Abstract
The influences of suspended particles (SPs) on NH4 + adsorption and nitritation occurring in the water system of the Three Gorges Reservoir (TGR) were evaluated in this study. The results indicated that the adsorption of NH4 + was significantly affected by the SPs concentration under the conditions typically present in the TGR. The amount of ammonia adsorbed per unit weight of suspended particles was inverse proportional to the concentration of suspended particles. However, the influences of the particle size and the organic matter concentration existing in SPs were insignificant under the experimental conditions. The effects of suspended particles on nitritation were determined by the use of ammonia-oxidizing bacteria (AOB) strain SW16, identified as Nitrosomonas nitrosa, which was isolated from sediment samples of the TGR. Suspended particle concentration in water–sediment solution played an important role in the nitritation process. The rate of nitritation enhanced with the increase of the suspended particle concentration. It was found that the critical factor controlling ammonia oxidizing rate was the AOB biomass resulting from the AOB growth rate. Moreover, results demonstrated that both particle size and organic matter content showed little effect on the nitritation process under the experimental conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Three Gorges Reservoir (TGR) is located on the mid-downstream Yangtze River, China. Despite its benefits in terms of power generation and flood control, the TGR has attracted attention for its potential impact on ecosystems and socioeconomic stability (Wu et al. 2004). Since the construction of the TGR began, eutrophication has occurred frequently in recent years, due to the changes of aquatic ecosystem of TGR in the Yangtze River (Wang et al. 2008a).
It is known that eutrophication, as a consequence of hyper-nutrification by N and P loads, is greatly influenced by the N and P cycling in aquatic environment (Jergensen and Richardson 1996; Nixon 1995; Paerl 1997). Nitrification, microbially mediated oxidation of ammonia to nitrate, is a key process in the N cycling in freshwater. It is a two-step process, first, an ammonia-oxidizing stage that transforms NH4 + to NO2 − by ammonia-oxidizing bacteria (AOB), and second, the nitrite-oxidizing stage that transforms NO2 − to NO3 − by nitrite-oxidizing bacteria (NOB), in which the ammonia-oxidizing stage is the critical stage controlling the nitrification (Bodelier et al. 1996; Costa et al. 2006). Nitrification is routinely included in water quality models because of its importance in mediation contents of dissolved oxygen and ammonia- and nitrate-nitrogen (Deb and Bowers 1983; DiToro and Connolly 1980). In developing a model framework, it is often assumed that nitrification occurs in the water column and that the process follows first-order kinetics with rates calculated as a function of water column ammonia contents (Ambrose et al. 1993; McCutcheon 1987; Scott and Abumoghli 1995). However, nitrification in freshwater is known to take place mainly on suspended particles (SPs) and bed sediments (Bonnet et al. 1997; Gresikowski et al. 1996; Pauer and Auer 2000). Consequently, the investigation of the relationship between SPs and nitrification rate in aquatic environment is of importance for developing the ecosystem model of nitrogen biogeochemical cycling (Kittiwanich et al. 2007).
Ammonium ion (NH4 +), an electrically positive ion species, is very easy to be adsorbed onto the solids. Thus, the adsorption of ammonia on SPs and sediments is also a critical factor to influence the ammonia ion fluxing into the water body. In this context, SPs and sediments play important roles in the sequestration and biogeochemical process of nitrogen (Liu et al. 2007). Hence, analysis of the effects of SPs and sediments on the ammonia adsorption and nitrification in water body is of great importance for assessing transport, transformation, and fate of nitrogen on aquatic systems.
Previous studies have separately investigated the adsorption of ammonium ion onto the sediments (Simon and Kennedy 1987) and nitrification taking place on sediments (Magalhaes et al. 2005). However, no research has focused on simultaneous ammonia adsorption and nitrification. The construction of the TGR has changed the characteristics of SPs in water body, and consequently the adsorption of ammonia and nitrification taking place on the SPs (Yang et al. 2002). The effects of SPs on the adsorption and nitrification of nitrifier in water column were poorly understood till now. The project of this study is to systematically evaluate the influences of suspended particles on the two ammonia biogeochemical processes occurring in the TGR: adsorption and nitrification. As the nitrifiers are composed of two kinds of bacteria species (AOB and NOB), the commonly controlling-rate step namely ammonia-oxidizing stage induced by AOB is used to reflect the nitrification rate. At a constant temperature and with the use of sediments prepared with different treatments, the study aims to explore the influences of various factors including sediment concentration, particle size fraction and organic matter content of sediments on the ammonia adsorption and oxidation.
Materials and methods
AOB strain and preparation of inocula
The culture medium used for the AOB contained 3.8 mM (NH4)2SO4, 10 mM NaCl, 1 mM KCl, 0.2 mM MgSO4, 0.1 mM KH2PO4, 1 mM CaCl2, and 1 ml trace elements solution (Koops and Moller 1992) per liter deionized water (Millipore: MilliQ). The sediment samples from which AOB was isolated were collected from the TGR. The preparation stages of the sediment suspension followed those described by Satoh et al. (2003). The AOB in a pure form was isolated subjected to the following process: the sediment suspension (10 ml) and freshwater were inoculated into a 300-ml Erlenmeyer flask containing 100 ml of AOB culture medium. Shaking (120 rpm) of the medium was continued at 28°C for 10 days. Five milliliter of liquor from each culture that produced more than 100 μg ml−1 of nitrite was inoculated to each flask containing 100 ml of AOB medium and subcultured for 7–10 days. Subculturing was repeated five times. The final subculture was diluted and plated on gellan gum plates and incubated at 28°C for 10 days. On the plates at dilutions of 10−4 and 10−5 CFU ml−1, colony formation was noted after 14 days of incubation at 28°C. The colonies from each plate were inoculated into a test tube containing 5 ml of the medium and shaken at 120 strokes min−1. After 10–15 days incubation, the isolate that showed the highest growth was noted as SW16. The purification of the strain SW16 was conducted using three types of heterotrophic mediums. The identification process of the strain SW16, including DNA extraction, PCR amplification, the sequencing of amplified 16S rRNA genes and data analysis for construction of a phylogenetic tree, was performed in the previous report (Takahashi et al. 2001).
Cells of the strain SW16 were harvested in the late growth phase from cultures grown in standard media for AOB (Koops and Moller 1992). Cell suspensions of strain SW16 were obtained by centrifugation, followed by washing and resuspension in sterile saline solution.
Sample preparation
Sediments were collected from the Three Gorges Reservoir with a van Veen stainless steel grab sampler (Eijkelamp, Netherlands) in 2007. These freeze-dried sediment samples were ground, homogenized and stored at −20°C prior to analysis. To test the effect of particle size distribution on ammonia oxidation, the sediments were size fractionated using wet-sieve method in the following fractions: >200, 125–200, 63–125, 25–63, and <25 μm. The dependency of ammonia oxidation on the contents of organic matter deposited on sediments was evaluated. Sediments with different organic matter contents were prepared by mixing the raw sediments and the organic-removed sediments according to the given mixing ratios of 0:3, 1:2, 2:1 and 3:0, yielding sediments with organic matter contents of 0.33, 0.45, 0.56 and 0.68%, respectively. The organic matter was removed from original sediments using H2O2 removal method previously described by Wang et al. (2008b). After slow addition of 10 ml 30% H2O2 solution, 20 g sediment sample was incubated at 40°C with intermittent agitation followed by evaporating to dryness. To remove organic matter thoroughly, the above steps were repeated for three times.
Analysis and enumeration
Total organic carbon (TOC) was determined in sediment samples that were acidified with 1.6% HCl and stored at −20°C (Liqui TOC analyzer, Elementar, Hanau, Germany) (Feng et al. 2007). Ammonia and nitrite were determined with reagent kits for photometric analysis (Merck-Spectroquant®, Merck, Darmstadt, Germany). AOB densities were estimated in water systems with the most-probable-number (MPN) techniques (5 test tube test) using microtiter plates (Lipponen et al. 2002). Although the MPN methodology has been criticized for underestimating bacteria population (Belser 1979), it enables a comparison of the potential for nitrite oxidization in different water samples.
Experimental approach
Adsorption experiments of ammonium chloride (NH4Cl) on sediment samples were firstly measured. Surface water used in this study was filtered through 0.45 μm microvoid filter film to remove suspended particulate matter and plankton. A series of conical flasks (100 ml) were added with a given mass of sediment samples and 30 ml of NH4Cl solutions in concentrations of 0.5, 1.0, 2.0, 5.0, 10.0 and 20.0 mg/l. Flasks were then sealed, and shaken at 150 rpm in a constant temperature shaker (T = 20°C). Aliquots of 1.0 ml were collected in an interval of 10 min until the equilibrium was obtained. The suspensions were centrifuged at 4,000 rpm for 20 min, and the NH4Cl concentration in supernatant was measured with photometric analysis.
To investigate the influence of suspended particles on the nitrification, rates of ammonia oxidizing were calculated by observing changes in concentrations of NO2 − over time. The original NH4Cl concentration in treated surface water was ~3 mgN/l. After adding a given mass of sediment samples and 300 ml water sample in 500 ml of conical flasks, the microcosms were inoculated with 2.5 ml of inoculum and incubated at 180 rpm in a constant temperature shaking incubator (T = 27°C) in the dark for 8 days. In preliminary experiments, inspection of the ammonia and nitrite data indicated that nitrification resulting from physicochemical reaction was negligible. Six milliliter of aliquots was removed from the microcosms once daily, in which 1 ml was used to analyze the density of AOB cells, and 5 ml underwent nitrite-nitrogen content determination after centrifuging. The centrifuged sediment pellet was flushed with equal deionized water and added to the microcosms replacing the removed water to maintain a constant volume of water and sediment.
Results and discussion
Ammonia adsorption in sediments
Influence of suspended particle concentration on ammonia adsorption
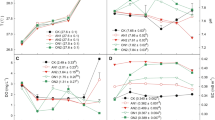
As NH4 + is an electrically positive ion species, it is very easy to be adsorbed onto the SPs which are negatively charged. The dependence of ammonia adsorption on different concentrations of SPs was evaluated. The preliminary experiments revealed that the adsorption of NH4 + onto SPs was very fast and approached equilibrium within 30 min, so that in this test the measure of supernatant NH4 + was conducted after 1 h of adsorption reaction. Figure 1 shows the adsorption of NH4 + at a range of suspended particle concentrations varying from 1.0 to 10 g/l. The variations of SPs used here are typically occurring in water systems of the Three Gorges Reservoir (Yangtze River Water Resources Commission 2000). At four suspended particle concentrations, the ammonia adsorption linearly increased with the increase in initial ammonia concentrations (R 2 = 0.98–0.99), but the increasing rate and the amount of ammonia adsorbed per unit weight of suspended particles were greater at lower concentrations of suspended particles. Under the experimental conditions of different suspended particle concentrations and initial ammonia concentrations, the ammonia adsorption was quite effective. Specifically, the transfer rate of ammonia from solution to sediments reached 99% at 2.0 mg/l of NH4 +–N irrespective of the SPs concentrations. Even with ammonia loading increasing to 20 mg/l of NH4 +–N, the adsorption efficiency still approached 92% for four SPs concentrations, resulting in the residual ammonia concentrations of 1.24–1.56 mg/l of NH4 +–N in supernatant solution. The high adsorption efficiency of ammonia under the experimental conditions may also explain the linear relationship between the ammonia adsorption and the initial ammonia concentrations.
According to the above observation that ammonia loading up to 20 mg/l of NH4 +–N was quickly and effectively fixed by the suspended particles with typical concentrations present in the TGR, it can be expected that once the external ammonia at a given concentration enters the water body of the TGR, it should be firstly adsorbed by the SPs. As thus, the ammonia adsorption by SPs may have significant effect on biological transformations of nitrogen cycling, such as nitrification/denitrification. This research result is in consistent with the observation previously made that the nitrification in Ems estuary, Onondaga Lake mainly took place on SPs and bed sediment (Bonnet et al. 1997; Gresikowski et al. 1996; Pauer and Auer 2000). Moreover, the ammonia fixed by the SPs will become an important internal nitrogen pollution source as a consequence of sedimentation of suspended particles in the TGR.
Ammonia adsorption with different particle sizes and organic matter contents in the SPs
The size distribution of suspended particles broadly fluctuated as a result of the change of the hydrodynamic conditions in various water bodies. The organic matter content of suspended particulate originated from various sources is different. The adsorption of ammonia on suspended particles with different sizes and organic matter contents was investigated, as shown in Figs. 2 and 3. At five suspended particle sizes and four organic matter contents, the ammonia adsorption linearly increased with the increase in initial ammonia concentration (R 2 = 0.99). Neither the particle size nor organic matter content of SPs showed significant effect on ammonia adsorption under the experimental conditions. This suggests that the variation of particle size and organic matter content will not change the adsorption capacity of ammonia under the typical environmental conditions in the TGR. However, the two factors may influence the nitrogen biological transformation occurring on SPs, and they were investigated in the following chapters.
Ammonia nitritation
Because the nitrification involves two kinds of nitrifiers, AOB and NOB, it is difficult to evaluate the individual contribution of AOB and NOB during the nitrification process. The step controlling the nitrification rate, namely, ammonia-oxidizing stage induced by AOB was chosen to reflect the nitrification rate. As thus, an AOB strain SW16 was isolated from sediments collected from the TGR. An almost-complete 16S rRNA gene sequence of strain SW16, containing less than 1% undetermined positions, was obtained. NCBI BLAST, the Basic Local Alignment Search Tool (BLAST), is a suite of programs designed to search all available sequence databases for similarities between a protein or DNA query and known sequences. In this study, BLAST was used to match the sequence relationships, providing scores that can distinguish real matches from background hits with a high degree of statistical accuracy. The result of BLAST program indicated that the isolate possessed a 16S rRNA gene sequence with 98% similarity to that of the species Nitrosomonas nitrosa in GenBank; hence, AOB strain SW16 was classified as Nitrosomonas nitrosa. Cell suspensions of strain SW16 used in this study were diluted to concentration approximately 2.3 × 108 cells per milliliter of solution (SE 0.063 × 108).
Influence of suspended particle concentration on nitritation
The nitritation experiments under different suspended particle concentrations were carried out using AOB strain SW16. The preliminary experiments revealed that the ammonia completely depleted within 8 days under typical environmental condition in the TGR (suspended particle concentration was 2 g/l and ammonia concentration in supernatant was 0.2 mgN/l), so that in this test the nitritation experiment was performed for 8 days. Figure 4 depicts the nitrite productions coupled with the ammonia oxidation. At the first 2 days there was no nitrite production, whereas in the following 6 days the nitrite production dramatically increased. The biological transformation was completed in 8 days with the presence of sediments, but only 34% of ammonia was transferred to nitrite with the absence of sediments. The rate of nitrite production enhanced with the increase of the suspended particle concentrations (Fig. 4). For example, the transformation rate of ammonia to nitrite in 6 days approached 40, 52, 76 and 91% with the suspended particles of 1, 2, 5 and 10 g/l, respectively. This suggests that the presence of suspended particle facilitates the nitrogen biological transformation, and the increasing SP concentration accelerates the nitritation process.
The AOB cell concentrations in sediment–water solutions were audited during the 8-day nitritation process, as shown in Fig. 5. The initial AOB densities, ca. 1.2 × 106 cells ml−1, were similar in solutions with different suspended particle concentrations with the addition of strain SW16 inoculum (1 ml). After the lag phase of 2-day, the AOB growth entered into the logarithmic phase, and then reached the stationary phase with the consumption of ammonia. Figure 5 exhibits that the growth rates of AOB were higher under the higher suspended particle concentrations. Specifically, after 6-day ammonia oxidation, the AOB densities reached to 3.20 × 106, 9.13 × 106, 10.5 × 106, 12.6 × 106 and 13.6 × 106 cells ml−1 at SP concentrations of 0, 1, 2, 5 and 10 g/l, respectively. This can explain the observation made above that the rate of nitrite production improved with the increase in suspended particle concentrations. Moreover, it can be seen from Figs. 4 and 5 that there is a significant correlation (t test, P < 0.05) between the ammonia oxidizing rate and the AOB growth rate.
Many groups of bacteria have been shown to exist predominantly as attached colloids onto surfaces in contact with liquids (Davies 2000; Palmer et al. 2007). The advantages gained by the living bacteria attached to a surface are thought to improve growth rate and activity of the microorganisms by the higher concentration of nutrients close to a surface and promoted genetic exchange (Donlan 2001). Moreover, there are many claims that surface attachment appears to protect nitrifying bacteria from a range of inhibitors (Foppen et al. 2008; Marina et al. 2000; Ng and Stenstrom 1987; Park et al. 2003). Marina et al. (2000) and Ng and Stenstrom (1987) reported that the addition of powdered activated carbon to activated sludge wastewater treatment process enhanced nitrification by adsorbing inhibitory compounds. Park et al. (2003) reported that the enhanced nitrification efficiency of activated sludge with the addition of powder activated carbon or zeolite was accomplished by the attached growth of nitrifier on-the-surface of carriers. The mechanism of the improvement in nitrification rate with the addition of nitrifier carriers is still not well understood, but the surface growth of nitrifier may be a factor affecting the nitrification efficiency. Previous researches have found that the nitrifying bacteria in water column of the lake and river were low with low or even no observed nitrification in water column (Cirello et al. 1979; Hall 1982; Pauer and Auer 2000). This is attributed to the low nitrifier biomass concentrations and the low growth rates of AOB in water column, probably as a result of low level of SPs, which was considered as a main habitat for the biological nitrification in water body (Bonnet et al. 1997; Gresikowski et al. 1996; Pauer and Auer 2000).
Influence of particle size and organic matter content on ammonia oxidation
To investigate the influences of suspended particle size and organic matter content on microbially nitrogen cycle under the typical Yangtze River conditions, the experiments of nitration in water-SP solution with different particle sizes and organic matter content SPs were performed. Figure 6 plots the nitrite production under different particle sizes, elucidating that the influence of particle size is not obvious under the experiment conditions. Specifically, for suspended particle size fractions of >200, 125–200, 63–125, 25–63 and <25 μm, the nitrite production was 1.73, 1.76, 1.77, 1.75 and 1.82 mgN/l, respectively, in 6 days. Previous studies (Ling et al. 2002; Muirhead et al. 2006; Oliver et al. 2007) found that bacteria (E. coli) would associate with soil particles and furthermore, cells would preferentially attach to a particular soil particle size fraction. It was found that E. coli preferentially attached to the soil particle size fraction of 30–16 μm when the observation was made with the soil particle size fractions of >31, 30–16, 15–4, 2–3 and < 2 μm, respectively. However, in this study, the discrimination of nitritation resulting from AOB attachment on the different size fractions was negligible to be observed. The mechanism involved in the discrepancy between this study and previous reports is not clear, providing a further issue to be concerned.
Figure 7 indicates the nitritation process of AOB under different organic matter contents containing in suspended particles. The ammonia oxidizing rates of AOB under different organic matter content sediments were not significant. Nevertheless, other researchers found that organic matter is frequently cited as factors that limit nitrification in activated sludge (Abeliovich 1992; Foppen et al. 2008; White and Gosz 1987). This may be due to the fact that under the experiment conditions the organic matter content containing in SPs is low, and their discrimination is not sufficient to cause the change of nitritation process.
Conclusion
Laboratory microcosm experiments were used to estimate the influences of suspended particles on the ammonia adsorption and the nitritation process. The adsorption of NH4 + was quite effective under the conditions of different suspended particle concentrations and initial ammonia concentrations, which were typically occurred in water systems of the Three Gorges Reservoir. Even with ammonia loading increasing to 20 mg/l of NH4 +–N, the adsorption efficiency still approached 92% for four SPs concentrations (1.0, 2.0, 5.0 and 10 g/l). For four studied suspended particle sizes and four experimented organic matter contents, the reflected influences on the adsorption of ammonia were not obvious under the experimental conditions. The quick and effective adsorption of ammonia under the experimental conditions implied that the ammonia pollution inflowing into water body would firstly be fixed on the suspended particles and consequently be loaded into the sediments.
AOB strain SW16, identified as Nitrosomonas nitrosa, was used to determine the nitritation process. Results showed that the suspended particles played an important role in the nitritation, and the ammonia oxidizing rate enhanced with the increase of suspended particle concentration. The significant improvement of nitritation under the high SP concentrations is attributed to the high nitrifier biomass concentrations resulting from the fast growth rates of AOB caused by the high level of SPs, which were considered as the main habitats for the biological nitrification in water body. Under the experimental conditions, no obvious changes in nitrite production were observed in nitrification process with the different particle size fractions and the organic matter concentrations loaded on sediments.
References
Abeliovich A (1992) Transformations of ammonia and the environmental impact of nitrifying bacteria. Biodegradation 3:255–264
Ambrose RB, Wool T, Martin JL (1993) The water quality analysis simulation program, WASP5, Part A: Model documentation. In: USEPA (ed) Athens
Belser LW (1979) Population ecology of nitrifying bacteria. Annu Rev Microbiol 33:309–333
Bodelier PL, Libochant JA, Blom CW, Laanbroek HJ (1996) Dynamics of nitrification and denitrification in root-oxygenated sediments and adaptation of ammonia-oxidizing bacteria to low-oxygen or anoxic habitats. Appl Environ Microbiol 62:4100–4107
Bonnet C, Volat B, Bardin R, Degrange V, Montuelle B (1997) Use of immunofluorescence technique for studying a Nitrobacter population from wastewater treatment plant following discharge in river sediments: first experimental data. Water Res 31:661–664
Cirello J, Rapaport RA, Strom PF, Matulewich VA, Morris ML, Goetz S, Finstein MS (1979) The question of nitrification in the Passaic River, New Jersey: analysis of historical data and experimental investigation. Water Res 13:525–537
Costa E, Perez J, Kreft JU (2006) Why is metabolic labour divided in nitrification? Trends Microbiol 14:213–219
Davies DG (2000) Physiological events in biofilm formation. pp 37–51. In: D Allison, P Gilbert, M Lappin-Scott, M Wilson (eds) Community structure and co-operation in biofilms
Deb A, Bowers D (1983) Diurnal water quality modeling: a case study. J Water Pollut Control Fed 55:1473–1488
DiToro DM, Connolly JP (1980) Mathematical models of water quality in large lakes. In: USEPA (ed) Lake Erie: EPA-600/3-80-065, ERL, ORD, Duluth MN
Donlan RM (2001) Biofilm formation: a clinically relevant microbiological process. Clin Infect Dis 33:1387–1392
Feng JL, Yang ZF, Niu JF, Shen ZY (2007) Remobilization of polycyclic aromatic hydrocarbons (PAHs) during the resuspension of Yangtse River sediments using a particle entrainment simulator. Environ Pollut 149:193–200
Foppen JW, Liem Y, Schijven J (2008) Effect of humic acid on the attachment of Escherichia coli in columns of goethite-coated sand. Water Res 42:211–219
Gresikowski S, Greiser N, Harms H (1996) Distribution and activity of nitrifying bacteria at two stations in the Ems estuary. Arch Hydrob Spe Iss Adv Limnol 47:65–76
Hall GH (1982) Apparent and measured rates of nitrification in the hypolimnion of a mesotrophic lake. Appl Environ Microbiol 43:542–547
Jergensen BB, Richardson K (1996) Eutrophication of coastal marine systems. American Geophysical Union, Washington, DC
Kittiwanich J, Yamamoto T, Kawaguchi O, Hashimoto T (2007) Analyses of phosphorus and nitrogen cyclings in the estuarine ecosystem of Hiroshima Bay by a pelagic and benthic coupled model. Estuar Coastal Shelf Sci 75:189–204
Koops HP, Moller UC (1992) The lithotrophic ammonia-oxidizing bacteria. In: Balows A, Truper HG, Dworkin M, Harder W, Schleifer KH (eds) The prokaryotes. Springer, New York, pp 2625–2637
Ling TY, Achberger EC, Drapcho CM, Bengtson RL (2002) Quantifying adsorption of an indicator bacteria in a soil–water system. Trans Am Soc Agric Eng 45:669–674
Lipponen MT, Suutari MH, Martikainen PJ (2002) Occurrence of nitrifying bacteria and nitrification in Finnish drinking water distribution systems. Water Res 36:4319–4329
Liu KK, Kao SJ, Wen LS, Chen KL (2007) Carbon and nitrogen isotopic compositions of particulate organic matter and biogeochemical processes in the eutrophic Danshuei Estuary in northern Taiwan. Sci Total Environ 382:103–120
Magalhaes CM, Joye SB, Moreira RM, Wiebe WJ, Bordalo AA (2005) Effect of salinity and inorganic nitrogen concentrations on nitrification and denitrification rates in intertidal sediments and rocky biofilms of the Douro River estuary, Portugal. Water Res 39:1783–1794
Marina IS, Wm Brian A, Zhiyao S (2000) Oxygen uptake rate inhibition with PACT sludge. J Hazard Mater B73:129–142
McCutcheon S (1987) Laboratory and in-stream nitrification rates for selected streams. J Environ Eng 113:628–646
Muirhead RW, Collins RP, Bremer PJ (2006) Interaction of Escherichia coli and soil particles in runoff. Appl Environ Microbiol 72:3406–3411
Ng AS, Stenstrom MK (1987) Nitrification in powdered activated sludge process. J Environ Eng 113:1285–1301
Nixon SW (1995) Coastal marine eutrophication: a definition, social causes and future concerns. Ophelia 41:199–219
Oliver D, Clegg C, Heathwaite A, Haygarth P (2007) Preferential attachment of Escherichia coli to different particle size fractions of an agricultural grassland soil. Water Air Soil Pollut 185:369–375
Paerl HW (1997) Coastal eutrophication and harmful algal blooms: importance of atmospheric deposition and groundwater as ‘new’ nitrogen and other nutrient sources. Limnol Oceanogr 42:1154–1165
Palmer J, Flint S, Brooks J (2007) Bacterial cell attachment, the beginning of a biofilm. J Ind Microbiol Biotechnol 34:577–588
Park SJ, Oh JW, Yoon TI (2003) The role of powdered zeolite and activated carbon carriers on nitrification in activated sludge with inhibitory materials. Process Biochem 39:211–219
Pauer JJ, Auer MT (2000) Nitrification in the water column and sediment of a hypereutrophic lake and adjoining river system. Water Res 34:1247–1254
Satoh K, Yanagida T, Isobe K, Tomiyama H, Takahashi R, Iwano H, Tokuyama T (2003) Effect of root exudates on growth of newly isolated nitrifying bacteria from barley rhizoplane. Soil Sci Plant Nutr 49:757–762
Scott JA, Abumoghli I (1995) Modelling nitrification in the river Zarka of Jordan. Water Res 29:1121–1127
Simon NS, Kennedy MM (1987) The distribution of nitrogen species and adsorption of ammonium in sediments from the tidal Potomac River and estuary. Estuar Coastal Shelf Sci 25:11–26
Takahashi R, Ohishi M, Ohshima M, Saitoh M, Omata K, Tokuyama T (2001) Characteristics of an ammonia-oxidizing bacterium with a plasmid isolated from alkaline soils and its phylogenetic relationship. J Biosci Bioeng 92:232–236
Wang J, Wang SR, Jin XC, Zhu SQ, Wu FC (2008a) Ammonium release characteristics of the sediments from the shallow lakes in the middle and lower reaches of Yangtze River region, China. Environ Geol 55:37–45
Wang LL, Niu JF, Yang ZF, Shen ZY, Wang JY (2008b) Effects of carbonate and organic matter on sorption and desorption behavior of polycyclic aromatic hydrocarbons in the sediments from Yangtze River. J Hazard Mater 154:811–817
White CS, Gosz JR (1987) Factors controlling nitrogen mineralization and nitrification in forest ecosystems in New Mexico. Biol Fertil Soil 5:195–202
Wu SR, Jin YM, Zhang YS, Shi JS, Dong C, Lei WZ, Shi L, Tan CX, Hu DG (2004) Investigations and assessment of the landslide hazards of Fengdu county in the reservoir region of the Three Gorges project on the Yangtze River. Environ Geol 45:560–566
Yang S, Zhao Q, Belkin IM (2002) Temporal variation in the sediment load of the Yangtze river and the influences of human activities. J Hydrol 263(1–4):56–71
Yangtze River Water Resources Commission (2000) Yangtze River sediments bulletin. Wuhan, Chinese
Acknowledgments
The research was funded by the National Basic Research Program of P. R. China (973 Project, 2003CB415204) and Program for New Century Excellent Talents in University (NCET-06-0130). We gratefully acknowledge the support of China Postdoctoral Science Foundation (20070410472) and special fund of State Key Laboratory of Water Environment Simulation (No. 08ESPCT-Z).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, H., Shen, Z., Guo, X. et al. Ammonia adsorption and nitritation in sediments derived from the Three Gorges Reservoir, China. Environ Earth Sci 60, 1653–1660 (2010). https://doi.org/10.1007/s12665-009-0299-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-009-0299-7