Abstract
Hamamayagi thermal spring (HTS) is located along the North Anatolian Fault Zone. The thermal spring has a temperature of 36°C, with total dissolved solids ranging from 485.6 to 508.5 mg/L. Hard, brittle, and gray limestones Permian aged are the reservoir rocks of the HTS. δ18O–δ2H isotope ratios clearly indicate a meteoric origin for the waters. The δ34S value of sulfate in the thermal water is nearly 4.1‰ and implies a diagenetic environment characterized by reduced sulfur compounds. The δ13C ratio for dissolved inorganic carbonate in the HTS lies between −1.78 and −1.62‰, showing that it originates from the dissolution of fresh-water carbonates. Quartz geothermometry suggests a reservoir temperature of 52–85°C for the Hamamayagi geothermal field, but chalcedony geothermometers suggest reservoir temperatures between 30 and 53°C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
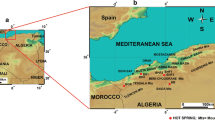
Hamamayagi thermal spring (HTS) is located in Ladik, south of Samsun in the Black Sea Region of Turkey (Fig. 1), along the North Anatolian Fault zone (NAFZ). The springs are used for swimming, bathing, and medical purposes. Geothermal studies in the Hamamayagi–Ladik region were started in 1985 by the General Directorate of Mineral Research and Exploration of Turkey (MTA). The spring area and its vicinity have been studied from the geological (Alp 1972; Göksu et al. 1974; Öztürk 1979; Ercan and Gedik 1983; Besbelli 1984; Serdar et al. 1984; Akdag 1992), petroleum (Gedik and Korkmaz 1984; Yoldaş et al. 1985), hydrogeological (Yenal et al. 1976; Akkuş et al. 1992; Özten and Yurtseven 1996; Erdem 2000; Başar 2003) and environmental points of view (Gülibrahimoglu et al. 2000). In Hamamayagi village, three wells were drilled to depths ranging from 178 to 298.7 m. The temperature of waters produced from these wells is 28–38°C (Akkuş et al. 2005).
The authors have performed hydrogeological, geochemical and isotopic studies of the HTS. The main objective of this paper is to discuss the hydrogeochemical evolution of HTS on the basis of physical and chemical properties, and environmental isotope compositions.
Geology and hydrogeology
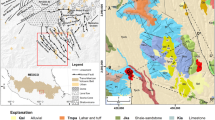
Formations in the study area were deposited at different times from Permian to Pliocene and generally consist of sedimentary rocks mixed with volcanic material. Akdag Formation of Permian age (Öztürk 1979) forms the basement and consists of hard, brittle, and gray colored limestones. Seyfe Formation of Lower Jurassic consists of sedimentary rocks with interbedded volcanics. Dogdu Formation of Late Jurassic-Early Cretaceous age, consisting of sandy limestone and gray limestone lies unconformably over the Seyfe Formation (Öztürk 1979). Yumaklı Formation of Late Cretaceous age consists of conglomerate, sandstone, sandy limestone, marl, shale, mudstone and tuff, and Tekkeköy Formation of Eocene age (Yoldaş et al. 1985) contains sedimentary and volcanic rocks. The Çerkeş Formation, consisting of bluish-gray colored marl, sandy limestone, conglomerate, shale and tuff, lies along the North Anatolian Fault (NAF). Alluvium of Quaternary age lies unconformably over the older units (Fig. 2).
According to Şengör, (1980), Şengör et al. (1983), and Şaroglu et al. (1987), two periods of paleotectonic and neotectonic activity occurred in the study area and its vicinity. The most important tectonic structure is the North Anatolian Fault which crosses just to the north of the study area and trends ESE-WNW. One fault (designated F2 in Fig. 2), located south of and parallel to NAF, is crossed by another (designated F3) trending NW–SE. The HTS discharges are mainly associated with the intersection of faults F2 and F3.
The main bodies of surface water in the study area are the Tersakan River and the Hamamayagi Stream. HTS is located about 25–30 m from the Hamamayagi Stream. The most important cold spring is KPC, located about 6 km south of the study area, and it has a perennial flow. The spring occurs at the contact of the Dogdu and Çerkeş Formations. Discharge rate of KPC is 3 L/s and its temperature is between 12 and 16°C. Hamamayagi geothermal field is located in the North Anatolian Fault Zone. The temperature of the thermal waters, used for balneological and medical purposes, is between 34.7 and 36.7°C. Discharge rate of thermal spring is 18 L/s (Akkuş et al. 1992).
The reservoir rock of the HTS is the Akdag Formation, a fractured permeable limestone. Sedimentary rocks belonging to the Seyfe Formation and the sandy limestones, and gray limestones of the Dogdu Formation have low permeability. The conglomerates, sandstone and sandy limestone of the Yumaklı Formation, and the sedimentary units of the Tekkeköy Formation are permeable. The silty and clayey (?) upper level of the Çerkeş Formation has very low permeability and therefore acts as a cap rock.
Materials and methods
To characterize HTS, spring and surface water samples were collected at four sample points (HTS is thermal spring, KPC is cold spring, UHS and DHS are stream water, Fig. 2) during the rainy, arid, and intermediate seasons for major anion, cation, δ18O, δ2H, 3H, δ34S, and δ13C analyses. Only one sample was collected from the each sampling point for the minor and trace elements analyses. Surface water samples were gathered along the Hamamayagi Stream (HS), 150–200 m upstream and downstream from the HTS. Kocapınar Fountain (KPC) was used to provide groundwater samples (Fig. 2).
The sampling process was started in November 2006 and finished in October 2007. High-density polyethylene bottles were used for water samples. One-liter samples were collected for major cation–anion and 100 mL samples for minor and trace element analyses. For δ18O, δ2H and 3H analyses, 1-L samples were gathered. A 1-litrepolyethylene box was used for δ13C and a 2-L dark glass box for δ34S. The temperature of the water, the pH, electrical conductivity, and total dissolved solid values (TDS) were measured at the sampling locations.
Determination of the major anion (Cl, SO4, HCO3 and CO3) and major cation (Ca, Mg, Na and K) compositions of the water samples was carried out at the water chemistry laboratories of the International Research and Application Center for Karst Water Resources (UKAM) at the Hacettepe University (Ankara). Trace elements (Cu, Fe, Cr, Ni, Pb, Co, Mn, Zn etc.) were analyzed at the Acme Analytic Laboratory (Canada-ISO Accredited Co.). δ18O, δ2H, and 3H values of the water samples were determined at the Isotope Laboratory of the Technical Research-Quality Control (TAKK) Directorate of the State Hydraulic Works (DSİ) (Ankara). δ13C and δ34S isotopes analyses were done at the Ottawa University G.G. Hatch Stable Isotope Laboratories (Canada). The precision of the analyses is ± 0.15‰ for 18O, ±2‰ for 2H, ±0.2‰ for 13C, and ±0.2‰ for 34S.
The cation–anion analyses were carried out according to APHA, AWWA, and WPCF (1989) standards. Ca2+, Mg2+, Na+, and K+ concentrations were analyzed by atomic absorption spectrometry. SO4 concentrations were determined by spectrophotometer together with alkalinity standard titrimetry. Cl− was analyzed by the AgNO3 titrimetric method. Minor and trace elements analyses were carried out with the ICP/MS (inductively coupled plasma mass spectrometry) method. PHREEQC chemical equilibrium software (Parkhurst and Appelo 1999) was used to determine the saturation states of secondary minerals with respect to the waters.
Results and discussion
Water chemistry
The results of chemical analyses of HTS, KPC, and HS waters are given in Table 1. Temperature of the thermal waters is between 34.7 and 36.7°C and that of KPC is between 12.1 and 16°C and that of HS between 10 and 28.4°C. The pH values are between 6.9 and 7.1 for thermal water, 7.13 and 7.3 for KPC, and 7.99 and 8.42 for HS. Electrical conductivity values are in the range 591–598 μS/cm in HTS, 527–598 μS/cm in KPC and 294–456 μS/cm in HS. TDS values are between 485.6 and 508.5 mg/L in the thermal spring, 284.8–481.7 mg/L in KPC, and 260.7–393.8 mg/L in HS.
The distribution of ions in the thermal waters, cold spring, and stream waters is plotted on the Piper diagram (Fig. 3) (Piper 1944). HTS water is of a Ca–Mg–Na–HCO3 type, while KPC and HS waters are predominantly Ca–HCO3 type.
Trace element concentrations of the Hamamayagi thermal and cold waters are presented in Table 2. All waters are generally similar in trace element contents, although As, B, Li, Rb, and Sr contents are higher in the thermal springs.
Mineral equilibration
It is important to know saturation indices (SI) for some carbonate (e.g. calcite, aragonite, dolomite and magnesite), sulfate (e.g. gypsum and anhydrite), and silica (e.g. quartz, amorphous quartz and chalcedony) minerals to predict which ones may precipitate during the extraction and use of thermal waters. Mineral saturation indices of the thermal spring were calculated by the PHREEQC Interactive 2.8 computer code (Parkhurst and Appelo 1999) on the basis of outlet temperature and pH. Saturation indices of carbonate, sulfate, and silica minerals were computed (Table 3; Fig. 4).
The results show that most of the waters are close to saturation with respect to calcite and aragonite. Calcite, aragonite, and dolomite are slightly oversaturated for HTS and borehole waters, which are a Ca–Mg–Na–HCO3 water type. HS water is oversaturated with respect to calcite and aragonite in the summer period because of evaporation. While anhydrite, gypsum, and halite are undersaturated, quartz is slightly oversaturated for all of the waters.
Geothermometer
Many geothermometers have been developed to estimate subsurface temperature (Fournier 1977; Arnorssón et al. 1983), and have become an important tool for estimating temperatures of hydrothermal reservoirs. HTS water and borehole samples from Akkuş et al. (2005) were used for the geothermometer applications. The applicability of cation geothermometers was evaluated on the Na–K–Mg diagram (Fig. 5) after Giggenbach (1988). The waters attain water–rock equilibrium and all of the samples plot in the “immature waters” (shallow or mixed waters) field (Fig. 5). The cation geothermometer temperature predictions for such waters should be treated with caution (Giggenbach 1988). For this reason, silica geothermometers were used for the spring water in this study. The amorphous silica geothermometer (Fournier 1977) presented temperatures below zero and the chalcedony (conductive cooling) (Arnorssón et al. 1983) geothermometer gave temperatures similar to the surface temperature of the spring. Chalcedony geothermometers (Fournier and Potter 1982; Arnorssón et al. 1983) and quartz (Fournier 1977) geothermometers indicated temperatures about 30–53°C and 61–83°C, respectively (Table 4).
Giggenbach’s (1988) Na–K–Mg diagram for the Hamamayagi thermal water. All the water samples are located near the Mg vertex
Isotopic characteristics
δ18O, δ2H, and 3H isotopes were analyzed to determine the origin of waters, recharge altitude, precipitation types, and groundwater circulation (Table 5). The δ18O–δ2H diagram shows that all of the waters in the study area lie close to the Global Meteoric Water Line (MWL) (Fig. 6a) (Craig 1961), indicating a meteoric origin. According to the δ18O-temperature and δ18O–δ2H relations, all water samples recharged at the approximately same elevation in the plain (Fig. 6b). But the thermal waters have more negative oxygen isotope values. Therefore, thermal waters recharged at the higher altitude than cold waters. The 18O values of the river water vary because of the effect of snowfall on the river in the winter. The deuterium excess can be used identify the vapour source. In general, winter precipitation originating from the sea is characterized by higher excess values because of lower relative humidity (Dansgaard 1964). Deuterium excess values (De) in all the waters are high in rainy seasons (Table 5), because recharge is from rainfall originating from winter evaporation. Low tritium, high electrical conductivity and high Cl− values in the HTS indicate that this spring is supplied by circulating fluids deeper than KPC (Fig. 6c, d). High tritium and low EC values in the KPC show that this spring water has shallow circulations. The 3H-temperature relation in the geothermal field is presented in Fig. 6e. Very low tritium values of the HTS indicate long residence times and deep circulation.
In order to determine the origin of sulfur (SO4) and carbon in the waters, all waters were analyzed for their δ13CVPDB (Versus Pee Dee Belemnite) and δ34SCDT (Canyon Diablo Triolite) (Table 5). As shown in Fig. 6f, the HCO3 content of the thermal spring shows an enriched value of δ13C with respect to the cold spring. δ13C contents are between −1.78 and −1.62‰ in the HTS, between −12.18 and −8.25‰ in the KPC, and between −10.10 and −8.31‰ in the HS, respectively. Carbon in the HTS originates from fresh water carbonates and CO2 gas of mantle origin. Carbon in the KPC and HS is controlled by CO2 in the soil and subsurface pores.
δ34SCDT values are in range −4.2 to −4.1‰ in HTS, 3.8‰ in KPC, 1.31, and 2.7‰ in HS. These values show that sulfur in the cold and hot springs has different origins. The δ34S ratio of sulfate from oxidation of juvenile sulfur is generally between −5 and +5‰ (Clark and Fritz 1997). Negative δ34S values are related to diagenetic environments which are characterized by reduced sulfur compounds (Krouse 1980). 34S isotope compositions in HTS come from volcanic rocks and shale that have reduced sulfur compounds. The sources of the sulfur in KPC and HS are probably limestone and shale in the study area.
Discussions
The studied thermal water has a low temperature (about 36°C) and is classified as a Ca–Mg–Na–HCO3 type. While HCO3 − (342 and 371.6 mg/L) is the most abundant anion in the thermal waters the concentrations of Cl− are quite low or between 4.5 and 12 mg/L. Although the SO4 2− value of HTS is very close to that of deep origin geothermal fluids, the low Cl− concentration of HTS is not consistent with this explanation. The thermal spring comes from a shallow reservoir where the horizontal flow is greater than the vertical flow (Nicholson 1993). While HCO3 − and TDS (>600 mg/L) have high values in the water, which is dominated water–rock interaction, TDS values of the HTS are low (<600 mg/L). This suggests that water–rock interaction is inefficient in the thermal spring water. The Na+ concentration is generally high (approximately 200–2,000 mg/L) in geothermal fields (Nicholson 1993). Na+ and K+ concentrations in the HTS are between 23.78 and 26.98 mg/L and 2.80–3.04 mg/L, respectively. The Na+/K+ ratio is between 8.5 and 8.87, Ca2+ and Mg2+ concentrations of HTS are 58.10–62.52 mg/L and 28.42–30.01 mg/L, respectively. While Ca2+ and Mg2+ values are high, the Na+/Ca2+ ratio is very low in the thermal spring. The F− value ranges over 0.336–0.518 mg/L in HTS. While high F− concentrations show water–rock interaction in volcanic rocks such as rhyolite and obsidian etc. (Mahon 1964), this value is very low for geothermal fluids because of the outcropping of generally sedimentary rocks near the thermal spring area.
Minor and trace element concentrations are slightly higher for HTS because of leaching of the reservoir rock compared with cold waters (Table 2). While As, B, Br, Cs, Li, Mo, Rb, and Sr values increase in thermal water, Al, Cd, Cr, Cu, Fe, Mn, Pb, and Zn values increase in the cold waters. Li+, Rb+, and Cs+, high in concentration for geothermal waters, are of low concentration for the HTS. As is well known, Li+ concentration rises also with temperature in geothermal waters (Kharaka and Mariner 1987). In the HTS the Li+ concentration is very low because of the low temperature (36°C) and lack of water–rock interaction. SiO2 concentrations in the HTS and cold water are 20.75 and 10.48 mg/L, respectively. These values are near to the SiO2 concentration of normal groundwater. In geothermal systems, B3+ and Cl− are used to determine the origin of waters and mixing between different reservoirs (Truesdell 1975, 1991; Arnorssón 1985; Arnorssón and Andrésdóttir 1995). Although B3+ and Cl− contents derived from secondary minerals are very high in geothermal waters having a temperature of >100°C (Ellis and Mahon 1964, 1967), B3+ concentration in the studied HTS is very low (343 ppb). The Cl−/B3+ value (20.41) of the HTS is close to surface water compositions.
Chemical analyses of thermal waters were used to estimate temperature of Hamamayagi geothermal area by several silica geothermometers. Results of silica geothermometers change between 30 and 83°C. Chalcedony geothermometers are assumed to be more applicable to the thermal spring under study. According to the chalcedony geothermometers, the temperature of the reservoir ranges between about 30 and 53°C.
Hydrogeochemistry, isotope geochemistry, and boron values show differences in geothermal areas because of lithological exchanges. Major ions, minor, and trace element concentrations lead to the conclusion that the reservoir is shallow in the Hamamayagi geothermal field. The shallow reservoir and active fault system causes large amounts of groundwater to enter the Hamamayagi geothermal area. This situation causes a decrease in water–rock interaction due to the reduced temperature of the geothermal waters, resulting in a low TDS in the fluid and low concentrations of B3+, Li+, and SiO2.
Conclusion
The HTS has a temperature of 36°C, a pH value of 6.9–7.7, electrical conductivity of 591–598 μS/cm, and total dissolved solids of 485.6–508.5 mg/L. Thermal spring and borehole waters fall into the field of immature waters in the Giggenbach diagram. Therefore, the silica geothermometers are more appropriate than cation geothermometers for the estimation of reservoir temperature. According to the chalcedony geothermometers, the temperature of the reservoir ranges between about 30 and 53°C. The saturation calculations for Hamamayagi thermal water show that anhydrite, gypsum, and halite minerals are undersaturated. Calcite, aragonite, and dolomite minerals are marginally oversaturated, nearly in equilibrium.
Sources of dissolved species in water were determined by using 18O, 2H, 3H, 13C, and 34S isotopes ratios. According to the δ18O–δ2H relation, all waters have a meteoric origin. Deuterium excess values show that the recharge is from precipitation affected by evaporation in the winter season. According to the δ18O-temperature relation, thermal spring recharges at higher altitude than cold spring. The thermal spring has lower TU values and higher EC than cold water. This implies that HTS have deeper circulation than cold water. The δ13CVPDB compositions vary between −1.78 and −1.62‰ for the HTS; −12.18 and −8.25‰ for the KPC; −10.10 and −8.31‰ for the HS, suggesting different origins. Carbon in the HTS originated from fresh water carbonates in study area. In addition, carbon in the HTS is thought to originate from CO2 gases derived from mantle, because of young volcanic rocks outcropping at the north of the NAFZ. Carbon in the KPC and HS comes from CO2 which is accumulated in soil pores. The δ34SCDT values in sulfate are between −4.2 and −4.1‰ in the HTS, 3.8‰ in the HTS and 1.31–2.7‰ in the HS. The δ34SCDT values suggest different sources for the waters. The sulfur composition in the HTS, for example, is derived from sulfate reduction. On the other hand, the source of sulfur in the KPC and HS is shale and limestone.
In the Hamamayagi geothermal field in the North Anatolian Fault Zone, shallow reservoir rocks and an active fault system provide large amounts of groundwater circulation. Therefore, the temperature of geothermal waters is low, as are water–rock interactions, total dissolved solids content and boron, lithium, and silica.
References
Akdag K (1992) Kavak (Samsun) Yöresi Kretase Filişlerinin Sedimantolojik İncelemesi, Doktora Tezi, K.T.Ü., Fen Bilimleri Enstitüsü, Trabzon
Akkuş İ, Akıllı H et al (2005) Türkiye Jeotermal Kaynakları Envanteri, Maden Teknik Arama Genel Müdürlügü Envanter Serisi-201, Ankara
Akkuş İ, Yıldırım N (1992) Havza Hamamayagi (Ladik) Yöresinin Jeolojisi ve Jeotermal Enerji Olanakları: MTA Der.Rap. No: 9899, 41.s (yayımlanmamış), Ankara
Alp D (1972) Amasya Yöresinin Jeolojisi. İ.Ü, Fen Fakültesi Monografileri, İstanbul
APHA (1989) American Public Health Assn (APHA), American Water Work Assn (AWWA), Water Pollution Control Federation (WPCF). Standard methods for the determination of water and wastewater, 5th edn. APHA Publishing, Washington DC, 1134 pp
Arnorssón S (1985) The use of mixing models and chemical geothermometers for estimating underground temperatures in geothermal systems. J Volcanol Geotherm Res 23:299–335
Arnorssón S, Andrésdóttir A (1995) Processes controlling the distribution of boron and chlorine in natural waters in Iceland. Geochem Cosmoschim Acta 20(59):4125–4146
Arnorssón S, Gunnlaugsson E, Svavarsson H (1983) The chemistry of geothermal waters in Iceland, III. Chemical geothermometry in geothermal investigations. Geochem Cosmoschim Acta 47:567–577
Başar H (2003) Kürtün Havzası (Samsun) Hidrojeoloji İncelemesi, H.Ü. Fen Bilimleri Enstitüsü Yüksek Mühendislik Tezi, Ankara
Besbelli B (1984) Bafra (NW Samsun) Güneyindeki Üst-Kretase Alt Tersiyer İstifinin Sedimantolojik İncelemesi, H.Ü. Fen Bilimleri Enstitüsü Yüksek Mühendislik Tezi, Ankara, pp 29–60
Clark I, Fritz P (1997) Environmental isotopes in hydrogeology. Lewis Publishers, New York, p 328
Craig H (1961) Isotopic variations in meteoric waters. Science 133:1702–1703
Dansgaard W (1964) Stable isotopes in precipitation. Tellus 16:436–468
Ellis AJ, Mahon WAJ (1964) Natural hydrothermal systems and experimental hot water–rock interactions. Geochem Cosmoschim Acta 28:1323–1357
Ellis AJ, Mahon WAJ (1967) Natural hydrothermal systems and experimental hot water–rock interactions, Part II. Geochem Cosmoschim Acta 31:519–538
Ercan T, Gedik A (1983) Pontidlerdeki Volkanizma, Jeoloji Mühendisligi Dergisi, Sayı: 18, MTA, Ankara, vol 3, p 11 (in Turkish)
Erdem K (2000) Taşova Ovasının Hidrojeoloji İncelemesi, Yüksek Mühendislik Tezi, A.Ü., Fen Bilimleri Enstitüsü, Ankara
Fournier RO (1977) Chemical geothermometers and mixing models for geothermal systems. Geothermics 5:41–50
Fournier RO, Potter RW (1982) An equation correlating the solubility of quartz in water from 25 to 900°C at pressures up to 10,000 bars. Geochim Cosmochim Acta 46:1969–1973
Gedik A, Korkmaz S (1984) Sinop Havzasının Jeolojisi ve Petrol Olanakları. Jeoloji Müh. Dergisi 19:53–79(in Turkish)
Giggenbach WF (1988) Geothermal solute equilibria. Derivation of Na–K–Mg–Ca geoindicators. Geochim Cosmochim Acta 52:2749–2765
Göksu E, Pamir HN, Erentöz C (1974) Samsun 1/500 000 ölçekli Türkiye Jeoloji Haritası ve İzahnamesi, MTA Yayınları, Ankara, pp 5–22
Gülibrahimoglu İ, Yılmaz B S et al (2000) Samsun İlinin Çevre Jeolojisi ve Dogal Kaynaklar, MTA, Jeoloji Etüt Dairesi, Rapor No: 10481, Ankara, 12–34, 84–97
Kharaka YK, Mariner RH (1987) Chemical geothermometers and their application to formation waters from sedimentary basins. In: Naeser D, McCulloh TH (eds) Thermal history of sedimentary basins, New York, pp 75–102
Krouse HR (1980) Sulphur Isotopes in Our Environment. In: Fritz P, Fontes J-Ch (eds) Handbook of environmental isotope geochemistry I. The terrestrial environment. Elsevier, Amsterdam, pp 435–472
Mahon WJA (1964) Fluorine in the natural thermal waters of New Zealand. N Z J Sci 7:3–28
Nicholson K (1993) Geothermal fluids, chemistry and exploration techniques. Springer, Berlin, p 263
Özten A, Yurtseven D (1996) Samsun- Havza Bekdigin Sıcak Su Sondajı (BK-2) Kuyu Bitirme Raporu, MTA Dergisi, Rap. No: 9925, (yayımlanmamış), Ankara
Öztürk A (1979) Ladik-Destek Dolayının Stratigrafisi. Türkiye Jeoloji Kur. Bült. 23(1):31–38 (in Turkish)
Parkhurst D, Appelo CAJ (1999) User’s guide to PHREEQC (Version 2)–A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. USGS Water Resources Investigation Report 99-4259
Piper AM (1944) A graphic procedure in the geochemical interpretation of water analyses, American Geophysical Union Transactions 25, pp 914–923
Şaroglu F, Emre Ö, Boray A (1987) Türkiye’nin Diri Fayları ve Depremsellikleri, MTA, Rap. No: 8714 (yayımlanmamış), Ankara
Şengör AMC (1980) Türkiyenin Neotektoniginin Esasları, Türkiye Jeo. Kur. Konf., Seri 2, 40, Ankara (in Turkish)
Şengör AMC, Yılmaz Y (1983) Türkiye’de Tetisin evrimi, Levha Tektonigi Açısından Bir Yaklaşım, Türkiye Jeo. Kur.,Yerbilimleri Dizisi, 1, Ankara
Serdar SH, Aydın M, Yazman M (1984) Orta Pontidlerin Jeolojisi, TJK, 38. Bilimsel Teknik Kurultayı Bildiri Özetleri, pp 45–46
Truesdell AH (1975) GEOTERM, a geothermometric computer program for hot-spring systems’. In: Proceedings of the second United Nations symposium on the development and use of geothermal resources, San Francisco, pp 831–836
Trusdell AH (1991) Effects of physical processes on geothermal fluids. In: Applications of geochemistry in geothermal reservoir development (coordinator F. D’Amore), pp 71–92
Yenal O, Usman N, Kanan E (1976) Türkiye Maden Suları İstanbul Üniversitesi İstanbul Tıp Fakültesi Tıbbi Radyoloji ve Hidro-Klimatoloji Kürsüsü Kagıt Basım İşleri A.Ş., İstanbul
Yoldaş R, Keskin B et al (1985) Samsun ve Dolayının (Kızılırmak- Yeşilırmak Arasındaki Bölgenin) Jeolojisi ve Petrol olanakları, Rap No: 8130, MTA Genel Müdürlügü Enerji Hammadde Etüd ve Arama Dairesi, Ankara, pp 19–38
Acknowledgments
This work was supported by the Scientific Research Fund of Karadeniz Technical University with Project number 2006.122.005.4. The authors thank Prof. Dr. Mustafa Afşin for his comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gultekin, F., Hatipoglu, E. & Ersoy, A.F. Hydrogeochemistry, environmental isotopes and the origin of the Hamamayagi-Ladik thermal spring (Samsun, Turkey). Environ Earth Sci 62, 1351–1360 (2011). https://doi.org/10.1007/s12665-010-0621-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-010-0621-4