed acid mine drainage wetland. Soil Sci Soc Am J 59:1773–1781
Kersten M, Förstner U (1986) Chemical fractionation of heavy metals in anoxic estuarine and coastal sediment. Water Sci Technol 18:121–130
Kheboian C, Bauer CF (1987) Accuracy of selective extraction procedures for metal speciation in model aquatic sediments. Anal Chem 59:1417–1423
Kim J, Davis AP, Kim KW (2003) Stabilization of available arsenic in highly contaminated mine tailings sediments using iron. Environ Sci Technol 37:189–195
KMA (Korea Meteorological Administration) (2003) Annual Climate Report 2003 (in Korea)
Lee PK, Kang MJ, Choi SH, Touray JC (2005) Sulfide oxidation and the natural attenuation of arsenic and trace metals in the waste rocks of the abandoned Seobo tungsten mine, Korea. Appl Geochem 20:1687–1703
Li X, Thornton (2001) Chemical partitioning of trace and major elements in soils contaminated by mining and smelting activities. Appl Geochem 16:1693–1706
Lottermoser BG, Ashley PM (1998) Environmental impacts of abandoned metalliferous mine sites, New England Orogen, New South Wales. Geol Soc Aust Abstr 49:283
Ma LQ, Rao GN (1997) Chemical fractionation of cadmium, copper, nickel, and zinc in contaminated soils. J Environ Qual 26:259–264
Manning BA, Fendorf SE, Goldberg S (1998) Surface structures and stability of arsenic(III) on goethite: spectroscopic evidence for inner-sphere complexes. Environ Sci Technol 32:2383–2388
Masscheleyn PH, Delaune RD, Patrick WH Jr (1991) Effects of redox potential and pH on arsenic speciation and solubility in a contaminated soil. Environ Sci Technol 25:1414–1419
McCarty DK, Moore JN, Marcus WA (1998) Mineralogy and trace element association in an acid mine drainage iron oxide precipitate; comparison of selective extractions. Appl Geochem 13:165–176
McGregor RG, Blowes DW, Jambor JL, Robertson WD (1998) The solid-phase controls on the mobility of heavy metals at the Copper Cliff tailings sediments area, Sudbury, Ontario, Canada. J Contam Hydrol 33:247–271
Moses CO, Herman JS (1991) Pyrite oxidation at circumneutral pH. Geochi et Cosmochi Acta 55:471–482
Mossop KF, Davidson CM (2003) Comparison of original and modified BCR sequential extraction procedures for the fractionation of copper, iron, lead, manganese and zinc in soils and sediments. Anal Chimi Acta 478:111–118
Palumbo-Roe B, Klink B, Banks V, Quigley S (2009) Prediction of the long-term performance of abandoned lead zinc mine tailings sediments in a Welsh catchment. J Geochem Explor 100:169–181
Quevauviller P, Rauret G, Griepink B (1993) Conclusions of the workshop-single and sequential extraction in sediments and soils. Int J Environ Anal Chem 51:231–235
Rodríguez L, Ruiz E, Alonso-Azcárate J, Rincón J (2009) Heavy metal distribution and chemical speciation in tailings sediments and soils around a Pb–Zn mine in Spain. J Environ Manag 90:1106–1116
Roussel C, Néel C, Bril H (2000) Minerals controlling arsenic and lead solubility in an abandoned gold mine tailings sediments. Environ Sci Technol 263:209–219
Sahuquillo A, López-Sánchez JF, Rubio R, Rauret G, Thomas RP, Davidson CM, Ure AM (1999) Use of a certified reference material for extractable trace metals to assess sources of uncertainty in the BCR three-stage sequential extraction procedure. Anal Chim Acta 382:317–327
Shaw SC, Groat LA, Jambor JL, Blowes DW, Hanton-Fong CJ, Stuparyk RA (1998) Mineralogical study of base metal tailings sediments with various sulfide concentrations, oxidized in laboratory columns and field lysimeters. Environ Geol 33:209–217
So CS, Yun ST, Lee JH (1993) Hydrothermal W-Mo mineralization of the Cheongyang mine, Republic of Korea : A fluid inclusion and stable isotope study. Econ Geol (in Japan) 88:63–82
Strömberg B, Banwart S (1999) Weathering kinetics of waste rock from the Aitik copper mine, Sweden: scale dependent rate factors and pH controls in large column experiments. J Contam Hydrol 39:59–89
Tessier A, Campell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51:844–851
Ure A, Quevaullier Ph, Muntau H, Griepink B (1993) Speciation of heavy metal in soils and sediments. An account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the Commission of the European Communities. Int J Environ Anal Chem 51:135–151
Wang S, Mulligan CN (2009) Effect of natural organic matter on arsenic mobilization from mine tailings sediments. J Hazard Mater 168:721–726
Webster JG, Nordstrom DK, Smith KS (1994) Transport and natural attenuation of Cu, Zn, As and Fe in the acid mine drainage of Leviathan and Bryant Creeks. In: Alpers CN, Blowes DW (eds) Environmental geochemistry of sulphide oxidation. American Chemical Society, Symposium Series 550, Washington DC, pp 244–260
Webster JG, Swedlund PJ, Webster KS (1998) Trace metal adsorption onto acid mine drainage Fe(III) oxyhydroxysulphate. Environ Sci Technol 32:1361–1368
Williamson MA, Rimstidt JD (1994) The kinetics and electrochemical rate-determining step of aqueous pyrite oxidation. Geochim Cosmochim Ac 58:5443–5454
Yang JS, Lee JY, Baek K, Kwon TS, Choi J (2009) Extraction behavior of As, Pb, and Zn from mine tailings sediments with acid and base solutions. J Hazard Mater 171:443–451
Yellishetty M, Ranjith PG, Lalit Kumar D (2009) Metal concentrations and metal mobility in unsaturated mine wastes areas of Goa, India. Resour Conser Recyc 53:379–385
Acknowledgments
This work was financed by the Core R&D Program of the Korean Ministry of Science and Technology, under contract M1-0324-00-0009-03-B31-00-003-00 with the Korea Institute of S&T Evaluation and Planning.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, PK., Kang, MJ., Jo, H.Y. et al. Sequential extraction and leaching characteristics of heavy metals in abandoned tungsten mine tailings sediments. Environ Earth Sci 66, 1909–1923 (2012). https://doi.org/10.1007/s12665-011-1415-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-011-1415-z