Abstract
Large salinated areas are distributed in the middle and east of the North China Plain (NCP), where the fresh water shortage is serious. In this study, two sites in Cangzhou (CZ) and Hengshui (HS) of Hebei Province were selected to study the dynamics of shallow groundwater level and salinity. Electrical conductivity (EC) of groundwater was combined with the isotope compositions of δ18O and δ2H to identify the origin of salinity. Results showed that the dynamics of groundwater level at both sites were mainly controlled by precipitation and evaporation. Soil texture and structure played a significant role in the dynamics of salinity. The summer precipitation diluted the EC of groundwater at the HS site with homogeneous soil of sand loam, suggesting the larger infiltration rate; however, it did not dilute the EC at the CZ site with heterogeneous soil of sand loam and silt loam, suggesting that the summer precipitation could not recharge the groundwater directly. In winter, the EC decreased rapidly due to the temperature gradient underground if the groundwater was above the threshold level (at least 3 m below the ground surface) after the rainy season. Isotopes of δ18O and δ2H showed that precipitation was the major recharge source for the groundwater at the two sites. The salt mainly comes from the dissolution of soil or rock at the CZ site. While, the evaporation effect was strong at the HS site leading to the increase of the salt concentration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater dynamics reflect a complex natural process that is influenced by various factors in an environment (Chen et al. 1988). The dynamics of groundwater depend greatly on climate, geology, and topography, which are regarded as the three main factors that control subsurface water flow in the hydrologic landscape (UNEP 1996; Sophocleous 2002). In plain areas with shallow groundwater under a semi-arid climate, precipitation infiltration and evapotranspiration in the vertical direction are the major recharge and discharge processes of the water cycle. The dynamics of the groundwater level and salinity interact intimately with each other during the recharge and discharge processes. Groundwater affects the soil formation processes and moisture and solute transport dynamics, and is one of the important factors ameliorating soil conditions (Kats 1976). Excessive recharge and high evaporation in arid and semiarid climates can lead to a high water table that will trigger salinity of the soil (Salama et al. 1999; FAO 1996). Salinization of aquifers has become a major concern that degrades water quality and endangers future water exploitation in arid and semi-arid regions of the world (Mehta et al. 2000, Gaye 2001).
In the NCP, where groundwater is the major water source for industrial, agricultural, and domestic use, the shortage of freshwater resources is very serious and threatens the sustainable development of agriculture. Especially in the middle and eastern parts of NCP, a large area of saline water is distributed in the shallow groundwater aquifer. Since the late 1950s, agriculture irrigation has raised the groundwater level, which has caused soil sanitization and enlarged the saline area to 69% of NCP. Though the exploitation of deep groundwater led to a decline in the shallow groundwater level which made the sanitization area decreased, the water problem is still serious. Monitoring the dynamics of the groundwater level and salinity and identifying the origin of the salinity are crucial for both water management and remediation in this salinated and water-deficient region.
To identify the sources of salinity, the traditional approach is the geochemical method. The applications of isotope tracers to hydrology, the fundamental relationships between δ18O and δ2H and between δ18O and salinity have been used in previous studies to identify different salinization pathways (Gaye 2001). These pathways include flushing of airborne salts by precipitation, dissolution of evaporitic minerals from the surface, soil, or aquifer components, the seawater intrusion, or the flow induced from pockets of connate brine (Lee et al. 1999; Mehta et al. 2000; Gaye 2001; Kim et al. 2003; Zhang et al. 1997). In the NCP, studies have shown that saline water may be caused by the continental salinity and seawater intrusion during the geological history (Fang and Chen 2005; Zhang 2005). Geochemical data of total dissolved solids (TDS) and isotope data of 2H, 18O, 36Cl, and 3H studies have indicated that the saline water is due to the dissolution of salt rock after the precipitation recharge in the middle and eastern plains, and that the condensation effect due to evaporation is a major influence factor in the salinization of groundwater (Zhou et al. 1998, 2001).
Above researches in the NCP were carried out on a large spatial scale, and it did not provide detailed information about the water and salt dynamics under different hydrologic conditions. In this study, two sites were selected as the typical areas of saline groundwater with shallow water table to study the water and salt dynamics over a 4-year period. Automatic monitoring loggers provided by joint Chinese and Japanese cooperation were set to monitor the water table, EC of groundwater, and soil temperature at different depths. According to the fluctuations of water table and EC, factors that affected the groundwater dynamics were analyzed. By combining the EC data with that of stable isotopes in the groundwater, the origin of the salinity in the groundwater was discussed in detail.
Site description
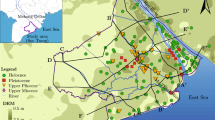
Two sites of CZ and HS of Hebei Province were selected. These two sites lie in the middle and east of the NCP and have groundwater depths of 0.4–5.0 m. The CZ site lies in the east alluvial coast plain, and the HS site lies in the middle of the flood and lake sedimentary plain (Fig. 1). Both sites have similar semi-arid continental climate conditions. During the period of 1971–2004, the annual mean precipitation was about 500–600 mm, and the precipitation from June to September accounted for 70–80% of the total annual precipitation. The annual evaporation is about 1,000–1,500 mm, and about 45% of the evaporation occurs from April to June. Especially in winter and spring, the evaporation is 16–38 times more than the precipitation.
Position of Cangzhou (CZ) and Hengshui (HS) sites and distribution of saline water with total dissolved solids (TDS) >2 g/l in the Hebei Plain (adapted from Zhang 2005)
The soil texture and vegetation at the two sites are described by Song et al. (2009). The soil of the CZ site is composed of silt loam and sand loam, indicating a heterogeneous soil structure, and the land is covered with high grass with roots depth about 200 cm. The soil of the HS site is mainly composed of sand loam showing a relatively homogeneous soil structure, and the land is covered with shorter grasses with roots depth about 30 cm (Fig. 2, Table 1). The unconsolidated sediments of Quaternary Q4 constitute the main stratigraphy in this area. The shallow groundwater aquifers include aquifer groups of Holocene Qh4 with a depth of 10–20 m, upper Pleistocene Qp3 with a depth of 50–70 m, and mid Pleistocene Qp2 with a depth of 80–160 m (Wang et al. 2008).
Methods
Data observation and monitoring
Precipitation was collected using a rain gauge and recorded manually at both sites. The data series of the CZ and HS site are from 1 January 2003 to 20 July 2007 and from 1 March 2003 to 30 October 2005, respectively. Evaporation was measured using an E601 evaporation pan and recorded manually at the CZ site from 2003 to 2007, excluding the winter. It was also observed using a 20-cm diameter (D-20 cm) evaporation pan during the whole year. The correlation between the values of these two evaporation was obtained to interpolate the absent E601 data (the Pearson correlation coefficient, r = 0.834, P < 0.01, n = 1092). The evaporation data were not obtained at the HS site.
The groundwater level was monitored using the automatic KADEC-MIZU II data loggers from Japan, which were provided by the National Institute for Environmental Studies of Japan. EC was monitored as an indicator of groundwater salinity. The measured value was calibrated under 25°C. The temperatures at soil depths of 10, 40, and 100 cm were measured simultaneously.
The water table, EC, and temperature were monitored every 30 min or 1 h from October 2003 to July 2007.
Sample collection and isotope analysis
Rainwater samples were collected with rain gauges at the two experimental stations. Samples were collected daily during precipitation events from 26 June 2005 to 21 September 2005 at the CZ Site and from 12 July 2005 to 7 October 2005 at the HS Site.
Groundwater samples were collected from 5 July 2005 to 30 December 2005 at the CZ site and from 6 July 2005 to 11 December 2005 at the HS site. Sampling frequency varied between 1 and 10 days. In general, samples were collected with a frequency of one per day during July and August.
All samples including precipitation and groundwater at both sites were collected in air-tight polyethylene vials of 50 or 100 ml and sealed with adhesive tape to prevent evaporation. Two bottles were collected for each sample if possible and brought back to the laboratory for analysis.
Hydrogen (δ2H) and oxygen (δ18O) compositions of the water samples were analyzed using a Finnigan MAT-253 mass spectrometer with the TC/EA method at the Institute of Geographical Sciences and Natural Resources Research, Chinese Academy of Sciences. The δ2H and δ18O results are presented in the standard δ-notation as per mil (‰) difference from Vienna standard mean ocean water (VSMOW). The uncertainty for δ2H and δ18O are ± 2 and ± 0.3‰, respectively.
Results
During the observation period, the annual precipitation was 620.6, 492.1, 581.2, and 430.9 mm in 2003, 2004, 2005, and 2006, respectively. They delegate the years with abundant, dry, average and dry rainfall, respectively. The surface water evaporation was transformed into the soil evaporation with an experimental coefficient. The coefficients from 2003 to 2006 were 0.671, 0.658, 0.674, and 0.637, respectively, and the corresponding soil evaporation was 1002, 1026, 1079 and 853 mm for each year (Fig. 3). The evaporation in 2006 was the lowest because the precipitation was lowest in that year. At the HS site, only the precipitation was recorded from 1 March 2003 to 20 October 2005 (Fig. 4).
The statistical value of the groundwater level, groundwater EC, and soil temperature at 10, 40 and 100 cm depth (G temp-10, G temp-40, and G temp-100) are listed in Table 2, and the results were showed in Figs. 5, 6, and 7, respectively. The mean water tables of the CZ and HS sites were 4.65 and 17.63 m, respectively. The mean EC value at the HS site was 12081 μs/cm, which was about 6 times larger than that of the CZ site with the EC value of 2107 μs/cm. According to the FAO classification, the average groundwater salinity value of the CZ site fell into the category of slightly saline and that of the HS site fell into the category of moderately saline. At both sites, the soil temperature increased with depth. The variation coefficient of the observation items excluding the water table at the HS site was larger than that at the CZ site.
The isotope values in precipitation and groundwater are shown in Table 3. The δ18O and δ2H values of precipitation at the CZ site ranged from −9.7 to −3.9‰ and −76 to −29‰, with mean values of −7.2 and −56‰, respectively. The δ18O and δ2H values in precipitation at the HS site ranged from −11.8 to −4.4‰ and −82 to −30‰, with mean values of −7.6 and −52‰, respectively. The δ18O and δ2H values of groundwater at the CZ site ranged from −8.4 to −5.4‰ and −63 to −51‰, with mean values of −7.1 and −58‰, respectively. The δ18O and δ2H values of groundwater at the HS site ranged from −9.6 to −6.2‰ and −70 to −59‰, with mean values of −7.7 and −65‰, respectively. The absolute isotope values of coefficients of variation of precipitation were larger than those of groundwater, suggesting that the effect of precipitation was attenuated during the process of precipitation recharging the groundwater.
Discussion
Dynamics of groundwater level and EC
The dynamics of water tables in the NCP are affected mainly by precipitation and evaporation, and salt movement had a close connection with the water table. The EC is positively correlated with the concentration of ions, which can thus be indirectly calculated from EC. Therefore, EC can be regarded as a water salinization index (Liu et al. 2003). The dynamics of groundwater are discussed from the viewpoint of different influencing factors.
Effects of precipitation and evaporation on dynamics of groundwater level
The groundwater level showed a declining trend from 2003 to 2007 at the CZ site (Fig. 5a). This was mainly caused by the over-exploitation of the deep aquifer in CZ City. It was reported that the groundwater depth in the center cone of the deep aquifer was 91 m in 2005, and the cone area was still enlarging in recent years in CZ City (Zhang 2007). At the HS site, the groundwater level tended to increase slightly during the study period (Fig. 5b).
The water tables at both sites varied within the period of a year, and they showed the similar seasonal variations due to the seasonal distribution of precipitation and evaporation. The water level increased to the highest in August or September after the precipitation events and then decreased slowly. However, some differences existed in the yearly fluctuation of groundwater. The lowest water level at the CZ site was in July because of the highest evaporation rate and the low precipitation recharge during this period (Fig. 3); while there were two obvious low and high values in each year at the HS site. The first low value was in March when there was no precipitation recharge (Fig. 4). After March, the water level increased quickly and reached the first high value in May, which indicated that the groundwater was recharged by the thawing of frozen water in the upper soils as the soil temperature increased (Fig. 7). The thawing water also had some effects on the groundwater level fluctuation at the CZ site; however, it increased just a little during the melting period (Fig. 5a). In June, the groundwater level declined to the second low value due to the evaporation, and then increased to the second high value due to the precipitation recharge.
Precipitation dilution and temperature effect on dynamics of groundwater salinity
The amount of precipitation is an important factor to groundwater salinity. In general, groundwater salinity will be diluted after the recharge of a precipitation event, as shown by the dynamics of the HS site with declining EC value after the precipitation (Fig. 6b). However, at the CZ site, the EC value did not change after the precipitation recharge, suggesting that the summer precipitation could not recharge the groundwater directly (Fig. 6a). This might be caused by the difference of soil texture and construction.
At the CZ site, the salinity decreased to the lowest level in January of the next year only after a year of abundant or average rainfall (2003 and 2005). This was caused by the redistribution of salt between the soil water and groundwater during the winter season. Fang (1982) studied the dynamic principle of water and salt in soil and the results showed that the water and salt in the soil profile moved upward during the freezing period while downward during the thawing period. During this process, temperature played an important role in the movement of soil water (Philip and De Vries 1957). As Figs. 6 and 7 show, the EC decreased with the decrease of temperature in October of 2003 and 2005, and after October, the temperature difference between the top soil (10 cm) and lower soil (40 cm) became larger and larger. The decrease of the soil temperature at the top soil depth destroyed the energy balance. As a result, water and salt moved upward until January when the EC reached the lowest value, and the soil temperature gradient was the largest. After that the temperature increased gradually, and the soil water and salt moved downward and began to recharge the groundwater gradually leading to the increase in the groundwater level and salinity.
The above phenomenon at the CZ site existed only in the winters after 2003 and 2005 because water and salt movements have a close connection with the water table. In years with the abundant rainfall (2003 and 2005), recharged by the precipitation the water table increased to the highest value of the year before winter. The water table was the highest in autumn with the values of 6.04 m and 5.20 m in 2003 and 2005 (Table 4), respectively, which created the condition for water and salt transfer between groundwater and soil water when the temperature decreased in the winter season. As a result, both the water table and EC in the winter season reached their smallest values of the year. Though the temperature gradient existed, the salinity transportation in water was very insignificant when the groundwater level was low with the mean values of 4.91 m and 4.23 m in the autumn season of 2004 and 2006, respectively (Fig. 5a). The EC value increased continuously from winter to spring. It seemed that desalinization of the groundwater occurred in winter if the water table in autumn reached a certain threshold level. According to the field observation data of groundwater level from 2003 to 2007, a water table of 5 m (with a groundwater depth of 3 m) was defined as the threshold value at the CZ site. When the groundwater rose above 3 m from the ground surface, the EC value decreased greatly in winter. This level depends to a large extent on the soil texture, its hydraulic properties, the general hydrogeologic and geochemical conditions, and the crops grown (Schmidt 1985).
The temperature had an effect on the dynamics of the groundwater EC in each year at the HS site because the water table was larger than 17 m with the largest groundwater depth of 3 m, which might exceed the threshold value (Figs. 6b, 7b; Table 4). However, the EC dynamics in the winters of 2005 and 2006 were not more abrupt than those of 2003 and 2004. Though the precipitation data during the study period are not available until now, it was concluded from the correlation between the precipitation and groundwater level (Fig. 5b) that the rainfall was abundant in 2005 and 2006. In addition, the effect of the precipitation amount was so obvious that the temperature effect was not reflected obviously in the EC curve (Fig. 6b). The salinity of groundwater was diluted under the control of a large amount of precipitation.
Hydrogeological conditions and soil texture effects on groundwater dynamics
Besides the above differences in groundwater dynamics, the dynamic curves of the water table and EC at the HS site were more abrupt than those of the CZ site (Figs. 5 and 6). Comparing the conditions of the two sites (Figs. 2, 3, and Table 1), the groundwater dynamics depended greatly on hydrogeological conditions and soil texture effects (Paul et al. 2004). In sites (e.g., the CZ site) where the soil is fine textured with sandy loam and silty loam interlayer, the local lateral groundwater flow is slow because the aquifer of this site is composed of the alluvial coastal sediments. Vertical groundwater dynamics (rise and fall) were slower than that in the coarse soils, however, the groundwater rise was higher and it might reach or be close to the soil surface under particular conditions. As a result, the range and coefficient of the water table were also higher (Table 2). For example, after a large storm with 109 mm precipitation on the 23rd July 2005 and several continuous weeks of infiltration of precipitation, the groundwater rose to 7.47 m on the 19th August 2005. By contrast, precipitation infiltrated more easily at the HS site and the lateral runoff condition was also better, which led to an abrupt increase of the water table. Salts could be retained in the fine-textured soils while they were easily flushed out of coarse soils under a large amount of precipitation. Fang and Chen (2005) have pointed out that the precipitation infiltrated and leached downward to recharge groundwater, which could refresh and dilute the salt of the soil water or groundwater during the rainy season. In the dry season, the shallow groundwater would be sanitized because of the evaporation and condensation effects. However, in this study it was concluded that this was not always what occurred and that the dilution effect was confined by the soil texture and construction. In addition, the temperature changes in winter had a great effect on the variation of groundwater EC when the water table reached a certain threshold value. As a result, if the drainage is taken at the beginning of the thawing period, it will help to desalinate the soil and groundwater.
Isotope evidence for origin of salinity in groundwater
Recharge source of groundwater
Precipitation is the major input to groundwater in the study area. The isotope compositions in precipitation can be used as a reference to study the features of groundwater.
Figure 8 shows the relationship between δ2H and δ18O in groundwater in 2005 at the CZ and HS sites, which were compared with the local meteoric water line (LMWL). Except for three points of the CZ site, other data lay below the LMWL, which suggested that the groundwater was mainly recharged by the precipitation. Especially, the points of HS site distributed further away from the LMWL than the points of the CZ site which displayed the stronger evaporation effect at the HS site. Zhou et al. (1998, 2001) provided evidence of the groundwater recharge source through the study of isotopes 3H, 2H, and 18O in the middle and east of the Hebei Plain and isotopes 2H and 18O at the CZ site. Zhang (2005) used the isotope 36Cl in the CZ City to study the groundwater source. These studies indicated that the shallow groundwater sourced from precipitation and experienced evaporation and condensation effects. The results of this paper are in agreement with the finding that the groundwater was mainly recharged by precipitation.
Relationship of δ2H-δ18O in groundwater at two sites: CZ and HS. The local meteoric water lines (LMWL) were obtained by regression with precipitation samples at two sites (Song et al. 2009)
Origin of salinity in groundwater
The ranges of the isotope compositions in groundwater at both sites are similar (Table 3). However, the relationships between the δ2H composition in groundwater and the EC values are different at the two sites (Fig. 9). The δ18O composition and the EC value have the similar characteristics which are not showed here. Research has verified that the δ2H or δ18O isotopes increase with increasing TDS because of the evaporation effect (Tantawl et al. 1998). At the CZ site, the δ2H values do not show this trend with the increasing EC value, obviously (Fig. 9a). The data points in this figure can be divided into two groups: Group I, for data from July to September, the δ2H had a large variation from −63 to −51‰ around the EC value of 2,330 μs/cm. From the above discussion, it was concluded that the evaporation effect occurred to some extent at this site. In Fig. 6a), the EC value increased from July to September 2005 from 2, 238 to 2, 339 μs/cm with small variation. This implies that the evaporation had slight effect on the salinity of groundwater; Group II, for data from October to December, the δ2H values had a small variation range with the opposite correlation between them. Because the groundwater moved upward to recharge the soil water in winter, as discussed above, the enrichment of isotopes might be caused by other processes. Due to less evaporation during this period (Fig. 4), the salinity of the groundwater might be sourced from the dissolution of soil and rock or the mixing effect in the processes of precipitation infiltration instead of a simple evaporation effect. As for the HS site, the data points from July to September showed a positive correlation between the isotopes of δ2H and the EC value. However, the trend from October to December was not obvious (Fig. 9b). In combination with Fig. 8, the significant effect of evaporation on the accumulation of salt was identified, and it was shown that the evaporation effect from July to September was stronger than that from October to December.
Conclusions
Two sites (CZ and HS) were selected for the study of water and salt dynamics in the shallow groundwater regions of the NCP. The results showed that the water table obviously reflected the precipitation and evaporation effects and that the temperature variation also has a slight effect on the dynamics of groundwater fluctuation during the winter period. The dynamics of the EC curve showed an obvious decreasing point in winter after the years of abundant rainfall at the eastern site (CZ) or in winter of every year at the middle site (HS). That was due to the existence of the temperature gradient underground leading to the redistribution of water and salt between the groundwater and the soil water. In particular, when the groundwater in autumn rose above a certain threshold level, the EC of groundwater decreased in winter. According to the observation data of groundwater dynamics, the depth of 3 m was defined as the threshold value. Precipitation might affect the dynamics of groundwater salinity if the precipitation can infiltrate into and recharge the groundwater quickly and directly. Salts were retained in the fine-textured soils, while they were easily flushed out of the coarse soils by the precipitation during the rainy season. In any event, hydrogeological conditions and soil textures played a great role in the different variation features under the same climate conditions. Based on the soil texture, the extents of precipitation, evaporation, and temperature effects led to different water and salt dynamics.
The δ2H and δ18O isotope compositions and the EC values provided evidence of the recharge sources of groundwater and the origin of groundwater salt. The groundwater at the eastern site (CZ) was recharged by precipitation and the groundwater salt originated mainly from the dissolution of rock, and the evaporation effect was relatively insignificant. The groundwater at the central site (HS) was recharged mainly by the precipitation of the same year, and the strong evaporation and condensation effects existed. As a result, the high salinity of groundwater was caused by evaporation. Though features of the dynamics under different conditions were analyzed in this paper, detailed information about the water and salt movement has not been obtained. However, the high frequency monitoring data will be useful for studying the detailed mechanism of the dynamics. We will consider the effects of water, salt, and temperature together in further study.
References
Chen B, Hong Z, Wang F (1988) Groundwater dynamics and prediction. Science Press, Beijing
Fang R (1982) Preliminary study on the dynamical regime of water and salt during the freezing and thawing period of soil. Acta Pedol Sin 19:164–172 (in Chinese)
Fang S, Chen X (2005) Influence of atmospheric precipitation on soil leaching and desalinization in the North China Plain. Acta Pedol Sin 42:730–736 cnki:ISSN:0564-3929.0.2005-05-004
Gaye CB (2001) Isotope techniques for monitoring groundwater salinization. Hydrogeol J 9:217–218
Kats DM (1976) The influence of irrigation on the groundwater. Kolos Pub, Moscow (in Russian)
Kim Y, Lee KS, Koh DC, Lee DH, Lee SG, Park WB, Koh GW, Woo NC (2003) Hydrogeochemical and isotopic evidence of groundwater salinization in a coastal aquifer: a case study in Jeju volcanic island, Korea. J Hydrol 270:282–294
Lee KS, Wenner DB, Lee I (1999) Using H− and O− isotopic data for estimating the relative contributions of rainy and dry season precipitation to groundwater: example from Cheju Island, Korea. J Hydrol 222:65–74
Liu C, Lin K, Kuo Y (2003) Application of factor analysis in the assessment of groundwater quality in a blackfoot disease area in Taiwan. Sci Total Environ 313:77–89
Mehta S, Fryar AE, Banner JL (2000) Controls on the regional-scale salinization of the Ogallala aquifer, Southern High Plains, Texas, USA. Appl Geochem 15:849–864
Paul LGV, Manfred D, Christopher M, Charles R, Nick VDG (2004) Spatial and temporal dynamics of groundwater table and salinity in Khorezm (Aral Sea Basin), Uzbekistan. University of Bonn, ZEF Series in Ecology and Development 23
Philip JR, De Vries DA (1957) Moisture movement in porous materials under temperature gradients. Trans Am Geophys Union 38:222–232
Salama RB, Otto CJ, Fitzpatrick RW (1999) Contributions of groundwater conditions to soil and water salinization. Hydrogeol J 7:46–64
Schmidt CM (1985) Estimation of ameliorative conditions of the irrigated lands in Uzbekistan. SANIIRI scientific report, Tashkent (in Russian)
Song X, Wang S, Xiao G, Wang Z, Liu X, Wang P (2009) A study of soil water movement combining soil water potential with stable isotopes at two sites of shallow groundwater areas in North China Plain. Hydrol Process 23:1376–1388
Sophocleous M (2002) Interactions between groundwater and surface water: the state of the science. Hydrogeol J 10:52–67
Tantawl MA, El-sayed E, Awad MA (1998) Hydrochemical and stable isotope study of groundwater in Saint Catherine-Wadi Feiran area, South Sinai, Egypt. J Afr Earth Sci 26:277–284
UNEP (1996) Groundwater: a threatened resource. UNEP Environ Libr (15):3–29
Wang S, Shao J, Song X, Zhang Y, Zhou X, Huo Z (2008) Application of MODFLOW and geographic information system to groundwater flow simulation in North China Plain, China. Eviron Geol 55:1449–1462
Zhang Z (2005) Groundwater in the vast North China Plain. Chin J Nat 27:311–315 (in Chinese)
Zhang Z (2007) Analysis on present situation and development trend of groundwater descent funnel in Cangzhou City. Groundwater 29:50–52 108
Zhang Z, Shi D, Ren F, Yin Z, Sun J, Zhang C (1997) Evolution of Quaternary groundwater system in North China Plain. Sci China Ser D 40:276–283
Zhou L, Liu C, Wang P (1998) The isotope composition of salt water of Quaternary Q4 in Hebei plain. Hydrogeol Eng Geol 3:4–8 (in Chinese)
Zhou L, Liu C, Jiang S, Zhang L (2001) The Tracing of 36Cl in Quaternary groundwater of the Cangzhou Area, Hebei plain. Bulletin Mineral, Petrol Geochem 20:418–420 (in Chinese)
Acknowledgments
The authors would like to thank engineers Liu Caitang, Li Lin, and Dr. Li Fadong who helped to set up the monitoring instruments at the experimental site, Chen Baogen who helped us to collect the data, and Zhang Wenmin, Qi Zhifang, Wu Jingfen, He Jie, et al., who all helped to observe the data and collect the samples. In particular, Professor Yu Jingjie and Professor Zhang Yinghua provided constructive suggestions on this research. The authors would also like to thank the editor and the anonymous reviewers for their valuable comments on the manuscript. This research was financial supported by the National Natural Funds Project “Water cycle study on the Taihang Mountain areas based on the isotope technology and water chemical” (40671034) and the corporation project between China and Japan “The observation of groundwater level in North China Plain”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, S., Song, X., Wang, Q. et al. Shallow groundwater dynamics and origin of salinity at two sites in salinated and water-deficient region of North China Plain, China. Environ Earth Sci 66, 729–739 (2012). https://doi.org/10.1007/s12665-011-1280-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-011-1280-9