Abstract
Phosphorus (P) fractions and their bioavailability in the sediments from the middle and lower reaches of the Yangtze River region were investigated using different chemical extraction methods. The results show that the contents of bioavailable P in the sediments extracted by different extraction procedures varied greatly. But their rank order was similar. Potentially releasable P (PRP) was the largest, followed by algal available P (AAP), NaHCO3 extractable P (Olsen-P), water soluble P (WSP), and readily desorbable P. PRP contributed approximately 60% to total P (TP) in most sediments, AAP 20%, Olsen-P 15%, WSP 2%, and readily desorbable P (RDP) 0.5%. For the heavily polluted sediments, their bioavailable P extracted from TP mainly originated from inorganic P (IP), IP mainly originated from NaOH–P, the bioavailable P concentrations can be evaluated by measuring the concentrations of TP, NaOH–P, and IP. For the slightly polluted sediments, the bioavailable P can only be evaluated by different chemical extractable methods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lake eutrophication has become a serious environmental problem in China, especially for shallow lakes in the middle and lower reaches of the Yangtze River region, it has resulted in serious blue-green algae blooms in the last few years (Qin 2002). As a major nutrient for aquatic ecology, phosphorus (P) is an important cause for the lake eutrophication (Dorich et al. 1984; Gibson 1997; Wu et al. 2001). In shallow lakes, P can be released from sediments into the overlying water under some environmental conditions, which had a significant impact on the water quality and may result in continuing lake eutrophication (Abrams and Jarrell 1995; Xie et al. 2003). However, not all of the P fractions can be released from the sediments and render lake eutrophication (Gonsiorczyk et al. 1998). Therefore, P fractions in lake sediments have been extensively studied to evaluate their bioavailability (Ruban et al. 1999).
Chang and Jackson (1957) partitioned soil P into labile P, aluminum-bound P, iron-bound P, calcium-bound P, reductant soluble P, occluded P, and organic P using sequential extraction procedure, and later this procedure was modified and applied to the P fractionation in lake sediments (Williams et al. 1976; Zhou et al. 2001). P bioavailability in lake sediments may be evaluated by selective chemical extraction methods except by the concentrations of total P (TP) and different P fractions (Andrieux and Aminot 1997; Gonsiorczyk et al. 1998; Moriarty 1998). Zhou et al. (2001) studied the P bioavailability in the sediments of West Lake and Taihu Lake in China and Lough Erne in Ireland using the concentrations of total P, water soluble P (WSP), readily desorbable P (RDP), algal available P (AAP), and NaHCO3 extractable P (Olsen-P), and the results showed that there was a significant correlation between the bioavailable P extracted using different chemical extractants and TP.
The sequential extraction procedure for P fractions in sediments is complicated and time-consuming. Results suggested that the bioavailable P concentrations in lake sediments can be more easily estimated by measuring the contents of WSP, RDP, AAP, and Olsen-P than by P fractions. The middle and lower reaches of Yangtze River region are central areas of freshwater shallow lakes in China, and most of the lakes in this region have been under eutrophication (Wang et al. 2005). Therefore, it is importance to evaluate the P bioavailability in sediments from this region. However, little is known about the correlation between the concentrations of bioavailable P extracted by different chemical extractants and different P fractions in sediments. The objective of this study was to evaluate the P bioavailability in sediments from this region, the relationships between their bioavailable P and P fractions were also discussed.
Materials and methods
Study areas
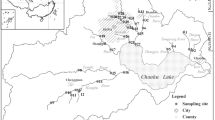
Ten lakes (Wuli Lake, Meiliang Lake, Gong Lake, East Taihu Lake, Yue Lake, Dongting Lake, Xuanwu Lake, Hongze Lake, Poyang Lake, and Chao Lake) were chosen in the middle and lower reaches of Yangtze River region in this study (Fig. 1). The basic characteristics of the region were described in our previous studies (Wang et al. 2005). Those chosen lakes represent the major lake types in the middle and lower reaches of Yangtze River region (Wang et al. 2006), and their geographic and limnological features are shown in Table 1.
Sediment samples and analyses
A total of 17 sediment core samples (0–10 cm) were collected in September 2003 with a core sampler of 30 cm length, 5 cm diameter cylinder tubes constructed of Plexiglas, and were taken to the laboratory in sealed plastic bags that were put in iceboxes, then freeze-dried, and ground for experiments. The general physical and chemical properties of the sediments are presented in Table 2. Cationic exchange capacity (CEC) was analyzed using EDTA-NH4 + method; total nitrogen (TN) was measured using the concentrated H2SO4 digestion method (Institute of soil science, Chinese Academy of Sciences 1978). Total organic carbon (TOC) in the sediments was analyzed with an Apollo 9000 TOC Analyzer (Tekmar Dohrman Co., USA) after pre-treatment in warm HCl 50% v/v to eliminate inorganic carbon (Zanini et al. 1998). The grain sizes were measured using a Mastersizer 2000 Laser Size Analyzer (Malvern Co., UK). Dried samples were put into beakers, 50 ml distilled water was then added and the beakers were frequently shaken for 5 min before analyses. The percentages of grain size groups including clay (0.02–4 μm), silt (4–63 μm), and sand fractions (63–500 μm) were determined (Das 1990). The concentrations of major elements in sediments were measured by ICP–OES (PE, ICP/6500, USA). The sample (0.1 g) was dissolved by a fusion with 0.3 g LiBO2 (1,000°C), and was re-dissolved with 5% HNO3. The solution was then used for the determination of the total concentrations of major elements (Jin et al. 2006).
P fractions and bioavailable P
The sediment samples were sieved with a standard 100-mesh sieve and the sequential extraction P fraction was carried out using the standards measurements and testing (SMT) procedure described in a previous study (Wang et al. 2005). Briefly, the following fractions were determined: iron-bound P (NaOH–P), calcium-bound P (HCl–P), inorganic P (IP), organic P (OP), and TP. NaOH–P is the P bound to Al, Fe, and Mn oxides and hydroxides that was extracted by NaOH (1 mol l−1), HCl–P was extracted by HCl (1 and 3.51 mol l−1), IP was extracted by HCl (1 mol l−1), and OP is the residual that was treated at 450°C to decompose organic matter and release P bound to organic matter. TP concentration in sediments was determined by treating the sample at 450°C, followed by HCl extraction (3.51 mol l−1). The soluble reactive P (SRP) concentrations were analyzed using the molybdenum blue method (APHA, AWWA, WPCE 1998). According to the SMT protocol, potentially releasable P (PRP) in the sediment can be obtained based on the sum of NaOH–P and OP (Ruban et al. 2001).
Water soluble P (WSP), readily desorbable P (RDP), algal available P (AAP), and NaHCO3 extractable P (Olsen-P) are the bioavailable P, which can be, respectively, determined by different chemical extraction methods (Zhou et al. 2001). Olsen-P is a good index to represent the status of nutrients in soils, which mainly includes the Fe, Al binding P (Ma et al. 2009). Thus, Olsen-P can be also regarded as a quantitative index of available P for algae to a certain extent (Zhou et al. 2001).
The analytical procedure was as follows: 0.5 g of dried sediment sample was placed into 100 ml acid washed screw cap centrifuge tubes for WSP with 50 ml deionized water added (Friend and Birch 1960; Psenner et al. 1985; Psenner 1988; Andrieux and Aminot 1997), for RDP with 50 ml 0.01 mol l−1 CaCl2 solution added (Reddy et al. 1980), for AAP mainly including Fe–P in sediments with 50 ml 0.1 mol l−1 NaOH solution added (Dorich et al. 1984, 1985), for Olsen-P with 50 ml 0.5 M NaHCO3 solution (pH 8.5) added (Olsen et al. 1954; Gonsiorczyk et al. 1998). All the centrifuge tubes were capped and incubated at 25 ± 1°C in an orbital shaker at 250 g, WSP for 2.0 h, RDP for 1.0 h, AAP for 4.0 h, and Olsen-P for 0.5 h. The solution was immediately centrifuged at 5,000g for 10 min, and the suspensions were filtered through 0.45 μm GF/C filter membranes, respectively, the filtrates were analyzed for PO4 3− using the molybdenum blue method (Jin et al. 1990) and the concentrations of WSP, RDP, AAP, and Olsen-P were calculated according to the concentrations of PO4 3−.
For all samples, triplicates were analyzed and the data were presented as the average in this study. The repeated experiments (three times) demonstrated that the high reproducibility of the methods, and the experimental error was within 7%.
Results
Sediment characteristics
The general features and chemical component contents of the studied sediments are presented in Table 2. TOC contents ranged from 0.18 to 9.21%, CEC from 8.88 to 36.10 meq (100 g)−1, TN from 0.03 to 0.54%, while Fe from 1.77 to 8.34%, Al from 4.84 to 12.04%, and Mn from 0.04 to 0.14%. The silt fraction (4–63 μm) dominated in the fractions for all studied sediments with the relative contribution from 64.30 to 81.00%, the clay fraction was the lowest, from 5.32 to 19.45%, and the sand fraction was the medium, from 8.15 to 31.07%. This suggests that the physical and chemical characteristics of the sediments varied greatly.
Concentrations of the bioavailable P and their contributions to TP
PRP, WSP, RDP, AAP, and Olsen-P concentrations of the 17 studied sediments varied greatly (Fig. 2), PRP ranged from 81 to 2,873 mg kg−1, WSP from 2 to 152 mg kg−1, RDP from 0.3 to 21 mg kg−1, AAP from 19 to 1,104 mg kg−1, and Olsen-P from 16 to 510 mg kg−1. Although the relative contributions of PRP, WSP, RDP, AAP, and Olsen-P to TP were different, which varied slightly (Table 3), PRP, AAP, Olsen-P, WSP, and RDP contributed approximately 60, 20, 15, 2, and 0.5%, to TP, respectively. This indicates that the bioavailable P concentrations in the studied sediments increased as TP increased, while the relative contribution of each bioavailable P to TP remained relatively constant.
Relationships between the bioavailable P and different fractions
The 17 studied sediment samples can be divided into two groups according to the Chinese environmental dredging common standard (Liu et al. 1999), and the sediments that their TP concentrations were over 500 mg kg−1 were considered as heavily polluted and should be dredged. The other were the slightly polluted sediments (mesotrophic or oligotrophic), in which TP concentrations were below 500 mg kg−1. This result is consistent with our previous reports (Wang et al. 2006). For example, Yue Lake and Xuanwu Lake were urban lakes and were hypereutrophic (Zhu et al. 2004). Hongze Lake and Poyang Lake were mesotrophic (Jin et al. 1995; Yuan et al. 2000). Meiliang Lake and Wuli Lake were identified to be the most polluted water bodies in China (Cai et al. 1995, 1997; Zhou et al. 1996; Shen et al. 2001). There were abundant aquatic plants in Gonghu Lake and East Taihu Lake, and all were in mesotrophication (Fan et al. 1997; Zhang and Qin 2001). Although Chao Lake was in hypereutrophication (Jin et al. 1990), the sediments (C-1, C-2, and C-3) were taken from the Mawei River estuary, the water quality of this area was better than that in other areas in Chao Lake, its TP and TN concentrations were lower, and the sediments were slightly polluted.
The relationships between the concentrations of PRP, WSP, RDP, AAP, Olsen-P, and different P fractions in the sediments are presented in Table 4. For all studied sediments, PRP, WSP, RDP, AAP, and Olsen-P had significant correlations with TP, NaOH–P, and IP, but not with HCl–P and OP. For those slightly polluted sediments, there were no significant correlations between the bioavailable P (PRP, WSP, RDP, AAP, and Olsen-P) and different P fractions. However, for those heavily polluted sediments, the correlations between the bioavailable P (PRP, WSP, RDP, AAP, and Olsen-P) and TP, NaOH–P, and IP were more significant than those for all studied sediments, their correlations with HCl–P and OP were also not significant.
Discussion
For all studied sediments, the OP concentrations increased at first as TP increased, and then remained constant (Fig. 3). There was a significant correlation between NaOH–P and IP (R 2 = 0.96, P < 0.01, n = 17), but there was no strong correlation between HCl–P and IP (Fig. 4). TP was the sum of IP and OP, therefore, the TP increasing was mainly resulted from the IP increasing and IP increasing mainly from the NaOH–P increasing for the heavily polluted lake sediments. Therefore, for the heavily polluted sediments, the bioavailable P extracted from TP mainly originated from IP, while IP mainly originated from NaOH–P. This result is similar to the reports in previous studies that the relative contribution of NaOH–P to TP ranged from 5 to 70% with high values in eutrophic lakes, and NaOH–P was an important source for bioavailable P in eutrophic sediments (Penn et al. 1995; Elizabeth et al. 2002).
Significant correlations between TP, IP, OP, NaOH–P and Fe, Al suggest Fe and Al binding to P in the studied sediments (Table 5). Fe and Al may significantly contribute to P bioavailability in sediments by increasing the cationic exchange capacity and reducing P fixation (Ruban et al. 2001), and the binding of P to Fe/Al may take place in the oxidized surface sediments or by precipitation from the overlying waters (Ribeiro et al. 2008). Organic matter could be associated with Fe and Al, possibly forming humic-Fe/Al complexes (Axt and Walbridge 1999). These complexes are thought to play a key role in binding P in sediments (Paludan and Jensen 1995). Humus coating of Fe/Al complexes may inhibit the crystallization of Al and Fe minerals, thus maintaining Fe/Al compounds in a non-crystalline state for P binding in sediments (Long and James 2002). Heavily polluted lakes in China were also reported with the large input of Al and Fe (Jin et al. 2006). In this study, different P fractions were all significantly correlated with TOC, TN, and clay (Fig. 4).
In lake system, the actual amount of P released from sediments was governed not only by the bioavailable P and different P fraction concentration but also by other factors, e.g., pH, Eh, and temperature (Bostrom 1988; Zhou et al. 2005). Among the bioavailable P, WSP was probably the closest simulation of the lake situation (Zhou et al. 2001). Our observations show that, although, the bioavailable P extracted by different extracted procedures varied greatly, their relative contributions to TP remained constant as TP increased for all studied sediments. This suggests that the bioavailable P concentration in the heavily polluted sediments from the middle and lower reaches of the Yangtze River region can be evaluated by different chemical extractable methods, and the concentrations of TP, NaOH–P, and IP can also be used. However, for the slightly polluted sediments, their bioavailable P concentration can only be evaluated by different chemical extractable methods.
Conclusions
In this study, phosphorus (P) fractions and its different kind of bioavailable P in 17 sediments from the middle and lower reaches of the Yangtze River region were investigated using different chemical extraction methods. The contents of different kinds of bioavailable P (PRP, WSP, RDP, AAP, and Olsen-P) in studied sediments varied greatly. Although their relative contributions of PRP, WSP, RDP, AAP, and Olsen-P to TP were different, one of their relative contributions varied slightly. Their rank order was PRP > AAP > Olsen-P > WSP > RDP. PRP contributed approximately 60% to total P, AAP 20%, Olsen-P 15%, WSP 2%, and RDP 0.5%. For those slightly polluted sediments, there were no significant correlations between the bioavailable P (PRP, WSP, RDP, AAP, and Olsen-P) and different P fractions. However, for those heavily polluted sediments, the correlations between the bioavailable P (PRP, WSP, RDP, AAP, and Olsen-P) and TP, NaOH–P, IP were significant. For the heavily polluted sediments, their bioavailable P mainly originated from IP, while IP mainly from NaOH–P, so their bioavailable P concentration can be evaluated by measuring their concentrations of TP, NaOH–P, and IP. For the slightly polluted sediments, their bioavailable P can only be evaluated by different chemical extractable methods.
References
Abrams MM, Jarrell WM (1995) Soil-phosphorus as a potential non-point source for elevated stream phosphorus levels. J Environ Qual 24:132–138
Andrieux F, Aminot A (1997) A two-year survey of phosphorus speciation in sediments of the Bay of Seine (France). Cont Shelf Res 17:1229–1245
AWWA, APHA, WPCE (1998) Standard methods for the examination of water and wastewater (18Ed.). American Public Health Association Press, Washington, DC
Axt JR, Walbridge MR (1999) Phosphate removal capacity of palustrine forested wetlands and adjacent uplands in Virginia. Soil Sci Soc Am J 63:1019–1031
Bostrom B (1988) Relations between chemistry, microbial biomass and activity in sediments of a polluted vs. a nonpolluted eutrophic lake. Verh Int Ver Limnol 23:451–459
Cai QM, Gao XY, Chen YW, Ma SW (1995) Dynamic variations of water quality in Taihu Lake and multivariate analysis of its influential factors. J Lake Sci 7(2):97–106
Cai QM, Gao XY, Chen YW, Ma SW, Dokulil M (1997) Dynamic variations of water quality in Taihu Lake and multivariate analysis of its influential factors. J Chinese Geogr 7:72–82
Chang SC, Jackson ML (1957) Fractionation of soil phosphorus. Soil Sci 84:133–144
Das BM (1990) Principles of geotechnical engineering, 2nd edn. PWS-KENT, Boston, MA
Dorich RA, Nelson DW, Sommers LE (1984) Availability of phosphorus to algae from eroded soil fractions. Agric Ecosyst Environ 11:253–264
Dorich RA, Nelson DW, Sommers LE (1985) Estimating phosphorus in suspended sediments by chemical extraction. J Environ Qual 14:400–405
Elizabeth PK, Janet SH, Aaron LM, George MH (2002) Changes in the sorption capacity of Coastal Plain sediments due to redox alteration of mineral surfaces. Appl Geochem 17:387–398
Fan CX, Ji J, Wu WH, Qing BQ, Chen KN, Chen YW (1997) Comprehensive evaluation and preliminary prediction for water quality and eutrophication of Gonghu Bay. Trans Limnol Oceanogr 3:18–24
Friend MT, Birch HF (1960) Phosphate responses in relation to soil tests and organic phosphorus. J Agric Sci 54:341–346
Gibson CE (1997) The dynamics of phosphorus in freshwater and marine environments. In: Tunney H (ed) Phosphorus loss from soil to water. CAB International Press, Oxon, pp 121–144
Gonsiorczyk T, Casper P, Koschel R (1998) Phosphorus binding forms in the sediment of an oligotrophic and an eutrophic hardwater lake of the Baltic district (Germany). Water Sci Technol 37: 51–58
Institute of soil science, Chinese Academy of Sciences (1978) Physico-chemistry analyze of Soil. Shanghai science and technology publishing company, Shanghai, pp 35–45
Jin XC, Liu HL, Tu QY (1990) Eutrophication of lakes in China. Chinese Environmental Science Press, Beijing
Jin XC, Liu SK, Zhang ZS (1995) Lakes in China research of their environment (volume two). China Ocean Press, Beijing
Jin XC, Wang SR, Pang Y, Wu FC (2006) Phosphorus fractions and the effect of pH on the phosphorus release of the sediments from different trophic areas in the Taihu Lake, China. Environ Pollut 139:288–295
Li WC (1996) Biological and environmental succession in Wuli bay of Taihu Lake along with the eutrophication processes. J Lake Sci 8(Suppl):37–45
Liu TJ, Chen MQ (2001) Wetland ecological function deterioration in Poyang Lake and counter measures. Chinese J Ecol 20(3):74–77
Liu HH, Jin XC, Jing YF (1999) Environmental dredging technology of lake sediment. Chinese Eng Sci 1:81–84
Long N, James S (2002) Phosphorus fractions and retention in drainage ditch sediments receiving surface runoff and subsurface drainage from agricultural catchments in the North Island, New Zealand. Agric Ecosyst Environ 92:49–69
Ma B, Zhou ZY, Zhang CP, Zhang G, Hu YJ (2009) Inorganic phosphorus fractions in the rhizosphere of xerophytic shrubs in the Alxa Desert. J Arid Environ 73:55–61
Moriarty C (1998) Studies of Irish lakes and rivers. Marine Institute Press, Dublin
Olsen SR, Cole CV, Watanabe FS (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USDA, Washington, DC
Paludan C, Jensen HS (1995) Sequential extraction of phosphorus in freshwater wetland and lake sediment: significance of humic acids. Wetlands 15:365–373
Penn MR, Auer MT, Van Orman EL, Korienek JJ (1995) Phosphorus diagenesis in lake sediments: investigation using fractionation techniques. Mar Freshwater Res 46:89–99
Psenner R (1988) Fractionation of phosphorus in suspended matter and sediment. Ergebnisse der Limnologie 30:98–113
Psenner R, Pucsko R, Sager M (1985) Fraktionierung organischer und anorganischer Phosphorverbindungen von Sedimenten. Versuch einer Definition ökologisch wichtiger Fraktionen. Archiv für Hydrobiologie Supplement 70:111–115
Qin BQ (2002) Approaches to mechanisms and control of eutrophication of shallow lakes in the middle and lower reaches of the Yangtze River. J Lake Sci 14:193–202
Reddy KR, Overcash MR, Khaled R (1980) Phosphorus adsorption–desorption characteristics of two soils utilized for disposal of animal wastes. J Environ Qual 9:86–92
Ribeiro DC, Martins G, Nogueira R, Cruz JV, Brito AG (2008) Phosphorus fractionation in volcanic lake sediments (Azores–Portugal). Chemosphere 70:1256–1263
Ruban V, Brigault S, Demare D (1999) An investigation of the origin and mobility of phosphorus in freshwater sediments from Bort-Les-Orgues Reservoir, France. Environ Monit Assess 1(4):403–407
Ruban VJF, López SP, Pardo G, Rauret H, Muntau PQ (2001) Harmonized protocol and certified reference material for the determination of extractable contents of phosphorus in freshwater sediments: a synthesis of recent works. Fresenius J Anal Chem 370:224–228
Shen L, Lin GF, Tan JW, Shen JH (2001) Genotoxicity of surface water samples from Meiliang Bay, Taihu Lake, and Eastern China. Chemosphere 41:129–132
Wang SR, Jin XC, Pang Y, Zhao HC, Zhou XN, Wu FC (2005) Phosphorus fractions and phosphate sorption characteristics in relation to the sediment compositions of the shallow lakes in the middle and lower reaches of Yangtze River. J Colloid Interface Sci 289:339–346
Wang SR, Jin XC, Zhao HC, Wu FC (2006) Phosphorus fractions and its release in the sediments from the shallow lakes in the middle and lower reaches of Yangtze River area in China. Colloid Surf A 273:109–116
Williams JDH, Jaquet JM, Thomas RL (1976) Forms of phosphorus in surficial sediments of Lake Erie. Fish Res 33:413–429
Wu FC, Qing H, Wan GJ (2001) Regeneration of N, P and Si near the sediment/water interface of lakes from Southwestern China Plateau. Water Res 35:1334–1337
Xie LQ, Xie P, Tang HJ (2003) Enhancement of dissolved phosphorus release from sediment to lake water by Microcystis blooms: an enclosure experiment in a hyper-eutrophic, subtropical Chinese lake. Environ Pollut 122:391–399
Yan WJ, Zhang JC, Ji M (1999) Characteristics of N, P formation in main rivers of Chao Lake Valley. J Soil Erosion Water Conserv 5(2):35–38
Yang L, Sun J (2001) Phosphorus pollution investigation of Wuli Lake and Meiliang Lake. Environ Monit Manag Technol 13(6):18–20
Yang ZF, Shi WG, Chen LQ, Chen Y, Zhou ZL (2003) Ecological environment succession and countermeasure of East Taihu Lake. Chinese Environ Sci 23(1):64–68
Yuan HQ, Pin XH, Yao XM (2000) Management of water environment of Hongze Lake. Environ Monit Manag Technol 12(1):26–27
Zanini L, Robertson WD, Ptacek CJ, Schiff SL, Mayer T (1998) Phosphorus characterization in sediments impacted by septic effluent at four sites in central Canada. J Contam Hydrol 33:405–429
Zhang YL, Qin BQ (2001) Study prospect and evolution of eutrophication in Lake Taihu. Shanghai Environ Sci 20(6):263–265
Zhou HX, Sheng GY, Sun C, Xu OY (1996) Distribution of organic contaminants Lake Taihu. Water Resour 30(9):2003–2008
Zhou QX, Christopher EG, Zhu YM (2001) Evaluation of phosphorus bioavailability in sediments of three contrasting lakes in China and the UK. Chemosphere 42:221–225
Zhou AM, Tang HX, Wang DS (2005) Phosphorus adsorption on natural sediments: Modeling and effects of pH and sediment composition. Water Res 39:1245–1254
Zhu M, Wang GX, Wang J (2004) Comparative analysis of changes of pollutants in sediment in Nanjing Xuanwu Lake before and after sediment dredging. J Nanjing Normal Univ (Eng Technol) 4(2):66–69
Acknowledgments
Authors wish to thank for the financial support from National Basic Research Program of China (2008CB418200), the National natural science foundation of China (No. 40703017, U0833603), and Chinese Research Academy of Environment Sciences (No. 2007KYYW27).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, S., Jin, X., Bu, Q. et al. Evaluation of phosphorus bioavailability in sediments of the shallow lakes in the middle and lower reaches of the Yangtze River region, China. Environ Earth Sci 60, 1491–1498 (2010). https://doi.org/10.1007/s12665-009-0284-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-009-0284-1